-
PDF
- Split View
-
Views
-
Cite
Cite
Jingyu Liu, Qian Zhu, Yan Pan, Sainan Hao, Zhaoxian Wang, Chuting Cui, Junwei Li, Yueying Huang, Liangjun Xia, Tiancheng Xu, Jie Cheng, Jie Shen, Youbing Xia, Electroacupuncture alleviates intrauterine adhesion through regulating autophagy in rats, Molecular Human Reproduction, Volume 29, Issue 11, November 2023, gaad037, https://doi.org/10.1093/molehr/gaad037
Close - Share Icon Share
Abstract
Autophagy is a well-conserved metabolic system that maintains homeostasis by relying on lysosomal breakdown. The endometrium of patients with intrauterine adhesion (IUA) and an animal model exhibits impaired autophagy. Autophagy is negatively correlated with inflammation. Activation of autophagy can inhibit the inflammatory response, while defects in autophagy will activate the inflammatory response. Here, we studied whether electroacupuncture (EA) inhibits inflammation and promotes endometrial injury repair by activating endometrial autophagy. The IUA animal model was established by mechanical injury plus lipopolysaccharide infection. EA stimulation was applied to the acupoints Guanyuan (CV4), bilateral Sanyinjiao (SP6), and Zusanli (ST36). The results indicated that EA could improve endometrial morphology, attenuate endometrial fibers, and enhance endometrial receptivity in the rat. EA could increase the autophagosomes of endometrial epithelial cells, increase the levels of LC3 and Beclin1, and decrease the level of p62. Additionally, EA may also suppress the nuclear factor kappa-B (NF-κB) signaling pathway and reduce the release of inflammatory factors. Additionally, the effect of EA was comparable to that of the autophagy agonist rapamycin, and the autophagy inhibitor 3-methyladenine reversed the therapeutic effect of EA. Therefore, we assume that EA may facilitate endometrial healing by activating autophagy and reducing NF-κB signal pathway-mediated inflammation.
Introduction
Intrauterine adhesion (IUA) is a common uterine factor in infertility, and approximately one in five women who miscarry suffer from this disease (Hooker et al., 2014). IUA is essentially endometrial fibrosis, which is defined as an abnormal healing process following injury beyond the scarless regeneration ability of tissue, whether traumatic or infectious (Yu et al., 2008). Currently, hysteroscopy is the gold standard for treatment; however, it can also lead to a series of complications (Amin et al., 2015). Despite numerous management approaches to prevent the recurrence of fibrotic synechiae, including hyaluronic acid gel, hormonal treatment, intrauterine devices, and human amnion, there remains a lack of evidence to demonstrate that any intervention is effective in preventing endometrial fibrosis after hysteroscopy (Healy et al., 2016). Therefore, it is critical to develop a safe and effective alternative treatment for patients with IUA.
IUA has been categorized as fibrotic endometritis according to etiology and pathogenesis (Czernobilsky, 1978). The activation and expression of the nuclear factor kappa-B (NF-κB) signaling pathway are critical to the pathophysiology of IUA (Xue et al., 2015). Inflammatory substances are generated locally in the endometrium as a result of inflammation, which stimulates the accumulation of extracellular matrix (ECM) and accelerates the process of uterine adhesion.
Autophagy is an intracellular degradation system in which a portion of the cytoplasm is wrapped in a bilayer membrane-binding structure called the autophagosome, which undergoes maturation and fusion with the lysosome for degradation (Klionsky and Emr, 2000; Levine and Klionsky, 2004). Autophagy is involved in the physiological functions of the endometrium, including periodic menstruation, embryo implantation, and decidualization during normal pregnancy (Yang et al., 2019; Devis-Jauregui et al., 2021). There are multiple levels of autophagy deficiency in the endometrium of patients with IUA (Zhou et al., 2022). Correlation research has indicated that autophagy can negatively regulate the inflammatory response, while autophagy defects can induce the inflammatory response (Deretic, 2021). Autophagy can control the NF-κB signaling pathway (Paul et al., 2012) and the release of inflammatory cytokines (Qian et al., 2017). There is an interaction between autophagy and the NF-κB pathway in IUAs, although the specific mechanism needs further study.
Acupuncture and moxibustion are important components of traditional Chinese medicine (Ye et al., 2017), which dredge the meridians and regulate the functions of the viscera (Zhang et al., 2015). Electroacupuncture (EA) therapy is a method of treating diseases by using EA instruments to generate pulse currents and acting on acupoints. With the ability to objectively manipulate the stimulus quantity, EA therapy can compensate for the limits of conventional acupuncture in terms of acupuncture frequency and stimulation intensity (Su et al., 2023) and can promote the circulation of Qi and blood and modify the muscular tension. Recently, numerous studies have indicated that EA is effective in the field of assisted reproduction (Dehghani et al., 2020; Guven et al., 2020). EA has a good clinical effect on low endometrial receptivity (Shuai et al., 2015), mainly by enhancing the microcirculation, improving morphological structure, regulating estrogen and progesterone receptors (Stener-Victorin et al., 1996; Chen and Hau, 2015), and increasing the clinical pregnancy rate (Zhong et al., 2019). As previously reported (Xia et al., 2019; Xi et al., 2021), EA showed a mending impact on endometrial damage. Other studies have suggested that EA can modulate autophagy, promote cell survival, and improve the pathological morphology of tissues to exert the protective effect of acupuncture (Xue et al., 2014). Here, we demonstrated the effects of EA on autophagy and the repair of endometrial injury in a rat model.
Materials and methods
Animals
All experiments complied with the ARRIVE guidelines 2.0 (updated guidelines for reporting animal research) (Percie Du Sert et al., 2020). Mature female and male Sprague-Dawley (SD) rats aged 8–12 weeks (weighing 200–300 g) were obtained from the Shanghai Xipuer-Bikai Lab Animal Co., Ltd. (Shanghai, China). All rats were maintained under controlled 12-h light-dark cycles with a standard diet and free access to water. The study was approved by the Animal Care and Use Committee of Nanjing University of Chinese Medicine (Reference Number: 202207A028). All rats were fed adaptively for 1 week, and those with normal estrous cycles were included in the experiment.
Experimental design
In the first part of the experiment, 45 female rats were randomly divided into three groups: control group, IUA group, and electroacupuncture group (EA) (n = 15). Ten male rats were used for cage mating with female rats. In the second part of the experiment, 40 female rats were randomly placed into four groups: IUA group, EA group, EA + autophagy inhibitor 3-methyladenine (3-MA) group, and autophagy agonist rapamycin (RAPA) group (n = 10). In order to determine whether the autophagy inhibitor 3-MA could counteract the therapeutic effects of EA, the rats in the electroacupuncture + inhibitor group were injected i.p. with the autophagy inhibitor 3-MA on the basis of EA treatment. Meanwhile, we used the autophagy agonist RAPA as a positive control and contrasted it with EA to compare the two treatments’ effects (Fig. 1).
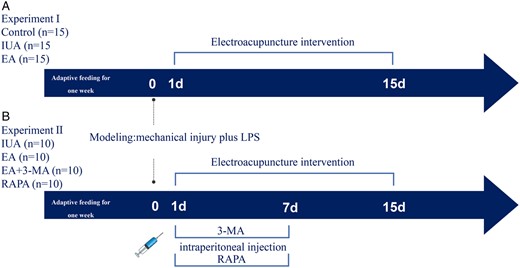
Schematic of the experimental design for a study on the effects of electroacupuncture on intrauterine adhesion in rats. (A) Experiment I grouping and experimental procedure. (B) Experiment II grouping and experimental procedure. The electroacupuncture group was treated with electroacupuncture the next day after molding, once a day for 15 days. The 3-MA and RAPA were injected i.p. the next day after molding, once a day for 7 days. IUA: intrauterine adhesion; EA: electroacupuncture; 3-MA: 3-methyladenine; RAPA: rapamycin.
Modeling
The IUA rat model was established by mechanical injury plus lipopolysaccharide (LPS) infection, as reported (Sun et al., 2019). The right uterine horn was modeled during estrus, which corresponded to the late reproductive phase in humans, based on vaginal smears (Wood et al., 2007). All the rats were anesthetized with 2–3% isoflurane, and then anesthesia was maintained with 1.5–2%. The uterus was exposed, and the horn was damaged with a curette that was applied to scrape up and down until the uterine wall became rough. Subsequently, a cotton thread containing LPS (Escherichia coli 0111: B4; Sigma, St. Louis, MO, USA) was placed into the damaged uterine cavity, and a 0.5-cm tail wire was left in the vagina to remove the cotton thread 48 h later. The incisions were sutured, and the animals were allowed to recover.
Treatments
EA was applied after modeling for three consecutive estrous cycles. Acupuncture needles (0.18 × 13 mm; Beijing Zhongyan Taihe Medical Instrument Co., Ltd., Beijing, China) were pierced to a depth of 2 mm at Guanyuan (CV4) and 5 mm at Sanyinjiao (SP6) and Zusanli (ST36). The location and depth of the acupoints are described in detail in Experimental Acupuncture and Moxibustion (Yu and Xu, 2012). CV4 is located 25 mm below the navel of the rat’s abdomen; SP6 is located on the hind limbs, 10 mm above the tip of the medial malleolus; and ST36 is located 3–4 mm below the midline of the knee and 1–2 mm laterally. Bilateral SP6 and ST36 were connected to an electrical stimulator (SDZ-II, Suzhou Medical Appliance Factory, Suzhou, China). A diagram of the EA intervention is shown in Supplementary Fig. S1. The EA stimulation was conducted once daily for 15 min for three consecutive estrus cycles. The EA + 3-MA group was injected i.p. with 3-MA (HY-19312; MCE, NJ, USA), 1.5 mg/kg/day on the basis of EA therapy. The RAPA group was injected i.p. with RAPA (B20714; Yuanye Bio-Technology Co., Ltd., Shanghai, China), 2 mg/kg/day.
Fertility testing
Female rats were mated with male rats at a ratio of 2:1 during estrus. The morning of the presence of vaginal plugs was considered Day 1 of pregnancy. On the eighth day of gestation, the number of embryos implanted in the rat was calculated. The diagrams of embryos on Day 8 of pregnancy are shown in Supplementary Fig. S2.
Hematoxylin–eosin staining
The uterus tissues were fixed overnight at 4°C in 4% paraformaldehyde, dehydrated in a graduated series of ethanol, cleaned in xylene, and then embedded in paraffin. The isolated endometrial tissues were cut into 6-μm-thick sections before staining with hematoxylin and eosin and viewing under light microscopy (IX73; Olympus, Tokyo, Japan). Image J (National Institutes of Health [NIH], Bethesda, MD, USA) software was used to measure the endometrial thickness (the vertical distance between the junction of endometrium and the myometrium). The average of the four fields was used in the statistical analysis.
Masson’s trichrome staining
Masson’s staining (G1340, Solarbio, Beijing, China) was applied to the uterus following the manufacturer’s instructions. Following fixation with Bouin buffer solution, the collagen fibers were stained pale blue with aniline blue solutions, and the muscle fibers were dyed red with Ponceau solutions. Image J software was used to assess the fibrosis area.
Transmission electron microscopy
Fresh endometrial tissues were fixed with 2.5% glutaraldehyde and 1% osmium tetroxide before being cut into 150-nm-thick slices and analyzed using an electron microscope (Tecnai G2 Spirit Bio TWIN, FEI, OR, USA). Sample processing and sectioning were completed by experts from the Analysis and Testing Center of Nanjing Medical University.
Immunohistochemistry staining
The endometrial tissue sections were deparaffinized, dehydrated, and rehydrated. Following that, citrate buffer was employed to retrieve the antigen and endogenous peroxidase activity was blocked using hydrogen peroxide (3%). Primary antibodies were applied to the endometrial sections after blocking with 5% bovine serum albumin (BSA), and primary antibodies, including anti-microtubule-associated protein1 light chain 3 (LC3) (1:300, 14600-1-AP, Proteintech Group, IL, USA); anti-Beclin1 (1:200, 66665-1-Ig, Proteintech Group, IL, USA); anti-p62 (1:200, 18420-1-AP, Proteintech Group, IL, USA); anti-transforming growth factor-β1 (TGF-β1) (1:400, 21898-1-AP, Proteintech Group, IL, USA); and anti-alpha-smooth muscle actin (α-SMA) (1:5000, 14395-1-AP, Proteintech Group, IL, USA) were added to the endometrial sections. Negative controls for immunohistochemistry are shown in Supplementary Fig. S3. Immunoreactivity was observed using the Mouse and Rabbit Specific HRP/DAB Assay Kit (SA1022, BOSTER, CA, USA) according to the manufacturer’s instructions.
ELISA
Protein was extracted from the endometrium using PBS solution containing protease inhibitor and then the standard, standard diluent, and sample were added sequentially, followed by the addition of the detection antibody to each well. The concentrations of IL-6, IL-1β, and tumor necrosis factor (TNF)-α in the endometrial tissues were quantified using the rat IL-6 ELISA Kit (RX302856R, Quanzhou Ruixin Biotechnology Co., Ltd, Quanzhou, China), the Rat Interleukin-1β High Sensitivity ELISA Kit (EK301BHS, MultiSciences, Hangzhou, China), and the Rat TNF-α ELISA Kit (EK382, MultiSciences, Hangzhou, China) in accordance with the specification, and the optical density value was calculated at 450/630 nm wavelength. By plotting the standard curve, the levels of IL-6, IL-1β, and TNF-α in the sample were determined.
Quantitative real-time PCR
Total RNA was extracted from the endometrium with TRIzol reagent (R0016, Beyotime, Shanghai, China) according to the manufacturer’s instructions. cDNA synthesis from RNA reverse transcription was performed with Hifair® III 1st Strand cDNA Synthesis SuperMix for qPCR gDNA digester plus (1114IES60, Yeasen Biotech Co., Ltd, Shanghai, China). The cDNA mixture preparation was shown in Supplementary Table S1. Quantitative Real-time PCR (qPCR) was performed using HieffTM qPCR SYBR Green Master Mix (Low Rox Plus) (11202, Yeasen Biotech Co., Ltd., Shanghai, China) on Sequence Detection software (Applied Biosystems, Foster City, CA, USA). The PCR primers are shown in Supplementary Table S2 (Generay Biotech Co., Ltd., Shanghai, China). The quantitative expression of mRNA was analyzed by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Western blot
Protein was extracted from the endometrium using radioimmunoprecipitation assay (RIPA) lysis buffer, and the protein concentration was quantified with an enhanced BCA protein detection kit (P0010, Beyotime, Shanghai, China). Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate 10 µg of total protein, and the separated protein was then transferred to polyvinylidene difluoride membranes. The SDS-PAGE gel formulation is listed in Supplementary Table S3. The membranes were incubated with primary antibodies overnight at 4°C with mild shaking after being blocked with 5% BSA for 2 h at room temperature. The details of all the antibodies used in this study are listed in Supplementary Table S4. The ECL (36208ES60, Yeasen Biotech Co., Ltd., Shanghai, China) developer solution was uniformly spread on polyvinylidene difluoride membrane for development. The bands were evaluated using the gel imaging system (5200, Tanon, Shanghai, China), and the protein intensity was quantified using Image J software.
Statistical analysis
SPSS 23.0 software was used for statistical analysis (IBM, NY, USA). Data are presented as the mean ± SEM. A one-way ANOVA was used to conduct comparisons among groups. Levene was used to test the homogeneity of variance; if it was, the least squares difference method was employed for comparison; otherwise, Dunnett’s T3 was utilized. A P-value <0.05 was considered statistically significant.
Results
Electroacupuncture restores endometrial morphology
As shown in Fig. 2A, the endometrial thickness of IUA rats became thinner and the number of glands decreased compared to the control group, indicating poor morphologic recovery of the injured uterus. However, after EA treatment, the endometrial thickness improved and more glands appeared (Fig. 2C and D).
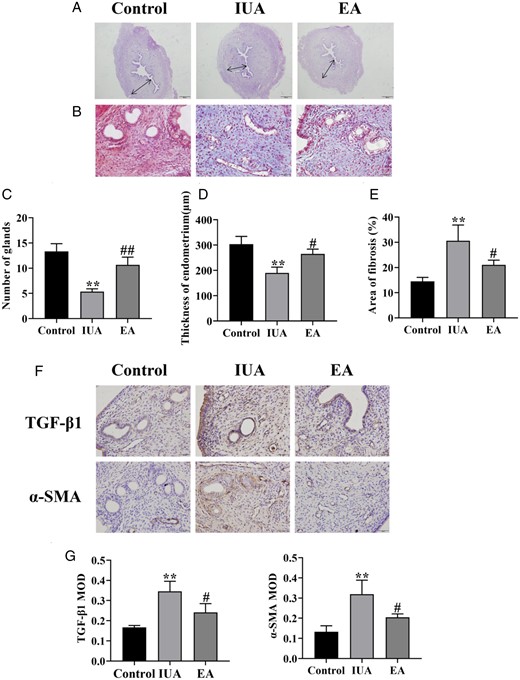
Comparison of endometrial morphology in rats. (A) Hematoxylin–eosin staining of rat uterine tissue (×40). (B) Masson’s staining shows endometrial fibrosis (×400). (C) Number of endometrial glands. (D) Endometrial thickness. (E) Endometrial fibrosis area. (F) Immunohistochemistry staining of TGF-β1 and α-SMA in endometrial tissues (×400). (G) The average MOD values of TGF-β1 and α-SMA. A one-way ANOVA was used to conduct comparisons among groups. Data are represented as the mean ± SEM (n =3). **P < 0.01, compared to the control group; #P < 0.05 and ##P < 0.01, compared to the model group. TGF-β1: transforming growth factor-β1; α-SMA: alpha-smooth muscle actin; MOD: mean optical density; IUA: intrauterine adhesion; EA: electroacupuncture.
The essence of IUAs is fibrosis. Collagen fibers are blue according to Masson staining, whereas red blood cells and muscle fibers are red. The endometrial fibrosis region was considerably larger in the IUA group compared to the control group, but the fibrosis area decreased after EA treatment (Fig. 2B and E).
TGF-β1 and α-SMA are important markers of fibrosis, which are closely related to the development of IUA. The endometrial tissues in the IUA group had higher protein levels of TGF-β1 and α-SMA than those in the control group. EA therapy decreased TGF-β1 and α-SMA protein in comparison to the IUA group (Fig. 2F and G). We concluded that EA might alleviate endometrial fibrosis and promote injury repair.
Electroacupuncture improves endometrial receptivity and increases embryo implantation rate
HoxA10 is a crucial regulator of embryo implantation, affecting endometrial decidualization (Ashary et al., 2020). The cytokine leukemia inhibitory factor (LIF) impacts trophoblast migration and invasion by acting on endometrial epithelial cells (Kondera-Anasz et al., 2004). The results demonstrated that the protein and mRNA expression levels of HoxA10 and LIF were both significantly downregulated after modeling, whereas their levels in the EA treatment group were significantly higher than in the IUA group (Fig. 3A–C).
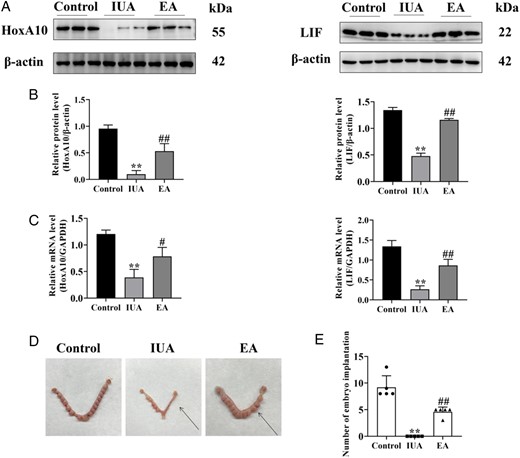
Electroacupuncture improves endometrial receptivity in a rat model. (A) HoxA10 and LIF protein bands were determined by western blot. The uncropped versions of these blots are presented in Supplementary Fig. S4. (B) Relative expression level of HoxA10 and LIF (n =3). (C) HoxA10 and LIF mRNA expression were analyzed by q-PCR (n =3). (D) Representative diagrams of embryos on Day 8 of pregnancy, with the arrow denoting the uterus after endometrial injury. (E) Number of embryo implantations in the damaged uterus (n =5). A one-way ANOVA was used to conduct comparisons among groups. Data are represented as the mean ± SEM. **P < 0.01, compared to the control group; #P < 0.05 and ##P < 0.01, compared to the model group. HoxA10: homeobox A10; LIF: leukemia inhibitory factor; IUA: intrauterine adhesion; EA: electroacupuncture.
Patients with IUA frequently suffer from diminished fertility. The results here showed that the control group had the largest number of embryo implantations, with 13, 8, 8, 8, and 9. There was no embryo implantation in the damaged endometrium in the model group. Furthermore, the number of embryo implantations in the EA group was higher than that of the IUA group, with n = 5, 5, 5, 5, and 3 (Fig. 3D and E).
Electroacupuncture rescues impaired autophagy in the endometrium
Autophagy is a non-apoptotic form of programmed cell death induced by high levels of cell stimulation that is essential for maintaining endometrial homeostasis (Popli et al., 2022). The formation of the autophagosome is crucial to the process of autophagy, and the autophagosome structure observed under transmission electron microscopy is the gold standard for detecting autophagy (Eskelinen et al. 2011). As illustrated in Fig. 4A, there were few autophagosomes in the IUA group. After EA treatment, the autophagosomes were well-developed, and the morphology of endometrial epithelial cells returned to normal, with distinct cell borders and normal mitochondria.
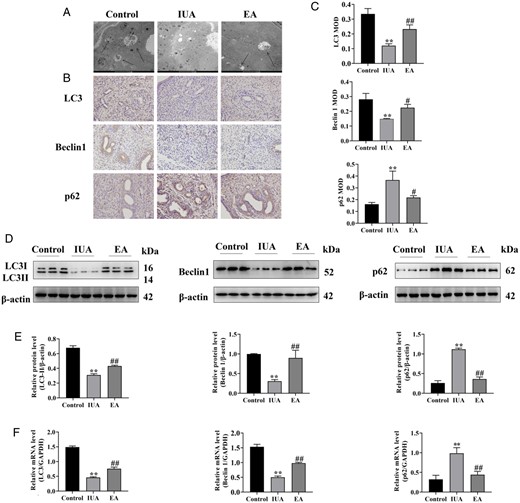
Electroacupuncture rescues impaired autophagy in the rat endometrium. (A) The autophagosomes of endometrial epithelial cells were observed by TEM. Arrowheads indicate autophagosomes. Scale bars: 5 μm. (B) Immunohistochemistry staining of LC3, Beclin1, and p62 proteins in endometrial tissues (×400). (C) The average MOD values of LC3, Beclin1, and p62 were calculated. (D) LC3, Beclin1, and p62 protein bands were determined by western blot. The uncropped versions of these blots are presented in Supplementary Fig. S5. (E) Relative protein expression levels of LC3, Beclin1, and p62. (F) LC3, Beclin1, and p62 mRNA expression were analyzed by q-PCR. A one-way ANOVA was used to conduct comparisons among groups. Data are represented as the mean ± SEM (n = 3). **P < 0.01, compared to the control group; #P < 0.05 and ##P < 0.01, compared to the model group. TEM: transmission electron microscopy; LC3: microtubule-associated protein 1 light chain 3; MOD: mean optical density; IUA: intrauterine adhesion; EA: electroacupuncture.
LC3 (an autophagosome marker), Beclin1 (an autophagy activation marker), and the p62 protein (an autophagy receptor and degradation substrate) are crucial to autophagy. Their expression in endometrial tissues was detected by immunohistochemistry analysis. The positive staining of LC3 and Beclin1 was weaker in the IUA group. After EA treatment, the positive staining was enhanced, indicating elevated content of LC3 and Beclin1. The level of p62, an important index of autophagic flux-related inhibition, showed the opposite results (Fig. 4B and C).
Additionally, the levels of LC3 and Beclin1 protein and mRNA expression in the IUA group were significantly lower than those in the control group, indicating defective autophagy in the IUA group. However, their levels considerably increased after EA therapy. Simultaneously, the levels of p62 protein and mRNA were also significantly higher in the IUA group as compared to the control group. Compared to the IUA group, the level of p62 protein and mRNA in the EA group was significantly decreased (Fig. 4D–F).
Electroacupuncture inhibits endometrial inflammation in rats with intrauterine adhesion
NF-κB is involved in the development of IUA. In IUA model rats, inhibitor of Kappa B Kinase alpha (IKKα), inhibitor of kappa B alpha (IκBα), and p65 phosphorylation levels were elevated compared to the control group, indicating the NF-κB signal pathway was activated. However, after EA treatment, these levels were considerably reduced (Fig. 5B and C).
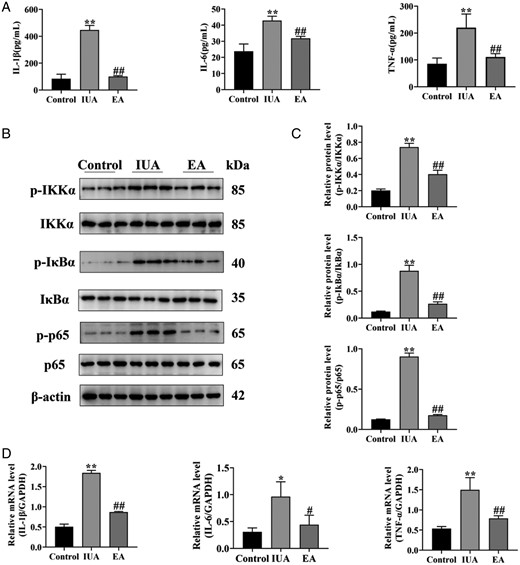
Electroacupuncture inhibits endometrial inflammation in rats with intrauterine adhesion. (A) IL-1β, IL-6, and TNF-α in endometrial tissue were detected by ELISA. (B) The phosphorylation levels of IKKα, IκBα, and p65 protein bands were determined by western blot. The uncropped versions of these blots are presented in Supplementary Fig. S6. (C) Relative level of IKKα, IκBα, and p65 protein expression. (D) IL-1β, IL-6, and TNF-α mRNA expression were analyzed by q-PCR. A one-way ANOVA was used to conduct comparisons among groups. Data are represented as the mean ± standard error (SEM) (n = 3). *P < 0.05 and **P < 0.01, compared to the control group; #P < 0.05 and ##P < 0.01, compared to the model group. IL: interleukin; TNF-α: tumor necrosis factor-alpha; IKKα: inhibitor of Kappa B Kinase alpha; IKBα: inhibitor of kappa B alpha; IUA: intrauterine adhesion; EA: electroacupuncture.
The expression of inflammatory cytokines can be regulated by the nuclear translocation of NF-κB. The levels of IL-1β, IL-6, and TNF-α proteins and mRNAs were increased in IUA, as measured by ELISA and qPCR, respectively, while the levels of these inflammatory cytokines were decreased after EA treatment (Fig. 5A and D). It has been hypothesized that EA can reduce the inflammatory response of IUA, which may be related to the inhibition of the NF-κB signal pathway and, consequently, the expression of inflammatory factors.
Mechanisms of electroacupuncture regulating autophagy and inhibiting endometrial inflammation in rats with intrauterine adhesion
Autophagy has been proven to negatively modulate the inflammatory response; however, it is unclear whether EA can achieve the therapeutic effects of IUA by activating autophagy and inhibiting inflammation. In order to further examine the correlation between autophagy and the inflammatory response, we used the autophagy inhibitor 3-MA and the autophagy agonist RAPA to suppress and activate autophagy, respectively. 3-MA, the most widely used autophagy inhibitor, is thought to be able to block Class III phosphatidylinositol 3-kinase (Class III PI3K) activity, which prevents autophagy from occurring and progressing (Wu et al., 2010). RAPA is a commonly used autophagy activator and a specific inhibitor of the mammalian target of rapamycin (Noda, 2017).
EA and RAPA greatly increased the protein and mRNA expression levels of LC3 and Beclin1 and decreased the protein and mRNA expression levels of p62; however, there was no statistically significant difference between the EA group and the RAPA group. In contrast, EA + 3-MA decreased the protein and mRNA levels of LC3 and Beclin1 and increased the protein and mRNA levels of p62; there was no statistically significant difference between the EA + 3-MA group and the IUA group (Fig. 6).
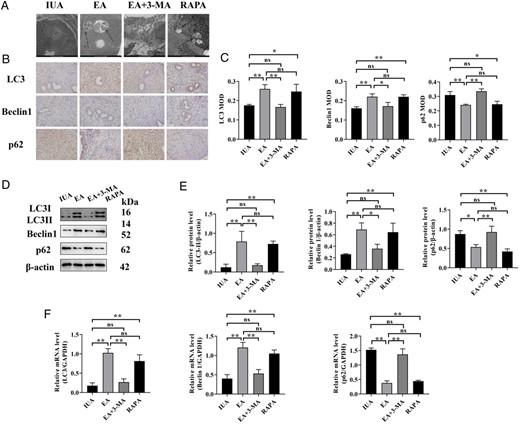
Endometrial autophagy of rats in each group. (A) The autophagosomes of endometrial epithelial cells were observed by TEM. Arrowheads indicate autophagosomes. Scale bars: 5μm. (B) Immunohistochemistry staining of LC3, Beclin1, and p62 proteins in endometrial tissues (×400). (C) The average MOD values of LC3, Beclin1, and p62 were calculated. (D) LC3, Beclin1, and p62 protein bands were determined by western blot. The uncropped versions of these blots are presented in Supplementary Fig. S7. (E) Relative expression levels of LC3, Beclin1, and p62. (F) LC3, Beclin1, and p62 mRNA expression were analyzed by q-PCR. A one-way ANOVA was used to conduct comparisons among groups. Data are represented as the mean ± SEM (n = 3). *P < 0.05 and **P < 0.01, ns: no significance. TEM: transmission electron microscopy; LC3: microtubule-associated protein 1 light chain 3; MOD: mean optical density; IUA: intrauterine adhesion; EA: electroacupuncture 3-MA: 3-methyladenine; RAPA: rapamycin.
EA and RAPA reduced the levels of IKKα, IκBα, and p65 phosphorylation and inhibited the expression of inflammatory factors, but there was no statistically significant difference between the EA group and the RAPA group. EA + 3-MA augmented the levels of IKKα, IκBα, and p65 phosphorylation and increased the expression of inflammatory factors; there was no statistically significant difference between the EA + 3-MA group and the IUA group (Fig. 7).
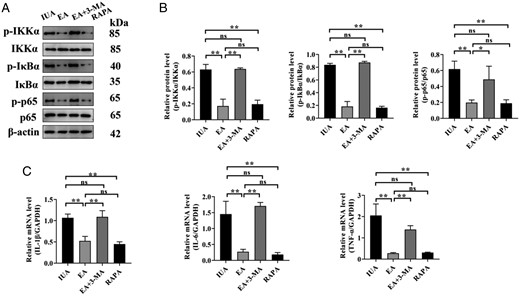
Endometrial inflammation of rats in each group. (A) The phosphorylation levels of IKKα, IκBα, and p65 protein bands were determined by western blot. The uncropped versions of these blots are presented in Supplementary Figs S8 and S9. (B) Relative level of IKKα, IκBα, and p65 protein expression. (C) IL-1β, IL-6, and TNF-α mRNA expression were analyzed by q-PCR. A one-way ANOVA was used to conduct comparisons among groups. Data are represented as the mean ± standard error (SEM) (n = 3). *P < 0.05 and **P < 0.01, ns: no significance. IKKα: inhibitor of Kappa B Kinase alpha; IKBα: inhibitor of kappa B alpha; IL: interleukin; TNF-α: tumor necrosis factor-alpha; IUA: intrauterine adhesion; EA: electroacupuncture 3-MA: 3-methyladenine; RAPA: rapamycin.
EA and RAPA increased endometrial thickness and the number of glands, decreased fibrosis severity, and lowered TGF-β1 and α-SMA expression; there was no statistically significant difference between the EA group and the RAPA group. Nevertheless, EA + 3-MA reversed the therapeutic tendency of EA; there was no statistically significant difference between the EA + 3-MA group and the IUA group (Fig. 8).
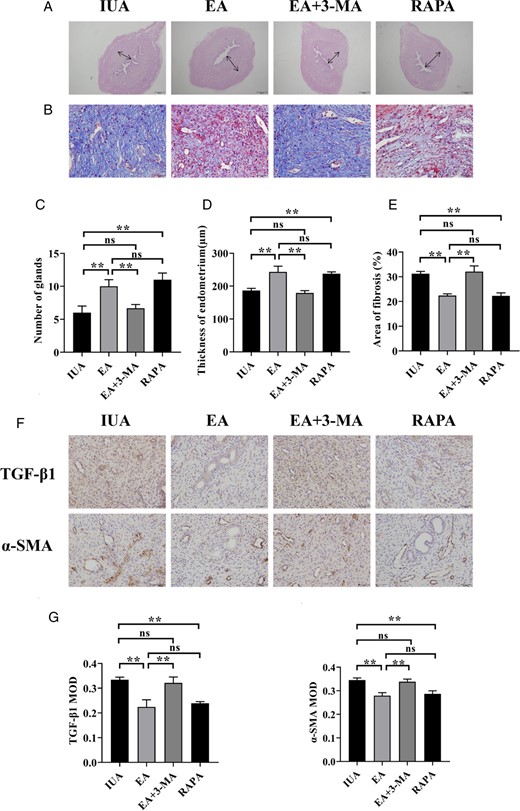
Endometrial repair effect for rats in each group. (A) H&E staining of rat uterine tissue (×40). (B) Masson’s staining shows endometrial fibrosis (×400). (C) Number of endometrial glands. (D) Endometrial thickness. (E) Endometrial fibrosis area. (F) Immunohistochemistry staining of TGF-β1 and α-SMA in endometrial tissues (×400). (G) The average MOD values of TGF-β1 and α-SMA. A one-way ANOVA was used to conduct comparisons among groups. Data are represented as the mean ± standard error (SEM) (n = 3). **P < 0.01, ns: no significance. TGF-β1: transforming growth factor-β1; α-SMA: alpha-smooth muscle actin; MOD: mean optical density; IUA: intrauterine adhesion; EA: electroacupuncture; 3-MA: 3-methyladenine; RAPA: rapamycin.
Discussion
IUA is a frequent post-hysteroscopy consequence that is known to have an impact on female fertility. Hormone therapy, hysteroscopy, and intrauterine devices have all recently been employed to treat IUA, although each has drawbacks. As a result, EA therapy has been investigated as a promising new technique to repair endometrial injury.
In this study, we constructed a physiological and clinical-related model of IUA rats with mechanical injury plus LPS infection by evaluating the degree of gland loss and fibrosis. Additionally, following EA therapy, not only did the number of endometrial glands increase remarkably but also the region of adhesion and the degree of fibrosis decreased. It has been reported that the reduction in collagen fibers is an important factor determining the repair effect of endometrial damage (Bai et al., 2019). We came to the preliminary conclusion that EA could promote the recovery of uterine morphology.
According to bibliometric research, CV4 and SP6 are the most widely used acupoints to improve endometrial receptivity, as they have a tight neurological connection to the uterus. Stimulation of these acupoints has been shown to improve uterine microcirculation, increase uterine blood flow rate, and decrease vascular resistance (Liu et al., 2018). ST36 was utilized in the current experiment; it can nourish Qi and blood and modulate the hypothalamic–pituitary–ovarian axis to promote pituitary and ovarian endocrine function (Jiang and Zou, 2017). Furthermore, ST36 exhibits anti-inflammatory properties (Liu et al., 2021). It is claimed that EA at these acupoints can regulate the function of the reproductive axis and promote endometrial repair.
The primary pathological manifestation of IUA is endometrial fibrosis, which is characterized by excessive ECM deposition and recombination to replace the normal endometrium. TGF-β1, an essential fibrogenic mediator, stimulates extensive ECM deposition in the endometrium and induces epithelial–mesenchymal transformation (Yao et al., 2019). TGF-β1 was found to be elevated in both human endometrial tissue and IUA animal models (Ning et al., 2018). α-SMA, the key marker of myofibroblasts, is induced by TGF-β1 signaling. Our results indicated that EA reduced the expression of TGF-β1 and α-SMA, suggesting that EA attenuated fibrosis in rats by inhibiting TGF-β1 signaling.
Endometrial receptivity is one of the important factors affecting embryo implantation, which is a significant parameter to evaluate female fertility. HoxA10 is a homeobox-containing transcription factor (Block et al., 2000) that is expressed in human endometrial epithelial and stromal cells, coinciding with the implantation window. LIF can act on epithelial and mesenchymal cells in an autocrine or paracrine manner, promote the establishment of endometrial receptivity (Tapia et al., 2008), and regulate the implantation and development of the embryo. There is evidence to suggest that the downregulation of HoxA10 reduces endometrial receptivity (Evans-Hoeker and Young, 2014; Rosario et al., 2014). Our results showed that EA greatly increased the expression of HoxA10 and LIF, demonstrating its potential to enhance endometrial receptivity. To further study the effectiveness of EA on reproductive capacity, the embryo implantation rate of rats was examined. The findings revealed a significantly lower rate of embryo implantation in the IUA group, demonstrating that IUA-related infertility is associated with reduced receptivity as a result of endometrial damage. Additionally, increased embryo implantation was observed in EA-treated rats, indicating that EA can be used as an important auxiliary approach in the field of reproduction.
Autophagy exists in endometrial tissue to maintain endometrial homeostasis (Devis-Jauregui et al., 2021). The dynamic change process in the endometrium involves continuous cell adaptation to shifting surroundings (Yang et al., 2019). Endometrial autophagy is inhibited when IUA occurs, and autophagy rescue is a part of the recovery process (Wei et al., 2020). The implementation of autophagy depends on double-membrane autophagosomes, which are formed within cells through the extension and elongation of endoplasmic reticulum-derived autophagosomes (Ohsumi, 2014). Our results revealed that autophagosomes were well-developed in the EA group, indicating that EA could improve the level of autophagy. LC-3 is a well-known autophagosome marker and is involved in the production of autophagosomes (Tanida et al., 2004). Beclin1 is involved in the initiation of autophagy by heightening vesicle nucleation (Suzuki et al., 2001). In this research, LC3 and Beclin1 levels decreased in the IUA group. Notably, the expression of LC3 and Beclin1 was obviously enhanced after EA therapy. To examine autophagic flux more carefully, we examined the level of the renowned autophagy substrate p62. The p62 protein, which is upregulated in cells with autophagy defects, can be employed as a carrier receptor for autophagy degradation of ubiquitinated substrate proteins (Lamark et al., 2009). The expression of p62 was markedly reduced in response to the rise in LC3 and Beclin1 expression. Collectively, all of these alterations suggested that EA was involved in the process of activating autophagy.
LPS, the primary pathogenic component of endotoxin, binds to Toll-like receptors to activate the NF-κB signaling pathway. The NF-κB pathway can stimulate inflammatory factors to be released and contribute to IUA inflammation (Wang et al., 2017). As a proinflammatory factor contactor, IL-1β is secreted in excessive amounts into the circulatory system, resulting in a disordered inflammatory response. IL-1 can also bind to TNF-α and interferon-γ to trigger TGF-β1-induced epithelial–mesenchymal transition (EMT), exacerbating fibrosis. IL-6 has the ability to transform acute inflammation into a persistent, profibrotic state that impairs tissue healing (Fielding et al., 2014). TNF-α induces a chain reaction of inflammatory signals and promotes fibrosis. In comparison to IUA, EA decreased the phosphorylation levels of critical NF-κB pathway proteins IKKα, IκBα, and p65, as well as the expression of IL-1β, IL-6, and TNF-α. It is suggested that EA can diminish the endometrial inflammatory response of IUA, which may be related to the suppression of the NF-κB signal pathway, hence reducing the expression of inflammatory factors.
There is a close relationship between autophagy and the inflammatory response, and autophagy efficiently suppresses the inflammatory response. p62 plays a key role in the regulatory interaction between autophagy and the NF-κB signaling cascade. p62 builds up as a result of the inhibition of autophagy, and its ZZ domain forms complexes with atypical protein kinase C by firmly binding to receptor interaction protein 1, promoting IκB protein phosphorylation and degradation, and then bringing about NF-κB nuclear translocation (Tao et al., 2020). The reduction in IUA autophagy levels prevents the restriction of autophagy on the inflammatory response, leading to NF-κB activation and the infiltration of a significant number of inflammatory components. To study the correlation between IUA autophagy and inflammation, the autophagy inhibitor 3-MA and the autophagy agonist RAPA were utilized, respectively. The study indicated that upregulation of autophagy can reduce the activation of the NF-κB pathway, while inhibition of autophagy can boost the inflammatory response, which is consistent with previous studies (Peng et al., 2019). Additionally, it was discovered that the effect of EA was comparable to that of the RAPA. Notably, on the basis of EA treatment, the addition of the autophagy inhibitor 3-MA can significantly reduce the level of autophagy and promote inflammatory response, possibly because 3-MA inhibits the effect of EA on boosting autophagy. It was demonstrated that the mechanism of action of EA was associated with the activation of autophagy.
Nevertheless, there remain certain limitations to this study. First, a limited sample size may not be sufficiently representative, which has a direct impact on the validity of the research findings. Second, autophagy acts as a double-edged sword in endometrium-related pathology with a very limiting knowledge regarding the mechanisms underlying its multifaceted role. The specific mechanism of the interaction between autophagy and inflammation needs to be further studied.
In conclusion, EA can promote endometrial injury repair by activating endometrial autophagy and suppressing the NF-κB pathway-mediated inflammatory response, which has the effect of relieving IUA.
Data availability
The data underlying this article are available in the article and in its online Supplementary Material.
Authors’ roles
Ji.L., Q.Z., Y.P., S.H., and Z.W. conducted the experiments and collected data. C.C., Ju.L., and Y.H. analyzed the data. Ji.L. drafted the article. J.C., J.S., L.X., and T.X. reviewed and edited the article. Y.X. took responsibility for the integrity of the data analysis. The final text has been reviewed and approved by all authors.
Funding
This work was financed by the National Natural Science Foundation of China (No. 81873371) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX22_1962).
Conflict of interest
The authors have declared no conflict of interest.



