-
PDF
- Split View
-
Views
-
Cite
Cite
Erik M Hegeman, Miles W A Fisher, Daniel J Cognetti, Benjamin F Plucknette, Joseph F Alderete, David Wilson, Marlin Wayne Causey, Traumatic Transradial Forearm Amputation Temporized With Extracorporeal Membrane Oxygenation: A Brief Report, Military Medicine, Volume 189, Issue 1-2, January/February 2024, Pages e27–e33, https://doi.org/10.1093/milmed/usad148
Close - Share Icon Share
ABSTRACT
Extracorporeal membrane oxygenation (ECMO) is typically used to provide mechanical perfusion and gas exchange to critically ill patients with cardiopulmonary failure. We present a case of a traumatic high transradial amputation in which the amputated limb was placed on ECMO to allow for limb perfusion during bony fixation and preparations and coordination of orthopedic and vascular soft tissue reconstructions.
This is a descriptive single case report which underwent managment at a level 1 trauma center. Instutional review board (IRB) approval was obtained.
This case highlights many important factors of limb salvage. First, complex limb salvage requires a well-organized, pre-planned multi-disciplinary approach to optimize patient outcomes. Second, advancements in trauma resuscitation and reconstructive techniques over the past 20 years have drastically expanded the ability of treating surgeons to preserve limbs that would have otherwise been indicated for amputation. Lastly, which will be the focus of further discussion, ECMO and EP have a role in the limb salvage algorithm to extend current timing limitations for ischemia, allow for multidisciplinary planning, and prevent reperfusion injury with increasing literature to support its use.
ECMO is an emerging technology that may have clinical utility for traumatic amputations, limb salvage, and free flap cases. In particular, it may extend current limitations of ischemia time and reduce the incidence of ischemia reperfusion injury in proximal amputation, thus expanding the current indications for proximal limb replantation. It is clear that developing a multi-disciplinary limb salvage team with standardized treatment protocols is paramount to optimize patient outcomes and allows limb salvage to be pursued in increasingly complex cases.
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a life-saving intervention for critically ill patients with refractory cardiopulmonary failure. Currently, indications for ECMO therapy for adults include acute respiratory distress syndrome (ARDS), cardiac failure, and extracorporeal support during cardiopulmonary resuscitations.1 ECMO is applied via two modalities, venovenous ECMO, which provides respiratory support, and venoarterial ECMO, which can provide respiratory and hemodynamic support.2 The indications for ECMO and extracorporeal perfusion (EP) continue to expand from cardiopulmonary resuscitation to transplantation, replantation, and limb salvage. Reports of traumatic extremity amputations attempting to undergo limb salvage, as well as extremity replantation that uses EP to prolong ischemia times, are the current areas of focus.
The decision to pursue limb salvage or replantation is complex and requires critical evaluation of patient-specific factors, the injury’s nature, and the available medical resources.3 Key patient factors essential for reimplantation consideration include resuscitation status, other traumatic injuries, medical comorbidities, age, and vocation. Injury factors include mechanism (laceration versus crush), level of injury, the extent of soft tissue injuries, and ischemia time. When evaluated in context, these factors allow treating surgeons to evaluate the available medical resources to determine if perusing limb salvage is appropriate.
Ischemia time, in particular, is of great concern when considering replantation and limb salvage surgery in the upper extremity. This is typically addressed through controlled hypothermia of the amputated extremity to decrease the metabolic rate and prevent ischemia reperfusion injury (IRI), which is defined as the acute inflammation of reperfused tissue following a period of ischemia.4 Reactive oxygen species and activated neutrophils are the main factors implicated in local and systemic damage through disruption of the cell membrane and activation of the cyclooxygenase and lipoxygenase pathways.4 IRIs can have devasting consequences if not mitigated and can lead to local sequelae of failed replantation, amputation, compartment syndrome, myonecrosis, and contractures. However, even more pressing are the potential systemic complications such as adult respiratory distress syndrome, acute kidney injury secondary to rhabdomyolysis, acute multi-organ failure, and death.4
Historically, upper extremity critical ischemia times were defined as 6 hours of warm ischemia time (without cooling) and 12 hours of cold ischemia time for hand and proximal replantations.5 These limits, however, are not well defined as there are reports of successful hand replantations after 54 hours of cold storage.6 As a general rule, the more proximal the amputation, the shorter the allowed ischemia time. This is due to increasing metabolic demands of proximal amputations secondary to greater muscle mass when compared to more distal amputations.
Currently, there are few clinical strategies aimed to reduce the effects of IRI besides controlled extremity hypothermia and proper patient resuscitation. Therapeutic delivery of hydrogen sulfide and copolymer surfactants has shown efficacy in animal models to improve myocyte survival and improve mouse hind-foot survival.7 Additionally, hyperbaric oxygen therapy has been shown to be effective in reducing skeletal muscle injury in a rat torniquet IRI model.8 However, these interventions have not yet entered into the treating algorithms for replantations, and further human trials are needed to validate their effectiveness.
The ultimate goals of care in replantation and limb salvage are to preserve life, tissue, and function and reconstruct functions that may have been lost because of injury.9 This is typically accomplished operatively by thoroughly examining and debriding the wound, restoring limb perfusion, rigid skeletal fixation, and viable soft tissue coverage. This requires a multidisciplinary surgical team that usually includes orthopedic, vascular, plastic, and extracorporeal membrane specialists and trauma surgeons to work together to optimize patient outcomes.
CASE PRESENTATION
A 21-year-old right hand–dominant male was involved in a restrained motor vehicle rollover accident at around 7:00 am. The patient reported waving his right arm outside his car window to get the attention of an oncoming vehicle before the collision at which time his arm was pinned underneath his car as it rolled over. In the field, Emerency Medical Services noted he was ambulating without assistance and had sustained a traumatic amputation just distal to the elbow with arterial bleeding and gross contamination. The amputated limb was cooled en route.
The patient arrived to the trauma bay at 7:35 am and was found to be hemodynamically stable with a Glasgow coma score of 15. Primary and secondary surveys revealed no other traumatic injuries, later confirmed with a computed tomography scan before transfer to the operating room (OR), which demonstrated no acute injuries to the head, chest, abdomen, or pelvis. Initial trauma radiographs taken at 7:45 am revealed a traumatic amputation of the right forearm distal to the elbow joint with a comminuted fracture of the proximal radius and a segmental fracture of the proximal and midshaft ulna. Immediately after radiographs were complete, his amputated limb was transferred to the OR for preparation; at approximately 8:15 am, the limb was removed from cooling (total cold ischemia time roughly 1 hour 15 minutes) and underwent a thorough debridement, identification of anatomic structures, assessment of soft tissue injuries, and cannulation of the radial and ulnar arteries in preparation for possible utilization of ECMO. Additionally at this time, the ECMO and orthopedic limb salvage teams, which included fellowship trained hand and oncology surgeons, were consulted in preparation to receive the patient in the OR. Figure 1 demonstrates his preoperative photographs and radiographs.
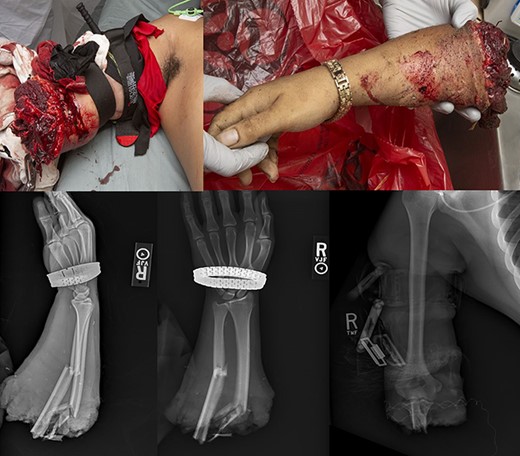
The patient endorsed a history of well-controlled schizoaffective disorder, anxiety, and depression treated with brexpiprazole and vortioxetine. He denied any other significant medical, surgical, or medication history. He wished to proceed to the OR for exploration and possible replantation.
At 8:37 am, the patient entered the OR, where a multidisciplinary team of orthopedic surgery, vascular surgery, anesthesiology, and ECMO providers received the patient. Working simultaneously, all teams prepared for a coordinated effort to pursue replantation and limb salvage. Based on the extent of soft tissue damage, proximal amputation level, and the possible need for long segment vascular and nerve repairs, the team elected to utilize ECMO as an adjunct to perfuse the limb. This immediately addressed the ischemia time and allowed for better decision-making in determining the optimum limb length, appropriate vascular bypass length, and method of nerve repair. As the amputated limb was already prepared, the patient underwent an exploration of the patient’s proximal limb to develop a plan for hemorrhage control and perfusion. Vascular surgery identified the proximal brachial artery. Given that the plan was to support the severed limb with ECMO, a 7 French sheath was placed in the radial artery and a micropuncture sheath in the ulnar artery. The proximal brachial artery was identified, and a 5 French micropuncture sheath was placed (Cook Medical; Bloomington, IN). These were connected using Check-Flo valves on the micropuncture and a 3-way stopcock. Due to the lack of venous outflow, the shunted limb was allowed free bleeding while shunted, and the basilic vein was identified in both the amputated limb and the proximal limb. These were then shunted using Check-Flo valves and a 3-way stopcock (Fig. 2). This allowed venous outflow through named structures in addition to the severed deep veins that were not amendable to reconstruction.
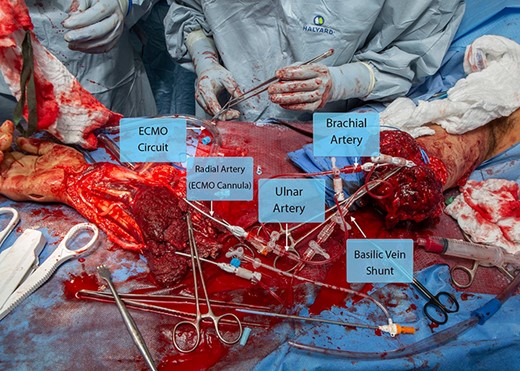
The amputated limb and residual extremity after arterial and venous shunting with ECMO cannula placement. Abbreviation: ECMO, extracorporeal membrane oxygenation.
At 9:30 am, the ECMO circuit was primed using whole blood, tissue plasminogen activator, glucose, heparin, and insulin. The 3-way shunting allowed the amputated limb to receive perfusion systemically through the ulnar artery while the ECMO allowed limb perfusion through the radial artery using 5 units of whole blood, 20,000 units of heparin, 100 mg of tissue plasminogen activator, 10 units of insulin, 10 g of dextrose, and 1 ampoule of bicarbonate. Figure 3 demonstrates the intraoperative use of ECMO, anastomoses, and reconstruction. Venous outflow and return to the ECMO circuit were performed with the limb in a basin. The circuit was monitored by the ECMO team who measured electrolytes and the acid base status and monitored the perfusion of the limb through the reservoir basin. Ultimately, the limb required a perfusion rate of 100-200 mL/min.
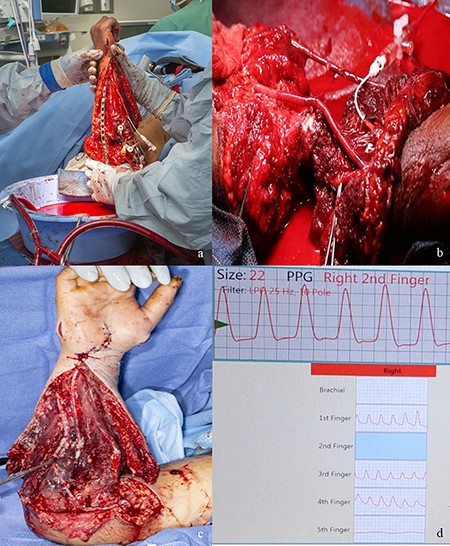
(A) The open reduction and internal fixation of the ulna while the open ECMO circuit is perfusing the amputated forearm. (B) Radial and ulnar artery anastomoses. (C) Postoperative day 6 appearance of the reconstruction after repeat debridement. (D) Vascular Doppler studies of the digital arteries postoperative revealing perfusion of the thumb through the ring finger with appropriate waveforms. Abbreviation: ECMO, extracorporeal membrane oxygenation.
At 9:37 am, the orthopedic team began an open reduction and internal fixation of the ulna and radius to provide a stable length for vascular surgery to perform definitive arterial and venous anastomoses to allow for proper bypass length; they were able to obtain proper length, alignment, and rotation, without shortening. The greater saphenous vein was harvested during the above procedures and a bypass was performed from the proximal brachial artery, proximal to the shunting, to the radial artery with reimplantation of the ulnar artery onto the bypass, roughly 8 cm in graft length. An additional 8 cm bypass was done from the basilic vein in the severed limb to the residual basilic vein to allow venous outflow, which was the only large vein amendable to bypass reconstruction as the deep venous structures were unable to be reconstructed.
After arterial and venous anastomoses were complete, attention was focused on musculoskeletal and soft tissue reconstruction. The patient underwent repair of all flexor and extensor tendons and allograft nerve reconstruction of the ulnar and median nerves, which had segmental defects of 12 cm. He underwent a brachialis rotational flap to cover his vascular anastomoses as there was muscle and skin loss overlying the antecubital fossa after debridement. Once these repairs and reconstructions were complete, he underwent hand and forearm fasciotomies with carpal tunnel and Guyon’s canal releases. His wounds were vacuumed open, and he was placed into a splint for soft tissue rest.
He was admitted to the surgical trauma intensive care unit; intubated for q1 hour neurovascular checks, extremity warming, antibiotics (ancef and gentamycin), and hemodynamic monitoring; and started on heparin and aspirin anticoagulation per the vascular team to minimize the risk of bypass thrombosis. Overall, this case took 11 and a half hours, 15 units of whole blood, 11 units of fresh frozen plasma, two units of cryoprecipitate, two units of platelets, and 7 L of crystalloid to complete.
On postoperative day 1, the vascular team assessed the extremity with digital photoplethysmography, and this demonstrated normal finger waveforms in digits 1 through 4. Additionally, a pulse oximeter was placed on the 2nd digit in the postoperative period, which demonstrated good waveform and oxygen saturation levels above 95%. The patient underwent serial debridement every 2-3 days with the orthopedic and vascular teams to debride necrotic tissue, visualize the anastomoses’ integrity, and plan for soft tissue coverage. Plans were being made for definitive closure with split-thickness skin grafting in the upcoming days.
Unfortunately, on postoperative day 9, his heparin drip was discontinued because of concerns of hypovolemic shock after a debridement and wound vacuum exchange and not restarted for 24 hours. During that period, there was evidence of vascular congestion and a dusky appearing forearm. His heparinization was restarted, but unfortunately, when he was reexplored on postoperative day 12, his hand had demarcated. Attempts were made for bypass thrombectomy and revision, but his microvascular thrombosis had progressed, and there was arterial bypass compromise. On postoperative day 14, the decision was made to pursue amputation secondary to a necrotic forearm, which would provide a nonfunctional hand. Figure 4 demonstrates photographs just before and after amputation.
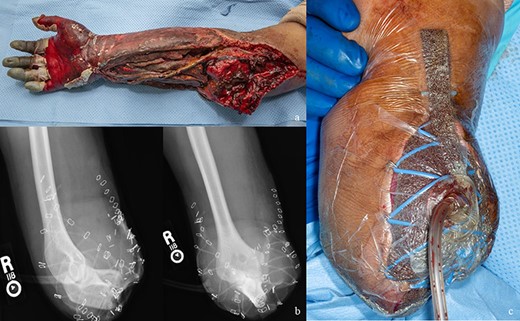
(A) Appearance of the replanted forearm before amputation. (B) Postoperative radiographs showing high transradial amputation. (C) Clinical photograph of transradial amputation.
DISCUSSION
This case highlights many important factors of limb salvage. First, complex limb salvage requires a well-organized, preplanned multidisciplinary approach to optimize patient outcomes.10,11 Second, advancements in trauma resuscitation and reconstructive techniques over the past 20 years have drastically expanded the ability of treating surgeons to preserve limbs that would have otherwise been indicated for amputation.12–14 Lastly, which will be the focus of further discussion, ECMO and EP have a role in the limb salvage algorithm to extend current timing limitations for ischemia, allow for multidisciplinary planning, and prevent reperfusion injury with increasing literature to support its use.15–18
The idea that major traumatic amputations, residual limbs, and free flaps could be stabilized and addressed stepwise over an extended period is attractive from a revascularization and reconstructive standpoint. This is particularly true in cases of prolonged ischemia time and inability to perform limb salvage in a timely manner due to austere environments, hospital capabilities, and the patient resuscitation status.
In this case, given the proximal amputation level, crush nature of injury, extensive soft tissue damage, and need for long segment bypass and nerve reconstructions, the risks of IRI are high. These factors led the treating team to utilize ECMO as an adjunct to restore vascular perfusion as quickly as possible. In this case, ECMO was performed when the patient was in the OR as he had a quick transition to the OR and fortunately lacked any other traumatic injuries. If he had other injuries or factors to delay replantation, then ECMO cannulation and application would have occurred in the OR prior to the patient arrival, as establishing timely perfusion to the amputated limb would likely increase odds of successful replantation and functional outcomes. Extracorporeal membrane oxygenation may expand the indications for proximal extremity replantation by reducing the effects of IRI. In patients who have other traumatic life-threatening injuries that take precedent, it would allow replantation to be pursued in a delayed fashion without concerns for cold or warm ischemia times. This would effectively provide the limb resuscitation while the patient underwent surgical or medical resuscitation. Inadequate patient resuscitation is currently cited as a contraindication to replantation.19 Another relative contraindication to replantation is a crush mechanism of injury.20,21 This is due to high rates of late failure likely associated with the IRI secondary to severe myonecrosis. However, by ECMO controlling the electrolyte concentrations and establishing vascular flow to the amputated limb, the effects may be mitigated.
A few case studies have demonstrated the ability to utilize ECMO and EP for proximal extremity replantation in a delayed fashion. Greaney described a case where ECMO was used to perfuse a replanted trans-humeral amputation for 72 hours in the setting of cardiopulmonary arrest with demonstrated viability before eventual amputation because of concerns of infection.15 Taeger reported the use of EP in two major lower extremity amputates with successful replantation after 12 and 15 hours of external storage.16 These examples demonstrate that there is a role for EP in amputation and replantation care that may expand and improve outcomes in properly selected patients, especially those with proximal amputations.
In addition to traumatic amputations, current ECMO and EP research is promising in extending the ischemia time and prevalence of IRI in free flap surgery. This application of ECMO allows for increased perfusion and decreased ischemia time after dissection of the flap. The reported literature has demonstrated successful use of EP for flap perfusion during the repositioning of the patient and in cases of flap salvage while minimizing prolonged ischemia time.17,18 Taeger et al. performed a comparative study of EP versus cold ischemic storage on porcine musculocutaneous flaps, which demonstrated that the EP group had lower rates of cellular apoptosis and more physiologic electrolyte concentrations compared to cold ischemia.17 They propose that EP may be a viable option to extend flap ischemia time and prevent reperfusion injury.
Although this concept is promising, there remain significant logistical obstacles before reliable clinical use. Eberlin, in a brief clinical report, outlined many of these challenges.22 First, ECMO is a specialized technology in which only select centers can perform, limiting its widespread use. Second, the utilization of ECMO for limb salvage is incredibly resource-intensive, as seen in our patient; this may not be feasible in all situations. Third, there are several unknown technical challenges such as the timing of ECMO cannulation, infection prophylaxis, and a lack of standardized ECMO treatment algorithms to determine optimum flow rates, ECMO solution component concentrations, cannulation techniques, and storage, which are current limitations and areas of research.
This case reveals some of these challenges. This case occurred at a specialized level 1 trauma center with an ECMO team with robust clinical experience and the resources to sustain a prolonged ECMO extremity resuscitation. When evaluating possible reasons for failure, this case highlights the need for appropriate anticoagulation and critical evaluation of the integrity of the venous structures, which are amendable to reconstruction. In this case, only the basilic vein was able to be reconstructed and provide venous outflow for the forearm. Unfractionated heparin (UFH) infusion is the anticoagulation method of choice for proximal replantation as infusion rates can be titrated to obtain target activated partial thromboplastic clotting times (aPTT), and it is easily reversible with protamine sulfate. This is especially important in trauma patients who often have unexpected returns to the OR. Low molecular–weight heparin is a viable option; however, it is harder to titrate to target aPTT levels and is not as readily reversible as UFH. It is usually reserved for cases of heparin-induced thrombocytopenia or allergies. In most cases of replantation, aspirin also initiated for additional platelet inhibition. The current literature suggests that in digital amputations LMWH has been shown to have less bleeding and hypocoagulability complications compared to UFH, although the rates of successful replantation were found to have no difference.23 There is no consensus data guiding the management for proximal replantations above the hand. We suggest continuing heparinization even with hypovolemic or inflammatory response conditions and only stopping for hemorrhagic complications with careful multidisciplinary consideration.
CONCLUSION
Extracorporeal membrane oxygenation is an emerging technology that may have clinical utility for traumatic amputations, limb salvage, and free flap cases. In particular, it may extend current limitations of ischemia time and reduce the incidence of IRI in proximal amputation, thus expanding the current indications for proximal limb replantation. It is clear that developing a multidisciplinary limb salvage team with standardized treatment protocols is paramount to optimize patient outcomes and allows limb salvage to be pursued in increasingly complex cases. Further animal models and clinical research are needed to elicit and overcome conceptual and technical challenges related to implementing ECMO in the clinical setting and validate its role in treating IRI.
ACKNOWLEDGMENTS
None declared.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author. All data are freely accessible.
CLINICAL TRIAL REGISTRATION
None declared.
INSTITUTIONAL REVIEW BOARD (HUMAN SUBJECTS)
Institutional Review Board (IRB) approved through the Brooke Army Medical Center institutional review board; reference number C.2013.134d/391,024.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC)
Not applicable.
INDIVIDUAL AUTHOR CONTRIBUTION STATEMENT
E.M.H. collected and analyzed the data and drafted the original manuscript. All authors read and approved the final manuscript.
INSTITUTIONAL CLEARANCE
Institutional clearance does not apply.
REFERENCES
Author notes
The views expressed in this material are those of the authors and do not reflect the official policy or position of the U.S. Government, the Department of Defense, or the Department of the Army.
- ischemia
- extracorporeal membrane oxygenation
- amputation
- amputation, traumatic
- critical illness
- limb
- limb salvage
- perfusion
- reconstructive surgical procedures
- reperfusion injury
- surgical replantation
- resuscitation
- trauma centers
- wounds and injuries
- transradial amputation
- free flap
- soft-tissue reconstruction
- patient-focused outcomes
- cardiorespiratory failure



