-
PDF
- Split View
-
Views
-
Cite
Cite
Shachar Shapira, Maya Nitecki, Dorit Tzur, Naama Schwartz, Barbara G Silverman, Oren Zack, Limor Friedensohn-Zuck, Occupational Exposure to Nonionizing Radiation and Risk for Malignancy in Young Adults, Military Medicine, Volume 188, Issue 7-8, July/August 2023, Pages e2424–e2430, https://doi.org/10.1093/milmed/usad020
Close - Share Icon Share
ABSTRACT
Nonionizing radiation (NIR) is considered “possibly carcinogenic to humans,” and therefore, exposure of young military personnel raises concerns regarding increased risk for cancer. The aim of our study was to compare the cancer incidence in exposed and nonexposed populations in order to gain better understanding of their risk.
A longitudinal retrospective cohort study, between 2009 and 2018, was conducted. Israel Defense Forces (IDF) aerial defense units service members, with NIR exposure (range of 2-300 GHz, below the International Commission of Non-Ionizing Radiation Protection guidelines), were compared with a similar sociodemographic group of service members without NIR exposure. Both groups were followed for cancer incidence (all-cause and specific malignancies). Kaplan–Meier analysis of cancer-free survival and univariate and multivariable logistic regressions for possible confounders and risk factors were performed. This analysis was repeated on a matched 1:1 control group.
Exposure and comparison groups included 3,825 and 11,049 individuals, respectively. Forty-one cases diagnosed with cancer were identified during the follow-up time (mean 4.8 [±2.7] years), 13 (0.34%) of which were reported in the exposure group, and 28 (0.25%) were reported in the comparison group. The odds ratio (OR) for cancer incidence in the exposure vs. control groups was 1.34 (95%CI, 0.70-2.60), P-value = 0.3807. The results remained unchanged after adjustment for sex, age at enrollment, service length, socioeconomic status, and military occupation (adjOR = 1.38 [95%CI, 0.67-2.82], P = 0.3818).
Our study did not find an increased short-term risk for cancer in young adults exposed to NIR radiation as compared with unexposed young adults.
INTRODUCTION
During the last decade, aerial defense platforms and capabilities have developed dramatically worldwide, with an increasing number of military personnel engaged in operating these complex systems. These systems enable detection, tracking, and interception of a variety of airborne threats such as missiles, jets, and unmanned aerial vehicles. The main component of these platforms is the radar, which is crucial for all neutralization capabilities. This occupational environment carries the risk for exposure to nonionizing radiation (NIR) (predominantly radiofrequency [RF] and/or microwave [MW] electromagnetic fields [EMFs]) emitted from radar and other communication systems. The principal effect of NIR on body tissues is thermal, primarily affecting the eyes and skin.1,2 Other potential nonthermal adverse health effects reported include impaired brain functioning;3 a blunted circadian heart rhythm and a lower 24-hour heart rate;1,4 hearing loss;5 changes in the secretion, composition, and flow of saliva;6 and neurodegenerative diseases.7 Short-term exposure to very high-level EMF has been suggested to pose a risk of fertility impairment,8 although studies on sperm counts have been equivocal.4 Since NIR has been shown in cell cultures and animal studies to generate oxygen-free radicals,9 leading to oxidative stress and a disturbance in DNA repair mechanisms,10 a possible neoplastic effect remains a health concern. In 2011, the International Agency for Research on Cancer (IARC) determined that there is limited evidence both in humans and in animals for the carcinogenicity of RF radiation.11,12 However, because of positive associations reported in observational studies between RF radiation exposure from wireless phones and formation of gliomas and acoustic neuromas, RF-EMFs were classified as “possibly carcinogenic to humans” (category 2B). This controversy surrounding health hazards associated with NIR exposure is a subject for continuous research. Therefore, we conducted a retrospective cohort study to evaluate the incidence of cancer among military personnel serving in aerial defense military units exposed to NIR as compared with service members without NIR exposure.
METHODS
Study Population
We performed a retrospective cohort study including all Israel Defense Forces (IDF) service members who have served in aerial defense units, with NIR exposures, since the establishment of these units in 2009. A comparison group of active duty soldiers serving between the years 2009 and 2018 was established out of the general IDF population based on similar sociodemographic properties (such as gender diversity, medical fitness profiles, and socioeconomic profiling of place of residence [socioeconomic status, SES]) and lack of occupational NIR exposure. Furthermore, the groups had similar exposure to various chemicals (such as fuels and solvents, which change per mission profiles). To limit possible environmental confounders, the comparison group served in the same geographic areas as the exposure group. Albeit similar, there were significant differences between the two raw groups (Table I).
| . | Comparison group . | Exposed group . | . |
|---|---|---|---|
| . | N = 11,049 . | N = 3825 . | P-value . |
| Age at exposure (years) | 19.8 (1.9) [19.3, 18.1-44.8] | 19.9 (2.6) [19.1, 18-46.3] | <0.0001 |
| Gender—male | 6,658 (60.26%) | 2,779 (72.65%) | <0.0001 |
| BMI (kg/m2) | 22.2 (3.8) [21.7, 13-44] | 22.1 (4) [21, 13-46] | 0.0586 |
| BMI ≥ 30 (kg/m2) | 555 (5.11%) | 210 (5.59%) | 0.2514 |
| Israeli native | 9,246 (83.82%) | 3,182 (83.28%) | 0.4349 |
| SES | 0.0013 | ||
| Low | 2,787 (25.3%) | 988 (25.92%) | |
| Medium | 5,959 (54.09%) | 1,945 (51.04%) | |
| High | 2,271 (20.61%) | 878 (23.04%) | |
| Main occupation | <0.0001 | ||
| Combat | 8,756 (79.32%) | 2,172 (56.86%) | |
| Maintenance | 245 (2.22%) | 676 (17.7%) | |
| Driver | 210 (1.9%) | 252 (6.6%) | |
| Officer | 555 (5.03%) | 209 (5.47%) | |
| Others | 175 (1.59%) | 49 (1.28%) | |
| Administration | 1,098 (9.95%) | 462 (12.09%) | |
| Year of exposure initiation | <0.0001 | ||
| 2009-2010 | 2,207 (19.97%) | 454 (11.87%) | |
| 2011-2012 | 1,886 (17.07%) | 870 (22.75%) | |
| 2013-2014 | 2,328 (21.07%) | 877 (22.93%) | |
| 2015-2016 | 2,505 (22.67%) | 929 (24.29%) | |
| 2017-2018 | 2,123 (19.21%) | 695 (18.17%) | |
| Service length (days) | 541.3 (298.4) [509, 90-3337] | 608 (404.6) [551, 90-3337] | <0.0001 |
| Mortality | 21 (0.19%) | 4 (0.1%) | 0.2660 |
| Follow-up time (years) | 4.8 (2.8) [4.7, 0.2-9.1] | 4.6 (2.6) [4.4, 0.2-9.1] | <0.0001 |
| Cancer incidence | 28 (0.25%) | 13 (0.34%) | 0.3794 |
| Age at cancer incidence | 25.2 (5.5) [24, 19.8-43.6] | 23.1 (2.3) [22.9, 19.5-28.6] | 0.3199 |
| . | Comparison group . | Exposed group . | . |
|---|---|---|---|
| . | N = 11,049 . | N = 3825 . | P-value . |
| Age at exposure (years) | 19.8 (1.9) [19.3, 18.1-44.8] | 19.9 (2.6) [19.1, 18-46.3] | <0.0001 |
| Gender—male | 6,658 (60.26%) | 2,779 (72.65%) | <0.0001 |
| BMI (kg/m2) | 22.2 (3.8) [21.7, 13-44] | 22.1 (4) [21, 13-46] | 0.0586 |
| BMI ≥ 30 (kg/m2) | 555 (5.11%) | 210 (5.59%) | 0.2514 |
| Israeli native | 9,246 (83.82%) | 3,182 (83.28%) | 0.4349 |
| SES | 0.0013 | ||
| Low | 2,787 (25.3%) | 988 (25.92%) | |
| Medium | 5,959 (54.09%) | 1,945 (51.04%) | |
| High | 2,271 (20.61%) | 878 (23.04%) | |
| Main occupation | <0.0001 | ||
| Combat | 8,756 (79.32%) | 2,172 (56.86%) | |
| Maintenance | 245 (2.22%) | 676 (17.7%) | |
| Driver | 210 (1.9%) | 252 (6.6%) | |
| Officer | 555 (5.03%) | 209 (5.47%) | |
| Others | 175 (1.59%) | 49 (1.28%) | |
| Administration | 1,098 (9.95%) | 462 (12.09%) | |
| Year of exposure initiation | <0.0001 | ||
| 2009-2010 | 2,207 (19.97%) | 454 (11.87%) | |
| 2011-2012 | 1,886 (17.07%) | 870 (22.75%) | |
| 2013-2014 | 2,328 (21.07%) | 877 (22.93%) | |
| 2015-2016 | 2,505 (22.67%) | 929 (24.29%) | |
| 2017-2018 | 2,123 (19.21%) | 695 (18.17%) | |
| Service length (days) | 541.3 (298.4) [509, 90-3337] | 608 (404.6) [551, 90-3337] | <0.0001 |
| Mortality | 21 (0.19%) | 4 (0.1%) | 0.2660 |
| Follow-up time (years) | 4.8 (2.8) [4.7, 0.2-9.1] | 4.6 (2.6) [4.4, 0.2-9.1] | <0.0001 |
| Cancer incidence | 28 (0.25%) | 13 (0.34%) | 0.3794 |
| Age at cancer incidence | 25.2 (5.5) [24, 19.8-43.6] | 23.1 (2.3) [22.9, 19.5-28.6] | 0.3199 |
SES, socioeconomic status; BMI, Body Mass Index. Continuous variables are presented with mean (standard deviation) [median, range].
| . | Comparison group . | Exposed group . | . |
|---|---|---|---|
| . | N = 11,049 . | N = 3825 . | P-value . |
| Age at exposure (years) | 19.8 (1.9) [19.3, 18.1-44.8] | 19.9 (2.6) [19.1, 18-46.3] | <0.0001 |
| Gender—male | 6,658 (60.26%) | 2,779 (72.65%) | <0.0001 |
| BMI (kg/m2) | 22.2 (3.8) [21.7, 13-44] | 22.1 (4) [21, 13-46] | 0.0586 |
| BMI ≥ 30 (kg/m2) | 555 (5.11%) | 210 (5.59%) | 0.2514 |
| Israeli native | 9,246 (83.82%) | 3,182 (83.28%) | 0.4349 |
| SES | 0.0013 | ||
| Low | 2,787 (25.3%) | 988 (25.92%) | |
| Medium | 5,959 (54.09%) | 1,945 (51.04%) | |
| High | 2,271 (20.61%) | 878 (23.04%) | |
| Main occupation | <0.0001 | ||
| Combat | 8,756 (79.32%) | 2,172 (56.86%) | |
| Maintenance | 245 (2.22%) | 676 (17.7%) | |
| Driver | 210 (1.9%) | 252 (6.6%) | |
| Officer | 555 (5.03%) | 209 (5.47%) | |
| Others | 175 (1.59%) | 49 (1.28%) | |
| Administration | 1,098 (9.95%) | 462 (12.09%) | |
| Year of exposure initiation | <0.0001 | ||
| 2009-2010 | 2,207 (19.97%) | 454 (11.87%) | |
| 2011-2012 | 1,886 (17.07%) | 870 (22.75%) | |
| 2013-2014 | 2,328 (21.07%) | 877 (22.93%) | |
| 2015-2016 | 2,505 (22.67%) | 929 (24.29%) | |
| 2017-2018 | 2,123 (19.21%) | 695 (18.17%) | |
| Service length (days) | 541.3 (298.4) [509, 90-3337] | 608 (404.6) [551, 90-3337] | <0.0001 |
| Mortality | 21 (0.19%) | 4 (0.1%) | 0.2660 |
| Follow-up time (years) | 4.8 (2.8) [4.7, 0.2-9.1] | 4.6 (2.6) [4.4, 0.2-9.1] | <0.0001 |
| Cancer incidence | 28 (0.25%) | 13 (0.34%) | 0.3794 |
| Age at cancer incidence | 25.2 (5.5) [24, 19.8-43.6] | 23.1 (2.3) [22.9, 19.5-28.6] | 0.3199 |
| . | Comparison group . | Exposed group . | . |
|---|---|---|---|
| . | N = 11,049 . | N = 3825 . | P-value . |
| Age at exposure (years) | 19.8 (1.9) [19.3, 18.1-44.8] | 19.9 (2.6) [19.1, 18-46.3] | <0.0001 |
| Gender—male | 6,658 (60.26%) | 2,779 (72.65%) | <0.0001 |
| BMI (kg/m2) | 22.2 (3.8) [21.7, 13-44] | 22.1 (4) [21, 13-46] | 0.0586 |
| BMI ≥ 30 (kg/m2) | 555 (5.11%) | 210 (5.59%) | 0.2514 |
| Israeli native | 9,246 (83.82%) | 3,182 (83.28%) | 0.4349 |
| SES | 0.0013 | ||
| Low | 2,787 (25.3%) | 988 (25.92%) | |
| Medium | 5,959 (54.09%) | 1,945 (51.04%) | |
| High | 2,271 (20.61%) | 878 (23.04%) | |
| Main occupation | <0.0001 | ||
| Combat | 8,756 (79.32%) | 2,172 (56.86%) | |
| Maintenance | 245 (2.22%) | 676 (17.7%) | |
| Driver | 210 (1.9%) | 252 (6.6%) | |
| Officer | 555 (5.03%) | 209 (5.47%) | |
| Others | 175 (1.59%) | 49 (1.28%) | |
| Administration | 1,098 (9.95%) | 462 (12.09%) | |
| Year of exposure initiation | <0.0001 | ||
| 2009-2010 | 2,207 (19.97%) | 454 (11.87%) | |
| 2011-2012 | 1,886 (17.07%) | 870 (22.75%) | |
| 2013-2014 | 2,328 (21.07%) | 877 (22.93%) | |
| 2015-2016 | 2,505 (22.67%) | 929 (24.29%) | |
| 2017-2018 | 2,123 (19.21%) | 695 (18.17%) | |
| Service length (days) | 541.3 (298.4) [509, 90-3337] | 608 (404.6) [551, 90-3337] | <0.0001 |
| Mortality | 21 (0.19%) | 4 (0.1%) | 0.2660 |
| Follow-up time (years) | 4.8 (2.8) [4.7, 0.2-9.1] | 4.6 (2.6) [4.4, 0.2-9.1] | <0.0001 |
| Cancer incidence | 28 (0.25%) | 13 (0.34%) | 0.3794 |
| Age at cancer incidence | 25.2 (5.5) [24, 19.8-43.6] | 23.1 (2.3) [22.9, 19.5-28.6] | 0.3199 |
SES, socioeconomic status; BMI, Body Mass Index. Continuous variables are presented with mean (standard deviation) [median, range].
Therefore, additionally, we matched aerial defense groups’ sociodemographic properties using a propensity score matching technique (Grady method), to create a 1:1 matched control group from the comparison group described. A sensitivity analysis was conducted on the preliminary exposure and comparison groups.
Participants who served less than 90 days in the aerial defense units or served in both exposed and comparison units were excluded.
Primary Outcomes
Primary outcome measure was all-cause malignancy incidence rate. Secondary outcome was incidence of specific types of malignancies.
Data Collection
Israeli adolescents are approached at age 17 years for assessment of their medical eligibility for mandatory military service, as previously reported.13 In this study, data were drawn from the IDF Medical Corps (IDFMC) database, including demographic parameters (gender, age at first assignment to exposed or comparison units, SES), exposure days (defined as days that were served in the relevant unit), and primary military occupation (combative, administrative, etc.). Since military medical follow-up was terminated after mandatory 3 years of service, the IDFMC database was linked with the Israeli National Cancer Registry (INCR), which adheres to the International Classification of Diseases for Oncology coding system, to identify cases of reportable diseases diagnosed in the exposure and comparison groups. In addition to the malignancy diagnosis date, the cancer type (hematological, skin, etc.) and cancer site were extracted as well. Morphologically similar malignancies were grouped together. At the time of our analysis, data in the Israeli National Cancer Registry were complete until the end of 2018.
Occupational NIR Exposure
Soldiers in the studied aerial defense units operate radar systems that emit RF-EMF in the range of 2-300 GHz (the precise spectrum is classified and cannot be disclosed). Access to the radar and any ensuing emission fields is restricted per the IDF reference values for NIR exposures and based on power density measurements. These measures are compliant with the risk mitigation considerations for occupational exposures as outlined by the International Commission of Non-Ionizing Radiation Protection (ICNIRP).14 The IDF embraces the threshold limit values as promulgated by the Israeli Ministry of Environmental Protection for nonoccupational personnel (applying an additional safety factor of 10 on ICNIRP guidelines = 1 W/m2). Regarding occupational reference values, the IDF adheres to the precautionary principle and applies an uncertainty factor of 2 on the ICNIRP occupational reference values (25 W/m2). Although there are no cumulative personal exposure measurements, all NIR exposed units are regularly monitored (at designated NIR exposure areas, at least every 6 months, and at every equipment deployment or technical changes, the earliest of these) to ensure that safety measures are kept.
Statistical Analysis
Study groups were initially compared using the chi-square test (or Fisher’s exact test) for categorical variables and t-test (or Wilcoxon two-sample test) for continuous variables. Follow-up time was calculated as follows: for participants with a diagnosis of cancer, follow-up time was from first assignment to duty and until the date of diagnosis. For cancer-free cases, follow-up time was from first assignment until the date of death or December 2018, the last date for which INCR data were complete, whichever came first. Kaplan–Meier analysis of cancer-free survival was performed, using the log-rank test to assess the difference in survival among study groups. Univariate and multivariable logistic regressions, with cancer incidence as the dependent variable, were performed to adjust for possible confounders and potential risk factors. As the proportional hazards assumption was violated, Cox regression was not implemented and logistic regression was performed instead. This analysis was performed on the matched groups as well. We also repeated the logistic regression analysis for incidence of specific malignancies such as hematological and testicular cancers. Odds ratios (ORs) are presented with 95% confidence intervals (95%CIs).
P-values under 0.05 and 95%CI exclusive of the null were considered statistically significant. Statistical analyses were conducted using SAS 9.4 software.
Ethical Considerations
The institutional review board of the IDF approved this study (protocol number 0938-2021) and waived the requirement for written informed consent based on preserving participants’ anonymity.
RESULTS
Study Population
The database included 16,310 active duty soldiers serving between the years 2009 and 2018. Forty individuals were excluded because of service in both exposed and comparison groups, resulting in 16,270 individuals, 4,157 of which served in aerial defense units and 12,113 served in comparison units, without occupational NIR exposure. We excluded 332 individuals in the exposure group and 1,064 in the comparison group, because of insufficient service (exposure) duration (<90 days). Ultimately, 3,825 and 11,049 individuals were included in the exposure and comparison groups, respectively, and were followed for a total of 3337 days (Fig. 1). The exposed group was followed for 17,710.3 person years, while the comparison group was followed for 53,475.6 person years. Characteristics of the study population are presented in Table I.
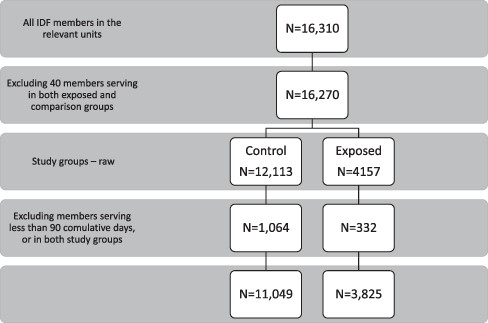
Study population flowchart. This figure describes the study population selection process. The linked database included 16,310 active duty soldiers serving between the years 2009 and 2018. Forty individuals were excluded because of service in both exposed and comparison groups, resulting in 16,270 active duty soldiers who served in the relevant units, 12,113 and 4,157 in the control and the exposed group, respectively. Study groups were selected based on the presence (exposed) or absence (control) of exposure to occupational nonionizing radiation. Beside exposure status, the control group was matched to the exposed group relying on sociodemographic similarities (units’ gender diversity, medical profiles, and socioeconomic profiling of place of residence). Three hundred thirty-two individuals in the study group and 1,064 in the control group were excluded, because of insufficient service duration (<90 days) or service in both units. Ultimately, 3,825 and 11,049 individuals were included in the exposure and control groups, respectively.
All-Cause Cancer Incidence
A total of 41 cases diagnosed with cancer were identified during the total follow-up period. In the exposure group, 13 cases (0.34%) were reported, and 28 cases (0.25%) were reported in the comparison group. The cancer incidence rate (per 1000 person years) was calculated to be 0.734 and 0.524, respectively, with an incidence rate ratio for both groups of 1.4 (95%CI, 0.73-2.71). Figure 2 shows the Kaplan–Meier curve for survival in both study groups, with no significant survival difference among groups (log-rank test P-value = 0.2645). The OR for cancer incidence in the exposure vs. comparison groups was OR = 1.34 ([95%CI, 0.70-2.60], P = 0.3807), suggesting no statistically significantly increased risk of cancer. After adjusting for gender, age at exposure onset, exposure length, SES, and combat occupation, these results remained unchanged (adjusted OR = 1.29 [95%CI, 0.64-2.62]; P = 0.4751). Younger age upon arrival to the unit (i.e., potential exposure onset) and length of follow-up were the only factors independently associated with the incidence of cancer (adjusted OR = 1.13 [95%CI, 1.04-1.23]; P-value = 0.0045; adjusted OR = 0.87 [95%CI, 0.77-0.99]; P-value = 0.0355, respectively). In a sensitivity analysis using a comparison with a 1:1 matched control group, no differences were seen among Kaplan–Meier curves of both groups (log-rank test P-value = 0.2693) (Figure S1).
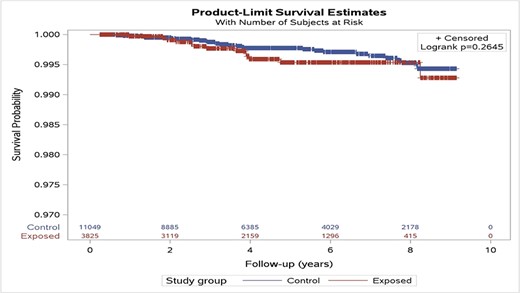
Kaplan–Meier curve for survival probability, stratified by study groups, original cohorts. This figure depicts the Kaplan–Meier curves for survival in the comparison (upper blue) and the exposed (lower red) groups. Based on the log-rank test, the differences among the curves were statistically insignificant (P = 0.2645).
Incidence of Specific Malignancies
We analyzed the specific malignancy distribution of the 41 cases reported overall during the follow-up period. A trend toward a higher incidence of hematological and testicular cancer was seen in the exposed group compared with the comparison group, without reaching statistical significance (hematological malignancy, adjusted OR = 2.76 [95%CI, 0.62-12.19]; P-value = 0.1810; testicular malignancy, adjusted OR = 7.2 [95%CI, 0.71-73.8]; P-value = 0.0957) (Table S1).
DISCUSSION
In this study, short-term cancer incidence in participants actively serving in aerial defense units involving occupational NIR exposures was not increased compared with the incidence in units without NIR exposures, throughout the follow-up period. These findings did not vary after adjustment for potential confounders and risk factors and upon a sensitivity analysis conducted with the 1:1 matched control group. A trend toward a higher rate of hematological and testicular malignancies was reported, albeit was statistically insignificant.
Human exposures to RF-EMF are nowadays ubiquitous. They are formed by personal devices (mobile and cordless phones, Bluetooth, and Wi-Fi), occupational exposures (including among others, high-powered pulsed radars), and environmental origins (such as mobile phone base stations, broadcast antennas, and medical applications). The factors most important in determining the induced fields are the distance from the source and the output power level.11,15 In children, who are considered sensitive to NIR, there are equivocal results regarding the effect of EMF on childhood leukemia.16 There is also inconsistent and insufficient evidence for an epidemiological association between NIR and malignancy in adults.17–19 Central Nervous System (CNS) and hematological malignancies are the most studied types of malignancies with regard to NIR exposure. Data from the INTEROCC study reported no association of cumulative occupational extremely low-frequency (ELF) exposures and glioma cases.20 Yakymenko et al. reviewed epidemiological data on exposure to radars and mobile communication systems9 and found that in some cases, long-term exposure (8 years) to low-intensity RF led to initiation and promotion of cancer, especially of hematolymphatic type. A higher risk for leukemia was observed in U.S. Navy servicemen who were exposed to radar during the Korean War (1950-1954), without an increase in cancer deaths.21 However, a follow-up study of the same cohort 40 years later found no excess cancer, except for nonlymphocytic leukemia in one high exposure occupational group out of three.22 Polish army personnel exposed to radar MWs (between 1971 and 1985) were found to have higher cancer incidence rates than nonexposed soldiers, mainly hematolymphatic types.23 Further data from U.S. Air Force personnel revealed that NIR caused a small but significant increase in neurologic cancer development.24 Degrave et al. compared cause-specific mortality in professional Belgian military personnel serving in radar exposed units between 1963 and 199425 and reported significantly higher cancer mortality rate in the radar exposed, mainly because of hemolymphatic malignancies. Noteworthy is that about a third of cancer patients worked on radars before 1970. Richter et al. described a shortened latency period and higher malignancy rates in Israeli radar technicians.26,27 However, a meta-analysis examining the association between MW radiation and RF and cancer in both environmental and occupational settings was conducted in 2016 and found an increased risk of lymphoma, leukemia, melanoma, breast, and brain/CNS cancers.28 Occupational military studies included in the analysis found increased risk for malignancy and mortality with decreasing age.23,25,29 Variani et al. later conducted a meta-analysis analyzing only occupational exposure to radar radiation and did not find an increased effect of occupational NIR exposure on the incidence of cancer and mortality rate.30 Similarly, we did not find a significant increase in any of the subtypes of cancer mentioned. However, it should be stated that a trend toward an increase in hematological malignancies and the study might have been underpowered and have insufficient follow-up time to detect these differences. This study also reports a trend toward an increase in testicular malignancy among those exposed to NIR, although overall statistically insignificant. In contrast to hematological malignancies, research linking testicular malignancies with NIR is sparse, relying on case series describing the disease cluster in policemen exposed to hand-held radar.31
Presented with epidemiological studies of potential brain tumors, leukemia, lymphoma, and other malignancies potentially associated with RF-EMF occupational exposure, the IARC Working Group noted that the studies had methodological limitations and the results were inconsistent, therefore concluding that there is limited evidence in humans and experimental animals for the carcinogenicity of RF and/or EMF.12,15 The recent Advisory Group to recommend priorities for the IARC Monographs 2020-2024 concluded that since the previous evaluation, several new epidemiological studies have been published on the association between RF-EMF and cancer, although the evidence remains mixed. They recommended that the RF evaluation be updated within the next 5 years. With regard to ELF-Magnetic Fields, the Advisory Group did not recommend an update because of a lack of new informative epidemiological findings, no toxicological evidence, and little supporting mechanistic evidence.32Our results of lack of statistically significant difference in cancer incidence are further supported by the U.S. Food and Drug Administration recent review of epidemiological and laboratory studies published between 2008 and 2018 with selected updates through August 2019, on malignancy and RF, concluding that there is insufficient evidence to support a causal association between RF exposure and tumorigenesis, because of lack of dose–response relationship, inconsistent findings, and lack of biological mechanistic plausibility.33
The results of this study are not in line with a recent case series assessment published by Peleg et al.28 suggesting that whole-body prolonged RF exposure in a military environment increased the risk for hemolymphatic malignancies. Notwithstanding, the exposure to RF in their study was assessed mainly from patients’ recalled reports and an engineer’s estimations and not by precise measurement of personal or unit exposure duration and intensity as unit exposure is provided in our study. Furthermore, this estimation did not account for exposure to known carcinogens such as ionizing radiation described among the cohort.26
Our study has several strengths. First, this cohort of predominantly young healthy adults is of importance because of the limited data regarding cancer risks in this age group and the relative rarity of comorbidities, which might independently affect the risk of cancer and serve as confounders. We also included a large and comprehensive exposed study group, composed of all active duty personnel serving in the Aerial defense units from the beginning of occupational NIR exposure. The reported results remained unchanged in a sensitivity analysis conducted on a matched control group, with similar sociodemographic characteristics and follow-up time, thus eliminating potential confounders. Additional strengths include the systemic data collection and measurement throughout the study using the valid pre-recruitment survey and the INCR, which covers the entire Israeli population.
The main shortcoming of most epidemiological data, both in the military and in mobile communication risk assessment, is a lack of a personal exposure dose measurement. Our aerial defense units are subject to specific restrictions and routine radiation monitoring, providing a good estimation of exposure dose, yet this remains unit level exposure (as opposed to individual exposure). Thus, the lack of continuous monitoring and personal dosage calculation presents a limitation. We emphasize that all radiation measurements documented during the studied time interval were within ICNIRP exposure recommendations for the general population, therefore allowing a stricter assessment of the association between NIR and adulthood malignancy. Another limitation is the relatively short follow-up period (a median of 4.4 years in the exposed group), which may be insufficient to diagnose some types of malignancies, although following our population for over 17,710 person years. Considering the rarity of cancer diagnosis in young adults, this study might have been underpowered to detect the effect of NIR because of an overall small number of cases despite the large cohort. However, it should be noted that our time interval is considered appropriate for the diagnosis of hematolymphatic malignancies, which is the main cancer risk identified in the literature. Although results of this study show no increased short-term cancer risk, further studies conducted on young adults are warranted to fully elucidate the effects of NIR on long-term cancer incidence in this population. Moreover, since the unexposed population does not include all the unexposed IDF personal, some risk of selection bias exists. Lastly, we had no available data regarding smoking status, alcohol consumption, dietary, and lifestyle habits, all of which may have contributed to cancer risk in both our exposed and comparison groups.
CONCLUSION
In this study, occupational exposure to NIR radiation did not increase the risk for cancer in young adults during the 9-year follow-up, as compared with unexposed individuals. Constant regulation of exposures by the IDF Occupational Health Administration as well as epidemiological surveillance is important for monitoring and reassessing possible health effects of modern radar systems.
ACKNOWLEDGMENTS
None declared.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at Military Medicine online.
FUNDING
None of the authors reports any financial or other conflict of interest with regard to the current study.
CONFLICT OF INTEREST STATEMENT
None declared.
INSTITUTIONAL REVIEW BOARD
The institutional review board of the IDF approved this study (protocol number 0938-2021) and waived the requirement for written informed consent based on preserving participants’ anonymity.
CLINICAL TRIAL REGISTRATION
None declared.
INDIVIDUAL AUTHOR CONTRIBUTION STATEMENT
L.F.-Z., O.Z., D.T., and N.S. conceived and designed the analysis; L.F.-Z., D.T., and B.G.S. collected the data; N.S., M.N., and D.T. performed the analysis; S.S., M.N., and L.F.-Z. wrote the paper; all authors read, revised, and contributed to the final manuscript.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
INSTITUTIONAL CLEARANCE
Institutional Clearance–Approved.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE
Not applicable.
REFERENCES
Author notes
The views expressed in this material are those of the authors and do not reflect the official policy or position of the Israel Defense Forces Medical Corps.



