-
PDF
- Split View
-
Views
-
Cite
Cite
Meera Srivastava, Ofer Eidelman, James Craig, Joshua Starr, Leonid Kvecher, Jianfang Liu, Matthew Hueman, Harvey B Pollard, Hai Hu, Craig D Shriver, Serum Biomarkers for Racial Disparities in Breast Cancer Progression, Military Medicine, Volume 184, Issue Supplement_1, March-April 2019, Pages 652–657, https://doi.org/10.1093/milmed/usy417
Close - Share Icon Share
Abstract
African American (AA) women are often diagnosed with more aggressive breast cancers and have worse survival outcomes than their Caucasian American (CA) counterparts. However, a comprehensive understanding of this disparity remains unclear. In this study, we attempted to identify the race-specific non-invasive protein biomarkers that may particularly benefit interventions aimed at reducing the risk of recurrence and metastasis in breast cancers (BrCa). Our technical strategy has been to discover candidate protein biomarkers in patient sera using a high throughput antibody microarray platform. A total of 240 subjects were selected, composed of controls and all immunohistochemistry-based subtypes of breast cancer cases, subdivided by pre- and post-menopausal status and by race. A global Wilcoxon analysis comparing no-cancer controls and cancer patients identified Pyk2, SAPK/JNK, and phosphatase and tensin homolog as present in higher concentrations in cancer patient serum. A paired t-test revealed that c-kit and Rb are significantly over-represented in AA cancer serum when compared to CA cancer serum. Interestingly, VEGFR2, a protein linked to BrCa metastasis and poor prognosis, was significantly over-represented in AA cancer serum compared to AA controls; however, this was not found in CA cancer serum compared to CA controls, suggesting a possible explanation for the higher incidence of aggressive BrCa in AA versus CA patients. Through examining race-specific differences in the protein landscape of BrCa patient serum, the identified proteins could lay the groundwork for the development of an all-inclusive “liquid mammogram test.”
INTRODUCTION
The incidence and mortality burdens of breast cancer differ markedly across racial/ethnic groups, but known risk factors do not explain this variation.1–3 While African American (AA) women are less likely to develop breast cancer, they also have a lower cancer-associated survival rate compared with Caucasian American (CA) women.3,4 Explanations for these disparities are complex and studies often include factors such as socioeconomic, cultural, and biological factors.5,6 However, even when accounting for these factors, the observed differences in the incidence and disease aggressiveness suggest a potential biological role for varying oncogenic signaling pathways of breast tumorigenesis between AA and CA women.7–12 Therefore, sensitive noninvasive biomarkers of disease progression are needed to determine its cause and to initiate adequate treatment before irreversible damage has occurred. In addition, a patient’s response to therapeutic interventions may be followed and analyzed by frequent measurements of such biomarkers.
As a non-invasive tool for monitoring aggressive breast cancer, bio-fluid proteomics has become popular in identifying biomarkers and optimal drug targets.13 To date, there is no specific and established noninvasive biomarker for BrCa which could allow early and accurate diagnosis of recurrence and metastasis without performing a biopsy. Furthermore, the markers for predicting recurrence and metastasis are limited in number and quite non-specific. Thus, the development of biomarkers derived from noninvasive serum proteomics is needed for more beneficial timely detection of recurrence and metastasis.
The goal of this study is to identify race-specific biological factors that may lead to the development of race-specific interventions that can reduce the risk of metastasis and recurrence in breast cancer. We focused our attention on identifying proteomic biomarkers in serum from large, well-defined breast cancer cohorts of AA and CA women from the Clinical Breast Care Project spearheaded by Murtha Cancer Center (MCC) at the Walter Reed National Military Medical Center (WRNMMC) involving multiple clinical and research centers. We hypothesized that breast cancer cells, or affected bystander cells, release proteins into the circulatory system that can be used as markers to predict differential progressions and outcomes for AA and CA breast cancer (BrCa) patients. Therefore, our particular aim of this current study was to identify and characterize the race and sub-type specific biomarkers for breast cancer from the serum specimen in using high-throughput antibody array.
METHODS
Patient Details
Patients were enrolled into the Clinical Breast Care Project (CBCP) at MCC at the Walter Reed National Military Medical Center (WRNMMC) following Institutional Review Board-approved, HIPAA-compliant protocols. A total of 240 subjects were selected, composed of controls and all immunohistochemistry-based subtypes of breast cancer cases, subdivided by pre- and post-menopausal statuses and by race. We attempted to obtain ten samples for each group; however, due to the lack of certain sample types in the AA population, other groups were increased in size. Immunohistochemistry (IHC) subtypes were determined as Luminal A (LA: ER+/HER2−/Ki67-), Luminal B1 (LB1: ER+/HER2-/Ki67+), Luminal B2 (LB2: ER+/HER2+), Her2+ (ER-/PR-/HER2+), and Triple Negative (TN: ER-/PR-/HER2-). The patient sera samples were pooled into 24 groups according to their subtype, race, and menopausal status (Table I).
| Patient/Tumor Characteristics . | AA samples (n = 120) . | CA samples (n = 120) . |
|---|---|---|
| Control, pre-menopausal | 10 | 10 |
| Control, post-menopausal | 10 | 10 |
| TN, pre- | 15 | 10 |
| TN, post- | 14 | 10 |
| HER2+, pre- | 6 | 10 |
| HER2+, post- | 1 | 10 |
| LA, pre- | 10 | 10 |
| LA, post- | 8 | 10 |
| LB1, pre- | 15 | 10 |
| LB1, post- | 22 | 10 |
| LB2, pre- | 5 | 10 |
| LB2, post- | 4 | 10 |
| Patient/Tumor Characteristics . | AA samples (n = 120) . | CA samples (n = 120) . |
|---|---|---|
| Control, pre-menopausal | 10 | 10 |
| Control, post-menopausal | 10 | 10 |
| TN, pre- | 15 | 10 |
| TN, post- | 14 | 10 |
| HER2+, pre- | 6 | 10 |
| HER2+, post- | 1 | 10 |
| LA, pre- | 10 | 10 |
| LA, post- | 8 | 10 |
| LB1, pre- | 15 | 10 |
| LB1, post- | 22 | 10 |
| LB2, pre- | 5 | 10 |
| LB2, post- | 4 | 10 |
AA, African American; CA, Caucasian American; TN, triple-negative subtype; Her2, human epidermal growth factor receptor 2 subtype; LA, Luminal A subtype; LB1, Luminal B1 subtype; LB2, Luminal B2 subtype.
| Patient/Tumor Characteristics . | AA samples (n = 120) . | CA samples (n = 120) . |
|---|---|---|
| Control, pre-menopausal | 10 | 10 |
| Control, post-menopausal | 10 | 10 |
| TN, pre- | 15 | 10 |
| TN, post- | 14 | 10 |
| HER2+, pre- | 6 | 10 |
| HER2+, post- | 1 | 10 |
| LA, pre- | 10 | 10 |
| LA, post- | 8 | 10 |
| LB1, pre- | 15 | 10 |
| LB1, post- | 22 | 10 |
| LB2, pre- | 5 | 10 |
| LB2, post- | 4 | 10 |
| Patient/Tumor Characteristics . | AA samples (n = 120) . | CA samples (n = 120) . |
|---|---|---|
| Control, pre-menopausal | 10 | 10 |
| Control, post-menopausal | 10 | 10 |
| TN, pre- | 15 | 10 |
| TN, post- | 14 | 10 |
| HER2+, pre- | 6 | 10 |
| HER2+, post- | 1 | 10 |
| LA, pre- | 10 | 10 |
| LA, post- | 8 | 10 |
| LB1, pre- | 15 | 10 |
| LB1, post- | 22 | 10 |
| LB2, pre- | 5 | 10 |
| LB2, post- | 4 | 10 |
AA, African American; CA, Caucasian American; TN, triple-negative subtype; Her2, human epidermal growth factor receptor 2 subtype; LA, Luminal A subtype; LB1, Luminal B1 subtype; LB2, Luminal B2 subtype.
Protein Profiling Using Antibody Microarrays
The serum samples collected from patients in each annotated set were pooled in equal volumes and labeled with Cy3. To obtain the relative expression level of same proteins in healthy female, we used a pooled FDA approved female serum sample (Sigma-Aldrich, MO, USA), and we named it as standard control. This healthy pooled female serum sample was labeled with Cy5 according to manufacturer protocol (Full Moon BioSystems, CA, USA). Equal volumes of pooled Cy3-labeled patient sera were mixed with Cy5-labeled control serum. Samples of Cy3-labeled control (i.e., healthy sample cohort) and Cy5 standard control sera were mixed and assayed on the antibody microarray to permit correction for differences in labeling efficiency of Cy3 vs. Cy5. Each sample was incubated with the antibody microarray slide (Full Moon Biosystems, CA, USA). The array were featured with 215 highly specific preselected antibodies designed for profiling different proteins and specific phosphorylation sites involved in various cancers and inflammation associated signaling pathways. The selection was made by the vendor and, all selected antibodies are associated with cancer-related NF-kB linked inflammatory pathway.
Fluorescence Detection
The fluorescence intensities from both Cy3 and Cy5 at each site on the antibody microarray were measured on a GenePix array reader (New Milton, New Hampshire, UK), and downloaded to an Excel spreadsheet. The relative expression of a particular protein was calculated by obtaining the ratio of Cy3 and Cy5 intensities from each site of the antibody of the same protein.
Data Analysis
There were total 215 microarray sites contains triplicate antibody spots. We collected data from six independent technical replicates of each pool of patients, and the final numbers were available for averaging and calculation of statistical significance. We were very careful and verified the spot intensities with the local background. Therefore, the false positive and false negative spots were rejected from the calculations of all spots with intensities below the local background, as well as all spots with signal-to-noise-ratio <3. Next, we calculated the standard deviation (SD) for each protein. Outliers were rejected if their deviations (SD) are larger than two from the average of the respective protein. The averages were recalculated by omitting outliers. We quantitated the volume-normalized protein levels by obtaining ratio between intensities of Cy3-labeled proteins in patient serum samples to the same protein, labeled with Cy5, in the standard control sera. The intensities ratio between Cy3 and Cy5 of control standard female sera which labeled with both Cy3 and Cy5 were calculated for measuring the labeling efficiency of each protein of interest.
Statistical Methods
Wilcoxon rank-sum analysis was performed to test for significant differential expression between each race and the healthy controls. A paired t-test was performed for comparisons between CA and AA signals, and fold change analysis was performed on the total protein and phospho-protein signals. The phospho-protein changes were calculated as a percentage of total protein signal. Since these are potentially secreted proteins in sera, we initially looked for over-expressed proteins as our primary interest.
RESULTS
Identification of Race- and Sub-type Specific Proteins as Candidate Biomarkers for Breast Cancer
A total of 240 patient cases were selected, composed of healthy controls and all immuno-histochemistry-based ER, PR, HER2, and Triple negative subtypes, subdivided by pre- and post-menopausal statuses and by race as described under “Methods” section. The patient serum samples were pooled into 24 groups according to their subtype, race, and menopausal status. A global Wilcoxon analysis comparing no-cancer controls and cancer patients identified several proteins of interest that were present in higher concentrations in cancer patient serum: Pyk2 (p = 0.007, 1.14 fold up-regulated (↑), SAPK/JNK (p = 0.029, 1.1), and PTEN (p = 0.045, 1.16↑) (Fig. 1). In addition, we identified several proteins that were present at elevated levels only in AA patients. A paired t-test revealed that c-kit (p = 0.013, 1.1↑) and Rb (p = 0.045, 1.2↑) are significantly over-represented in AA cancer serum when compared to CA cancer serum. When comparing AA to control AA groups, VEGFR2 (p = 0.03, 1.6↑) was found to be up-regulated in the blood serum of cancer patients. Besides, we have also compared the CA cancer patients’ serum samples with its corresponding healthy individuals, to obtain the race specific biomarker which only includes the Caucasian patients. In CA patients, elevated levels of heat shock transcription factor 1 (HSF1; p = 0.03, 1.3↑) and neurotrophic receptor tyrosine kinase 2 (Trkb; p = 0.03, 1.2↑) were found in comparison to CA controls.
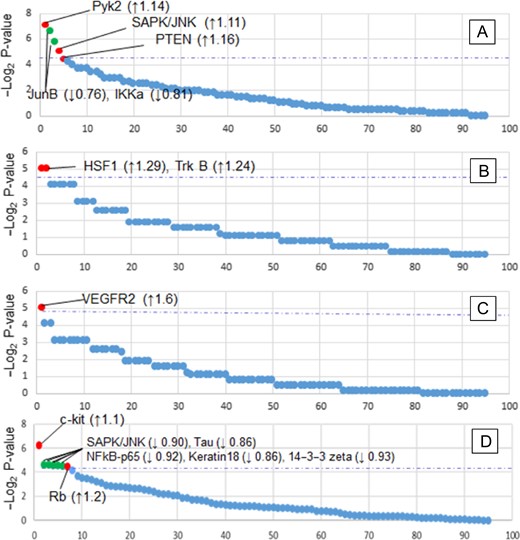
Global protein statistical analysis. (A–C) Wilcoxon rank-sum analysis of (A) Control samples vs. all cancer samples, (B) CA_cancer vs CA_control, (C) AA_cancer vs AA_control. (D) Paired t-test of CA cancer vs AA cancer samples. Red points indicate proteins with statistically significant increased levels in the serum; green points for significantly decreased levels; blue dotted lines indicate the threshold of significance (p = 0.05). Protein fold changes are indicated within parentheses.
Among the tested phosphorylated proteins, phospho-species JunB (p-Ser-259, p = 0.0006), JunB (p-Ser-79, p = 0.0009), and ICAM-1 (p-Tyr-512, p = 0.0031) were significantly different between the negative controls and cancer patients (Table II).
| Protein . | Phosphorylated Amino Acid . | p-Value . | Ratio . | Control* % . | Cancer* % . |
|---|---|---|---|---|---|
| JunB | p-Ser-259 | 0.0006 | ↑ 1.8 | 11 | 21 |
| JunB | p-Ser-79 | 0.0009 | ↑ 1.8 | 13 | 24 |
| ICAM-1 | p-Tyr-512 | 0.0031 | ↑ 1.6 | 36 | 59 |
| STAT3 | p-Tyr-705 | 0.0508 | ↑ 1.4 | 21 | 29 |
| Myc | p-Ser-373 | 0.0521 | ↑ 1.5 | 6 | 10 |
| p70 S6 Kinase | p-Ser-424 | 0.0525 | ↓ 1.1 | 45 | 42 |
| Tau | p-Ser-404 | 0.0531 | ↑ 1.5 | 39 | 57 |
| Beta-Catenin | p-Ser-37 | 0.0572 | ↑ 1.5 | 12 | 18 |
| Protein . | Phosphorylated Amino Acid . | p-Value . | Ratio . | Control* % . | Cancer* % . |
|---|---|---|---|---|---|
| JunB | p-Ser-259 | 0.0006 | ↑ 1.8 | 11 | 21 |
| JunB | p-Ser-79 | 0.0009 | ↑ 1.8 | 13 | 24 |
| ICAM-1 | p-Tyr-512 | 0.0031 | ↑ 1.6 | 36 | 59 |
| STAT3 | p-Tyr-705 | 0.0508 | ↑ 1.4 | 21 | 29 |
| Myc | p-Ser-373 | 0.0521 | ↑ 1.5 | 6 | 10 |
| p70 S6 Kinase | p-Ser-424 | 0.0525 | ↓ 1.1 | 45 | 42 |
| Tau | p-Ser-404 | 0.0531 | ↑ 1.5 | 39 | 57 |
| Beta-Catenin | p-Ser-37 | 0.0572 | ↑ 1.5 | 12 | 18 |
Specific sites of phosphorylated protein were calculated as a percentage of the global protein signal. *The percentage of phosphorylation in control samples was compared to percent phosphorylation in cancer patients by a two-tailed t-test to obtain the shown p-values.
| Protein . | Phosphorylated Amino Acid . | p-Value . | Ratio . | Control* % . | Cancer* % . |
|---|---|---|---|---|---|
| JunB | p-Ser-259 | 0.0006 | ↑ 1.8 | 11 | 21 |
| JunB | p-Ser-79 | 0.0009 | ↑ 1.8 | 13 | 24 |
| ICAM-1 | p-Tyr-512 | 0.0031 | ↑ 1.6 | 36 | 59 |
| STAT3 | p-Tyr-705 | 0.0508 | ↑ 1.4 | 21 | 29 |
| Myc | p-Ser-373 | 0.0521 | ↑ 1.5 | 6 | 10 |
| p70 S6 Kinase | p-Ser-424 | 0.0525 | ↓ 1.1 | 45 | 42 |
| Tau | p-Ser-404 | 0.0531 | ↑ 1.5 | 39 | 57 |
| Beta-Catenin | p-Ser-37 | 0.0572 | ↑ 1.5 | 12 | 18 |
| Protein . | Phosphorylated Amino Acid . | p-Value . | Ratio . | Control* % . | Cancer* % . |
|---|---|---|---|---|---|
| JunB | p-Ser-259 | 0.0006 | ↑ 1.8 | 11 | 21 |
| JunB | p-Ser-79 | 0.0009 | ↑ 1.8 | 13 | 24 |
| ICAM-1 | p-Tyr-512 | 0.0031 | ↑ 1.6 | 36 | 59 |
| STAT3 | p-Tyr-705 | 0.0508 | ↑ 1.4 | 21 | 29 |
| Myc | p-Ser-373 | 0.0521 | ↑ 1.5 | 6 | 10 |
| p70 S6 Kinase | p-Ser-424 | 0.0525 | ↓ 1.1 | 45 | 42 |
| Tau | p-Ser-404 | 0.0531 | ↑ 1.5 | 39 | 57 |
| Beta-Catenin | p-Ser-37 | 0.0572 | ↑ 1.5 | 12 | 18 |
Specific sites of phosphorylated protein were calculated as a percentage of the global protein signal. *The percentage of phosphorylation in control samples was compared to percent phosphorylation in cancer patients by a two-tailed t-test to obtain the shown p-values.
DISCUSSION
The analysis of serum protein markers serves as a method for early detection of breast cancer and as a tool for determining treatment options which could result in saving the lives of patients suffering from aggressive breast tumors.14 Single protein markers, whose levels show differences between diseased and control serum, such as prostate-specific antigen (PSA), have been useful for monitoring prostate cancer or heart disease.15,16 For complex disorders that affect multiple organs, such as breast cancer, there is a lot of interest in developing quantitative serum proteomic signatures from an analysis of all the detectable proteins at once. The rationale comes from compelling evidence in the cancer field that a serum protein signature consisting of multiple proteins can serve as a much better predictor of clinical state than the analysis of each individual protein, one at a time.17 It is also important to mention that a novel biomarker could also be used as a potential target for therapy. It is established that many commercially available diagnostic tests (for example, HER2 for BrCa) are also associated with the therapy.18
There are only a few examples of BrCa biomarker proteins, such as MUC-1, carcinoembryonic antigen, tissue polypeptide antigen, and HER-2, which regrettably have limited impact on bed-side decisions such as prognosis, surveillance and monitoring of treatment for breast cancer.19,20 The major restriction in biomarker research and advancement is that the serum biomarker projects currently in the pipeline suffer from a serious and intrinsic “constriction” due to the complexity of serum samples and to the intrinsically cumbersome nature of the available technology. The use of intracellular signaling proteins that are leaked due to cell injury, necrosis, and inflammation into the circulation requires access to serum from a large number of well-characterized BrCa patients, as well as sensitive and quantitative well-established platforms for simultaneous analysis of hundreds of low abundance proteins. Therefore, we sought to identify and validate robust serum biomarkers that can track the health status of BrCa patients. Our current technical strategy enables the discovery of candidate protein biomarkers in patient sera through the use of a high throughput antibody microarray platform. In this pilot study, we identified a number of protein molecules which may have relation with race specific progression of breast cancer.
In regard to novel race-specific serum signatures, c-Kit, a highly expressed gene in mammary epithelium cells, was found to be over-represented in AA BrCa patient serum compared to CA BrCa patients. Considering that c-Kit is required for growth and survival of the cells of origin and is associated with Brca1-mutation-associated breast cancer, its over-representation in AA BrCa patients may be an attributing factor for the higher incidence of aggressive BrCa in AA patients.21 This knowledge allows future diagnostic tests to account for differences in c-Kit expression between AA and CA BrCa patients, which may increase the test’s accuracy. For instance, a future BrCa diagnostic test in the process of development may find higher c-Kit expression in BrCa patients versus control; however, if a disproportionate number of AA BrCa patients were examined compared to CA BrCa patients, the results may inaccurately suggest c-Kit to be a valid BrCa biomarker.
Another novel race-specific serum signature finding is the under-representation of Rb in AA BrCa patients compared to CA BrCa patients. Retinoblastoma proteins are negative regulators of the G1 to S cell cycle transition and inhibit tumor progression.22 Therefore, the lower prevalence of Rb in the serum of AA BrCa patients compared to CA BrCa patients may further explain why BrCa tends to be more aggressive in AA patients.
Furthermore, VEGFR2, a protein linked to BrCa metastasis, poor prognosis, and racial disparities.23,24 In this current study, it was found to be significantly over-represented in AA cancer serum compared to AA controls. This was not the case in CA cancer serum compared to CA control, suggesting that AA BrCa patients may be at a higher risk for increased VEGFR2 expression and subsequently, more aggressive BrCa. Similarly to c-Kit, this novel finding of race-specific differences in VEGFR2 expression in BrCa patient serum allows future diagnostic tests to increase their accuracy by accounting for inherent differences in VEGFR2 expression between AA and CA BrCa patients. This may also suggest that AA BrCa patients may respond more positively to VEGFR2 inhibitors than AA BrCA paitents, which could be useful information for VEGFR2 inhibitors in the context of BrCa treatment.
Additionally, Pyk2, a protein shown to potentiate breast cancer proliferation and invasion, was found to be over-represented in BrCa patients versus control individuals, with no significant difference between AA BrCa versus CA BrCa patients.25,26 Specifically, Pyk2 enhances epithelial-to-mesenchymal transition (EMT) and BrCa invasion.25,26 This suggests that Pyk2 may be a useful non-race-specific serum biomarker for BrCa.
Since blood draws are commonplace in clinical practice, devising a blood test for BrCa patients which can supplement or replace currently existing invasive techniques would greatly benefit both the patient and the clinician. Currently, investigators are adopting panels of serum biomarkers in an attempt to increase the prediction of the particular stage of cancer.27,28 Biomarkers for breast cancer may be used for risk stratification, early detection, treatment selection, prognostication and monitoring for recurrence. All these areas of clinical management would benefit from sensitive and specific, noninvasive, cost-effective biomarkers. We have used the antibody microarray platform composed of antibodies against low abundance phospho-signaling proteins since laboratory tests used in the clinical setting are targeted at expressed phosphoproteins. In addition, therapeutics are now being developed that work at the level of the proteome, such as targeted small molecules and recombinant humanized monoclonal antibodies as part of cancer immunotherapy.29
In this pilot study on a limited cohort of patients, a serum proteomics platform empowered by the detection of low abundance phosphosignaling proteins from many independent intracellular pathways enabled the identification of race-specific markers. Hence, this approach is expected to overcome the multifactorial etiology of breast cancer progression which constrains the linkage of novel biomarkers with breast cancer due to differences in race-specific protein signatures. Importantly, to accurately identify race-specific protein signatures, the current study contains a higher percentage of African American (n = 120, total n = 240; 50% AA subject) patient cohort compared to other similar race-specific studies.1,7,30 Our studies have preliminarily identified non-invasive, race-specific serum-based biomarkers, such as VEGFR2, c-Kit, and Rb, which will help us and future investigators understand why the breast cancer burden differs in women of different race/ethnicities. Moreover, the underlying serum biomarkers may also be used to define and clarify how different signaling pathways affect breast cancer and may provide a basis for new personalized treatment and prevention strategies.
CONCLUSIONS
The data from our pilot study support the hypothesis that there may be a biological basis for racially distinct breast cancer, such as differences in VEGFR2, c-Kit, and Rb serum signatures. The identified proteins could lay the groundwork for the development of a “liquid mammogram test.” Our research continues to verify these potential markers with a reverse phase protein array. This will allow us to quantify for a higher level in-depth cancer stage-specific prediction and survival. The availability of a more predictive, race-specific, noninvasive, biomarker panel for progressive breast cancer would allow for earlier and more accurate diagnosis, and therefore, provide better insight for patient-specific precise treatment. Therefore, successful identification of the serum biomarkers will help to introduce the personalized precision medicine, and hence, the treatment will increase cancer-associated survival and improve our ability to monitor therapeutic responses, and help identify new therapeutic interventions.
Previous Presentations
Presented as a poster at the 2016 Military Health System Research Symposium, Kissmmee, FL, USA. August 2016 (Abstract number: MHSRS-16–1199).
Funding
This study was supported by a grant from US Marine Corps Installation grant #G1706R from United States Army Medical Research Acquisition Activity through the Henry Jackson Foundation for the Advancement of Military Medicine, Rockville, MD, USA. This supplement was sponsored by the Office of the Secretary of Defense for Health Affairs.
Acknowledgments
We thank the patients who participated in this study. The views expressed in this article are those of the author and do not reflect the official policy of the Department of Defense, or US Government.
References
Author notes
The views expressed in this paper are those of the authors and do not necessarily represent the official position or policy of the US Government, the Department of Defense, or the Department of the Air Force.



