-
PDF
- Split View
-
Views
-
Cite
Cite
Yuan-Lin Ding, Rui-Chao Yan, Hao-Kai Chen, Xu-Guang Guo, A meta-analysis of the accuracy of Xpert MTB/RIF in diagnosing intestinal tuberculosis, Laboratory Medicine, Volume 55, Issue 2, March 2024, Pages 238–244, https://doi.org/10.1093/labmed/lmad072
Close - Share Icon Share
Abstract
A detection method with high efficiency and accuracy is urgently needed in clinical work. The purpose of our study was to determine the diagnostic accuracy of the Xpert MTB/RIF assay for intestinal tuberculosis (ITB).
We searched PubMed and 4 other databases from their establishment to July 19, 2022, for published essays of diagnostic performance in which Xpert MTB/RIF was used to test patients with clinically suspected ITB. An assessment of the quality of the selected literature was conducted using QUADAS-2. We built forest plots by MetaDiSc software.
The pooled Xpert MTB/RIF sensitivity was 48%, and the specificity was 99%. Moreover, the positive likelihood ratio for ITB diagnosis was 21.61. The negative likelihood ratio was 0.54. There were substantial variations between the study estimates of sensitivity (I2 = 87.6%) and specificity (I2 = 82.4%).
Intestinal TB is detected with limited diagnostic sensitivity by Xpert MTB/RIF but with high specificity. An Xpert-positive result may facilitate the rapid identification of ITB cases. Nevertheless, a negative result has less certainty in excluding the disease.
Introduction
With HIV infection and the utilization of anti-immunosuppressive drugs, the scope of tuberculosis (TB) infection is expanding daily. As an important extrapulmonary TB (EPTB), intestinal TB (ITB) accounts for approximately 30% of extrapulmonary tuberculosis cases.1 In India, where the burden of TB is highest, 20% of TB cases are extrapulmonary, with peritoneal TB accounting for nearly 13%.2 Pakistan has the fifth largest burden of TB, and a study in Pakistan showed that EPTB accounted for nearly 30% of all open TB cases, with 21% of EPTB originating in the abdomen.3 The most common cumulative site of peritoneal TB is the gastrointestinal tract, accounting for 43% to 65% of all cases of peritoneal TB.4-17 With high incidence, ITB occurs frequently in developing countries. Intestinal TB can cause diarrhea, abdominal pain, abdominal mass, constipation, and a series of nonspecific clinical manifestations,18 which leads to its clinical diagnosis being controversial and challenging. In addition, there are significant challenges in separating Crohn’s disease (CD) from ITB, resulting in high morbidity and mortality due to misdiagnosis or delayed diagnosis.19 As a qualitative assay, Xpert MTB/RIF is a timely polymerase chain reaction (PCR) kit that detects both Mycobacterium tuberculosis and rifampicin (RIF) resistance simultaneously. Furthermore, RIF resistance can be an indication for MDR-TB (multidrug resistant TB, refers to resistance to both isoniazid [INH] and RIF).20 That is to say, this assay can assist in determining MDR-TB by detecting the presence or absence of RIF resistance, assuming that there is INH resistance. At present, although there has been widespread application of Xpert MTB/RIF for detecting pulmonary TB, it is rarely used for ITB. A meta-analysis was conducted to assess the diagnostic accuracy of Xpert MTB/RIF in ITB.
Methods
Sources of Data and Strategies for Retrieval
A total of 5 databases were used, including PubMed, Embase, Web Of Science, the Cochrane Library, and the China National Knowledge Infrastructure (CNKI), from the time they were founded to July 19, 2022. The accuracy of Xpert MTB/RIF in ITB detection was evaluated by retrieving published articles. The search was performed by searching MeSH terms and EMTREE terms obtained by “Xpert MTB/RIF” and “intestinal tuberculosis” in PubMed and Embase. We also further explored possible candidate studies from the references to the included articles.
Inclusion Criteria
Our inclusion criteria were as follows: (1) articles that evaluated Xpert MTB/RIF detection as an ITB diagnostic tool; (2) cohort studies conducted prospectively or retrospectively; (3) reference criteria were clearly defined and suitable for our study; and (4) complete and extracted data: true positive (TP), false positive (FP), false negative (FN), and true negative (TN). Studies with too few samples (<10) conference summaries, articles without complete 2 × 2 contingency table data, and articles written in languages other than English and Chinese were excluded.
Standard of Reference
The study used composite reference criteria (CRS) or Mycobacterium culture as references. Composite reference criteria include clinical manifestations, histopathology, biochemical test results, smears, culture, other nucleic acid amplification tests, and treatment response to anti-TB drugs.
Filtering and Selection of Literature
Two investigators independently completed the process of searching relevant literature, reviewing the abstract, reviewing the full text of the article, and extracting data from the 2 × 2 contingency table. The 2 investigators then checked whether the extracted data were consistent, and if they were not, the above steps were repeated. Only when the data were consistent were the analysis of the data and the creation of the figures performed. A third investigator was consulted to resolve the differences between the 2 investigators. All reviewed articles in this study underwent this review process.
Data Extraction
Among the data we extracted were author, year, study design, country, specimen origin, TP, FP, FN, and TN values as well as reference criteria (mycobacterial culture and CRS) and type of specimens. In the same manner, the necessary information was independently extracted by 2 investigators from each included article and resolved through discussion with a third researcher. In the papers that we reviewed, the 3 studies from China give the complete 2 × 2 contingency table data directly in the article for us to extract. The other 5 studies from India provide partial data as well as sensitivity, specificity, positive predictive value, and negative predictive value, which allows us to calculate the 2 × 2 contingency table data.
Assessment of Study Quality
QUADAS-2, a quality assessment tool to assess the accuracy of selected articles, was used to ensure quality and avoid the possibility of bias, which was controlled by 2 investigators separately. There are 4 domains in QUADAS-2: patient selection, index tests, reference standards, and flow and timing.
Data Analysis
The TP, FP, FN, and TN values for each included article were obtained as well as the combined sensitivity, and specificity estimates and 95% CI for Xpert MTV/RIF were calculated. We graphically generated the results by mapping the sensitivity and specificity of forest figures. The area under the curve (AUC) was calculated from summary receiver operating characteristic (sROC) curves. To assess heterogeneity between studies and benchmarks, I2 statistics were used. We used MetaDiSc software to build pooled forest figures for overall diagnostic sensitivity and specificity (95% CI) for each study.
Results
Identification of Studies
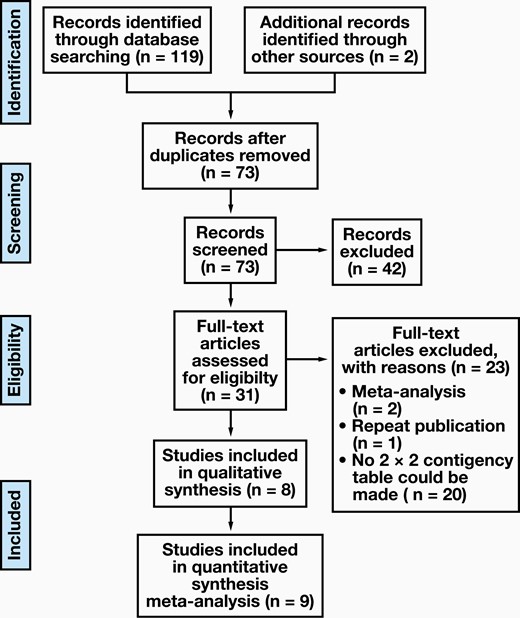
Through the retrieval strategy, 119 candidate articles were retrieved from the databases mentioned above. Thirty-one studies were included for full-text article assessment after scanning abstracts and titles and eliminating duplicate articles. Based on the inclusion criteria, 8 articles qualified for inclusion (FIGURE 1 shows a flow diagram of study identification and inclusion), including 4 prospective studies and 4 retrospective studies.21-28
Characteristics of the Study
TABLE 1 summarizes the characteristics of the trials and participants included in the study. By carefully reviewing all the included literature, we concluded that “colonic biopsy” and “colonoscopic tissue samples” are essentially the same, both being biopsies taken from intestinal tissue during colonoscopy. The reason for the different presentation in TABLE 1 is that we adopted a different presentation for each of the included studies. Similarly, “intestinal mucosal biopsy” and “intestinal tissue” essentially both refer to biopsy on the mucosa of intestinal tissue for the same reasons as above.
| Author . | Year . | Study design . | Country . | Reference standard . | Bacterial species . | Type of specimens . | TP . | FP . | FN . | TN . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mei et al23 | 2016 | Prospective | China | Clinical diagnosis | MTB | Intestinal mucosal biopsy | 19 | 0 | 13 | 48 |
| Kumar et al28 | 2017 | Prospective | India | CRS | MTB | Colonic biopsy | 3 | 0 | 34 | 61 |
| Bellam et al25 | 2019 | Retrospective | India | Histopathology | MTB | Colonic biopsy | 8 | 0 | 17 | 15 |
| Song et al24 | 2020 | Retrospective | China | Culture | MTB | Intestinal mucosal biopsy | 12 | 0 | 5 | 21 |
| Xiong et al26 | 2020 | Prospective | China | Clinical diagnosis | MTB | Stool | 84 | 0 | 55 | 115 |
| Alex et al27 | 2020 | Retrospective | India | Clinical suspicion and histological | MTB | Intestinal tissue | 5 | 7 | 2 | 25 |
| Fei et al21 | 2021 | Prospective | China | CRS/culture | MTB | Biopsy | 4 | 0 | 24 | 43 |
| Surgical | 10 | 0 | 4 | 3 | ||||||
| Paulose et al22 | 2021 | Retrospective | India | CRS | MTB | Colonoscopic tissue samples | 14 | 0 | 21 | 391 |
| Author . | Year . | Study design . | Country . | Reference standard . | Bacterial species . | Type of specimens . | TP . | FP . | FN . | TN . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mei et al23 | 2016 | Prospective | China | Clinical diagnosis | MTB | Intestinal mucosal biopsy | 19 | 0 | 13 | 48 |
| Kumar et al28 | 2017 | Prospective | India | CRS | MTB | Colonic biopsy | 3 | 0 | 34 | 61 |
| Bellam et al25 | 2019 | Retrospective | India | Histopathology | MTB | Colonic biopsy | 8 | 0 | 17 | 15 |
| Song et al24 | 2020 | Retrospective | China | Culture | MTB | Intestinal mucosal biopsy | 12 | 0 | 5 | 21 |
| Xiong et al26 | 2020 | Prospective | China | Clinical diagnosis | MTB | Stool | 84 | 0 | 55 | 115 |
| Alex et al27 | 2020 | Retrospective | India | Clinical suspicion and histological | MTB | Intestinal tissue | 5 | 7 | 2 | 25 |
| Fei et al21 | 2021 | Prospective | China | CRS/culture | MTB | Biopsy | 4 | 0 | 24 | 43 |
| Surgical | 10 | 0 | 4 | 3 | ||||||
| Paulose et al22 | 2021 | Retrospective | India | CRS | MTB | Colonoscopic tissue samples | 14 | 0 | 21 | 391 |
CRS, composite reference criteria; MTB, Mycobacterium tuberculosis.
| Author . | Year . | Study design . | Country . | Reference standard . | Bacterial species . | Type of specimens . | TP . | FP . | FN . | TN . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mei et al23 | 2016 | Prospective | China | Clinical diagnosis | MTB | Intestinal mucosal biopsy | 19 | 0 | 13 | 48 |
| Kumar et al28 | 2017 | Prospective | India | CRS | MTB | Colonic biopsy | 3 | 0 | 34 | 61 |
| Bellam et al25 | 2019 | Retrospective | India | Histopathology | MTB | Colonic biopsy | 8 | 0 | 17 | 15 |
| Song et al24 | 2020 | Retrospective | China | Culture | MTB | Intestinal mucosal biopsy | 12 | 0 | 5 | 21 |
| Xiong et al26 | 2020 | Prospective | China | Clinical diagnosis | MTB | Stool | 84 | 0 | 55 | 115 |
| Alex et al27 | 2020 | Retrospective | India | Clinical suspicion and histological | MTB | Intestinal tissue | 5 | 7 | 2 | 25 |
| Fei et al21 | 2021 | Prospective | China | CRS/culture | MTB | Biopsy | 4 | 0 | 24 | 43 |
| Surgical | 10 | 0 | 4 | 3 | ||||||
| Paulose et al22 | 2021 | Retrospective | India | CRS | MTB | Colonoscopic tissue samples | 14 | 0 | 21 | 391 |
| Author . | Year . | Study design . | Country . | Reference standard . | Bacterial species . | Type of specimens . | TP . | FP . | FN . | TN . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mei et al23 | 2016 | Prospective | China | Clinical diagnosis | MTB | Intestinal mucosal biopsy | 19 | 0 | 13 | 48 |
| Kumar et al28 | 2017 | Prospective | India | CRS | MTB | Colonic biopsy | 3 | 0 | 34 | 61 |
| Bellam et al25 | 2019 | Retrospective | India | Histopathology | MTB | Colonic biopsy | 8 | 0 | 17 | 15 |
| Song et al24 | 2020 | Retrospective | China | Culture | MTB | Intestinal mucosal biopsy | 12 | 0 | 5 | 21 |
| Xiong et al26 | 2020 | Prospective | China | Clinical diagnosis | MTB | Stool | 84 | 0 | 55 | 115 |
| Alex et al27 | 2020 | Retrospective | India | Clinical suspicion and histological | MTB | Intestinal tissue | 5 | 7 | 2 | 25 |
| Fei et al21 | 2021 | Prospective | China | CRS/culture | MTB | Biopsy | 4 | 0 | 24 | 43 |
| Surgical | 10 | 0 | 4 | 3 | ||||||
| Paulose et al22 | 2021 | Retrospective | India | CRS | MTB | Colonoscopic tissue samples | 14 | 0 | 21 | 391 |
CRS, composite reference criteria; MTB, Mycobacterium tuberculosis.
Study Quality
A QUADAS-2 quality assessment was used to evaluate the included studies, and the results are presented in FIGURE 2. Four areas of the included articles were examined in QUADAS-2: patient selection, reference standard, index test, and flow and timing.
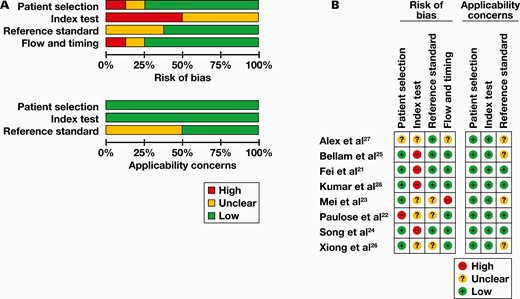
Quality assessment of the included studies. A, Overall quality assessment of included studies. B, Quality assessment of the individual studies.
Diagnostic Accuracy of ITB Detected by Xpert MTB/RIF
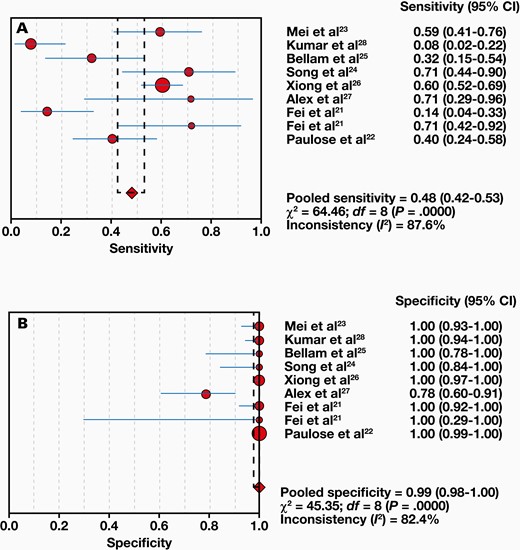
Eight studies compared 1063 samples with the reference standard, and a range of 8% to 71% sensitivity and 78% to 100% specificity was found for Xpert MTB/RIF. The combined sensitivity and specificity of the Xpert MTB/RIF assay were 48% (95% CI, 42%-53%) with I2 = 87.6% and 99% (95% CI, 98%-100%) with I2 = 82.4%, respectively (FIGURE 3). Moreover, the positive likelihood ratio (PLR) for ITB diagnosis was 21.61 (95% CI, 4.40-106.24). The negative likelihood ratio (NLR) was 0.54 (95% CI, 0.38-0.78) (FIGURE 4). FIGURE 5 shows the diagnostic odds ratios (DORs) for individual studies and the pooled DORs along with 95% CI. Compared with the reference criteria, sROC for Xpert MTB/RIF can be used to explain continuous diagnostic test results (FIGURE 6).
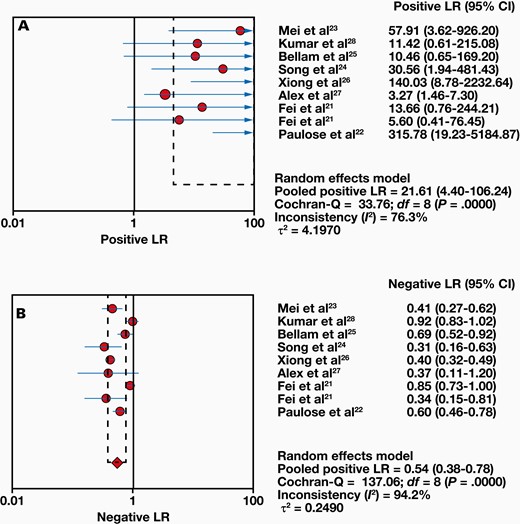
Forest plots of positive likelihood ratio (LR) (A) and negative LR (B).
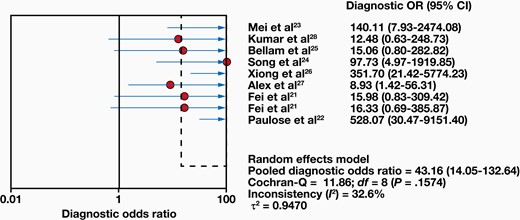
Forest plots of diagnostic odds ratio (OR) from Xpert MTB/RIF for the diagnosis of intestinal tuberculosis.

Forest plots of the summary receiver operating characteristic curve. Area under the curve (AUC) ± SE = 0.8474 ± 0.0777; Q* ± SE = 0.7787 ± 0.0731.
Discussion
Previous studies have noted the importance of making a rapid and accurate diagnosis for ITB because it has nonspecific clinical manifestations and is easily confused with Crohn’s disease, leading to misdiagnosis and missed diagnosis. Rapid details and information on drug resistance make Xpert MTB/RIF a valuable diagnostic method. Considering that many national programs that address TB of the lungs and lymph nodes have already adopted Xpert MTB/RIF and the challenge of ITB diagnosis, we proposed the idea of using Xpert MTB/RIF to detect ITB. There have been systematic reviews and meta-analyses of Xpert MTB/RIF for the diagnosis of abdominal TB, but none are specific to ITB.
Some limitations of the study included the limited number of studies conducted on ITB in just 1 region (India or China). Another reason for the low number of studies may be related to the difficulty of obtaining materials and the need for clinical enteroscopy. In addition, there was considerable heterogeneity in our study, because some studies were prospective whereas others were retrospective. Thus, there was methodological heterogeneity. Reference standard differences can also contribute to heterogeneity in study. To determine whether the results would be considerably affected by a particular study, sensitivity analysis was also carried out, eliminating 1 study at a time and combining the other studies. We found that when Xiong et al’s study26 was excluded, the sensitivity of the combination fluctuated greatly. We considered that this was because the samples used in this study were from stool. By reviewing the original article,21 we found that the exact location of the surgery was not specifically elaborated; we think that for the purpose of this assay, this ambiguity and potential difference in sampling location are likely not significant. The pathological changes of ITB mostly occur in the submucosa of the intestinal wall, and due to mucosal swelling, the endoscopic biopsy tissue tends to be smaller and the sampling area shallow and small, which may lead to false-negative results from Xpert MTB/RIF. In contrast, surgical pathological resection is more extensive and may reduce the possibility of missed diagnoses. Therefore, we tabulated biopsy and surgery separately in the text. Regarding the distinction between “small intestine,” “colon/large intestine,” and “stool,” we regret that we cannot elaborate on it in more detail at present because there are few studies on ITB.
According to our meta-analysis, the sensitivity of Xpert MTB/RIF for ITB detection has varied widely in different studies selected. Therefore, the pooled sensitivity (48% [95% CI, 42%-53%]) was not very high. The limited pooled sensitivity suggests that a negative Xpert assay for ITB has less certainty in excluding the disease. For better diagnosis and elimination of disease, PLR greater than 10 and NLR less than 0.1 are generally considered clinically significant.29 We found that the PLR value and NLR value of ITB diagnosed by the Xpert MTB/RIF method were 21.61 (95% CI, 4.40-106.24; P < .001) and 0.54 (95% CI, 0.38-0.78; P < .001).
Conclusion
Overall, Xpert has limited sensitivity but high specificity for the detection of ITB. Based on the data mentioned above, Xpert MTB/RIF is promising as an earlier, faster, and more accurate method for detecting ITB than other tests.
Abbreviations
- TB
tuberculosis
- ITB
intestinal TB
- EPTB
extrapulmonary TB
- CD
Crohn’s disease
- PCR
polymerase chain reaction
- MDR-TB
multidrug resistant TB
- RIF
rifampicin
- INH
isoniazid
- CNKI
China National Knowledge Infrastructure
- TP
true positive
- FP
false positive
- FN
false negative
- TN
true negative
- CRS
composite reference criteria
- AUC
area under the curve
- sROC
summary receiver operating characteristic
- DORs
diagnostic odds ratios
- PLR
positive likelihood ratio
- NLR
negative likelihood ratio
Conflict of Interest Disclosure
The authors have nothing to declare.
References
Author notes
Yuan-Lin Ding and Rui-Chao Yan Contributed equally.



