-
PDF
- Split View
-
Views
-
Cite
Cite
Joshua Fernandez De La Vega, Arian Pourmehdi Lahiji, Caitlin Raymond, Song Han, Harshwardhan Thaker, Jianli Dong, The first reported case of double trisomy 10 and 20 in a product of conception, Laboratory Medicine, Volume 55, Issue 2, March 2024, Pages 245–248, https://doi.org/10.1093/labmed/lmad052
Close - Share Icon Share
Abstract
Double trisomies are rare findings among products of conception and are often lethal to the developing embryo or fetus.
Here we describe a double trisomy case with symptoms of threatened miscarriage at 9 weeks gestation. Ultrasound revealed an anembryonic pregnancy. Pregnancy was terminated by dilation and curettage at gestational age 11 weeks and 6 days. Histologic examination and chromosome microarray were performed on a formalin-fixed product of conception (POC) sample to identify the cause of the anembryonic pregnancy.
Chromosome microarray analysis revealed a female chromosome complement with double trisomies 10 and 20, arr(10,20)x3, consistent with a karyotype of 48,XX,+10,+20.
To the best of our knowledge, this is the first reported case of double trisomy 10 and 20 in a POC. Due to nonspecific histopathological findings, chromosomal microarray is a powerful tool in identifying and differentiating chromosomal aneuploidies.
Greater than 50% of miscarriages have been described as having abnormalities of their chromosome complement, with single trisomy being the most common.1,2 Double trisomies (trisomy of 2 separate chromosomes) are rare, with most studies finding incidence rates below 3% in products of conception (POC).1-4 Only a few hundred double trisomies have been reported to date, with the most commonly reported combinations being chromosomes 2 and 16, 16 and 21, and 18 and 21.4 Double trisomy usually results in miscarriage, although rare cases of liveborn neonates with double trisomy have been reported, including combinations of autosomes 13, 18, and 21 with sex chromosomes X and Y. This suggests the lethality of the double trisomy depends on which chromosomes are involved,3,4 and notably, double trisomies typically involve the presence of additional copies of low gene density chromosomes. Indeed, to date there are no reports of double trisomy involving chromosome 19, which has the highest reported gene density.4 Here, we present the first reported case of double trisomy of chromosomes 10 and 20, known to have intermediate gene densities.5 We discuss related ultrasound and histopathologic findings, the differential diagnosis of a nonviable pregnancy, and patient follow-up after miscarriage with unique chromosome microarray result.
Clinical History
This study was approved by the University of Texas Medical Branch Institutional Review Board. A 40-year-old Hispanic G4P1203 woman at approximately 9 weeks gestation by last menstrual period presented to the emergency department (ED) with vaginal spotting and abdominal cramping. The patient had a history of pregestational diabetes and chronic hypertension. Beta-human chorionic gonadotropin (beta-hCG) level was 34,099 mUI/mL at presentation, and ultrasound revealed gestational sac diameter of 1.46 cm. Six days later, a beta-hCG level of 35,000 mUI/mL was observed. This low incremental increase prompted additional ultrasound studies in the following 2 weeks, which indicated no expansion of the gestational sac, the largest observed diameter being 1.87 cm, and no discrete yolk sac or fetal pole. A diagnosis of anembryonic pregnancy was made, and at 11 weeks and 6 days gestation, the patient underwent a dilation and curettage procedure.
Histologic examination of the POC revealed enlarged and dysmorphic chorionic villi with occasional central cisterns and syncytiotrophoblast hyperplasia (FIGURE 1A and 1B). These nonspecific findings were consistent with a genetically abnormal and nonviable pregnancy.
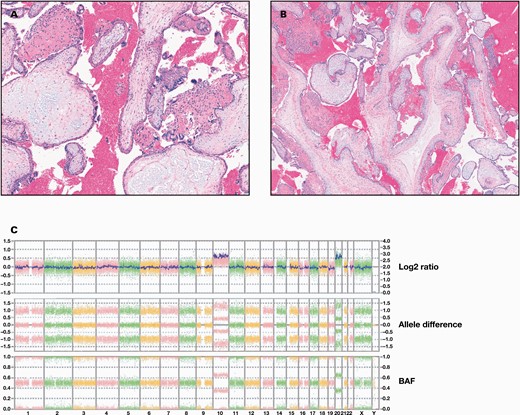
Histologic examination and chromosome microarray analyses of the product of conception (POC) sample. A and B, Histologic examination of the POC sample. H&E stain of the sample shows hydropic chorionic villi with syncytiotrophoblast hyperplasia (A) and dysmorphic villi with central cisterns (B). C, Whole genome view of chromosome microarray analysis. The result is a female with double trisomy 10 and 20, arr(10,20)x3, consistent with a karyotype of 48,XX,+10,+20. Chromosome microarray was performed using Affymetrix OncoScan microarray (Thermo Fisher Scientific).
Laboratory Role in Diagnosis
Chromosomal microarray analysis (CMA) is used to determine copy number variants in a sample and has become a first line assay for prenatal chromosomal analysis.6,7 There are 2 major types of CMA: comparative genomic hybridization, which returns information about DNA copy numbers, and single nucleotide variant (SNV), which returns information about copy number as well as base pair genotypes using SNV probes.8-11 In this case, CMA was performed on the formalin-fixed POC sample using Affymetrix OncoScan microarray (Thermo Fisher Scientific). The result of the SNV CMA was arr(10,20)x3, consistent with a karyotype of 48,XX,+10,+20 (FIGURE 1C).
Although SNV CMA can provide limited information about the unique polymorphisms presents in a sample, it is not ideal for determination of parental origin of trisomy. Parental origin is typically determined by short tandem repeat testing. Short tandem repeat testing identifies the numbers of a tandem repeat in parental samples, which can then be compared to those present in the POC sample to determine origin. Parental samples were not available in this case, and short tandem repeat testing was not performed.
Discussion
Incidence rates of double trisomy among miscarriages with chromosomal abnormalities have been found to fall between 0.21% and 2.8%, although the incidence may be higher among extremely early miscarriages that occur prior to awareness of pregnancy.1-4,12 The mean gestational age of cases of double trisomy is lower than cases of single trisomy (8.7 ± 2.2 weeks vs 10.1 ± 2.9 weeks),3,4 suggesting a more severe impact on viability and development. Indeed, the double trisomies that are reported typically involve chromosomes with low or low-intermediate gene density, such as chromosomes 13, 16, 18, and 21. Few cases of double trisomy involving chromosomes with intermediate or high gene density have been reported, indicating a selection bias towards lower gene doses.3,4 The lethality of double trisomy is hypothesized to be related to the triplosensitivity of involved genes. Triplosensitivity is a term indicating whether the presence of a third copy of a gene in a diploid organism results in pathogenicity. Although a triplosensitivity score is typically assigned to a specific gene, the density of genes across an entire chromosome can indicate the likelihood of pathogenicity in the event of aneuploidy, and chromosomes with fewer genes tend to have more favorable outcomes. Notably, rare cases of liveborn neonates with double trisomy universally involve combinations of autosomes 13, 18, and 21—all of which have low or low-intermediate gene density—with sex chromosomes X and Y.4,5 To the best of our knowledge, our case is the first reported double trisomy of the intermediate gene density chromosomes 10 and 20 in a POC sample.
Previous studies of parental origin in double trisomy have indicated a maternal predominance and an association with advanced maternal age.4,13 The most common mechanism implicated in trisomy is meiotic nondisjunction, in which homologous pairs of chromosomes or sister chromatids fail to separate in meiosis I or meiosis II. Mechanisms associated with meiotic nondisjunction include certain patterns of homologous chromosome recombination, which may disrupt the adhesion and separation of chromosomes and chromatids; reduced cohesion between sister chromatids due to degradation of cohesion proteins; meiotic spindle assembly dysfunction associated with decreased expression of assembly checkpoint proteins; and increased histone acetylation leading to impaired chromatin condensation.14 Our patient’s age of 40 years is associated with an increased risk for the aforementioned factors and may have played a role in the pathogenesis of this case.
Double trisomies present with a spectrum of ultrasound morphological findings, ranging from empty gestational sacs to developing embryos and fetuses, although findings trend towards empty gestational sacs.3,15 The finding of an empty sac without an identifiable embryo may be a result of more severe gene imbalance that is incompatible with embryo and fetal development; however, given the rarity of double trisomies, there are limited data to base conclusions on. Similarly, histopathological examination of POCs with double trisomies is rarely reported in the literature. One case of 48,XXX,+18 was found to have enlarged chorionic villi with trophoblastic pseudoinclusions,15 yet these findings are nonspecific, and there is no characteristic histomorphology or imaging finding that reliably distinguishes aneuploidy from other aberrances of chromosome complement. Therefore, further workup with genetics and genomics assays is a necessity to determine the genetic complement of the POC. Chromosomal microarray analysis is ideally suited to determine copy number differences and can provide definitive evidence of aneuploidy, although it is not sufficient to determine parental origin. In this case, CMA determined double trisomy of 10 and 20.
Determining the exact genetic abnormality present in POC is critical, as this can have significant consequences in the postpartum period for the patient. Molar pregnancies, whether complete or partial, are associated with a higher risk of gestational trophoblastic disease, in which abnormal trophoblastic cells proliferate in an uncontrolled fashion.16 Gestational trophoblastic disease can be either benign or malignant, but successful treatment relies on early detection. Patients that carry a molar pregnancy must be followed closely in the postpartum period to ensure that levels of beta-HCG are downtrending. Thus, differentiating between a molar pregnancy and an aneuploidy such as double trisomy is critical. In our patient, the level of beta-HCG was tracked for 2 months following her miscarriage, with no evidence of gestational trophoblastic disease.
In conclusion, double trisomy is a rare finding in POC, present in a minute percentage of cases. We present the first reported case of double trisomy 10 and 20, which is notable for the relatively higher gene density of these chromosomes than is typically observed in double trisomy. Consistent with the hypothesis of greater triplosensitivity and gene density producing greater pathogenicity, this case featured an anembryonic gestation and dysmorphic chorionic villi. Although suspicious for a genetically abnormal pregnancy, these nonspecific findings were further investigated with CMA, and a diagnosis of double trisomy was made.
Case Follow-up
The patient’s postoperative period was uncomplicated, with beta-hCG values gradually decreasing to normal levels over the course of 2 months. The subsequent 6 months also showed negative beta-hCG values, indicating that there were no complications.
Abbreviations
- POC
product of conception
- beta-hCG
beta-human chorionic gonadotropin
- CMA
chromosomal microarray analysis
- SNV
single nucleotide variant
Conflict of Interest Disclosure
The authors have nothing to disclose.
Data Availability
All data used in the preparation of this manuscript will be made available upon reasonable request to the corresponding author.
References
Author notes
Joshua Fernandez De La Vega and Arian Pourmehdi Lahiji Contributed equally.



