-
PDF
- Split View
-
Views
-
Cite
Cite
Vít Gloser, Maciej A. Zwieniecki, Colin M. Orians, N. Michele Holbrook, Dynamic changes in root hydraulic properties in response to nitrate availability, Journal of Experimental Botany, Volume 58, Issue 10, July 2007, Pages 2409–2415, https://doi.org/10.1093/jxb/erm118
Close - Share Icon Share
Abstract
Changes in root hydraulic resistance in response to alterations in nitrate supply were explored in detail as a potential mechanism that allows plants to respond rapidly to changes in their environment. Sunflower (Helianthus annuus cv. Holiday) plants grown hydroponically with limited nitrate availability (200 μmol l−1) served as our model system. Experimental plants were 6–9-weeks-old with total dry mass of 2–4 g. Root pressurization of intact plants and detached root systems was used to elucidate the temporal dynamics of root hydraulic properties in sunflower plants following changes in external nitrate availability. The response was rapid, with a 20% decrease in hydraulic resistance occurring within the first hour after the addition of 5 mM nitrate and the magnitude of the effect was dependent on nitrate concentration. The change in root hydraulic resistance was largely reversible, although the temporal dynamics of the response to nitrate addition versus nitrate withdrawal was not symmetric (a gradual decrease in resistance versus its fast increase), raising the possibility that the underlying mechanisms may also differ. Evidence is presented that the observed changes in root hydraulic properties require the assimilation of nitrate by root cells. The hydraulic resistance of roots, previously stimulated by the addition of nitrate, increased more than in control plants in low nitrate under anoxia and that suggests a key role of aquaporin activity in this response. It is proposed that a rapid decrease in root hydraulic resistance in the presence of increased nitrate availability is an important trait that could enhance a plant's ability to compete for nitrate in the soil.
Introduction
Nitrogen supply is highly variable in both time and space and is often a limiting factor for plant growth (Chapin, 1980; Marschner, 1995). An important source of nitrogen for plants is nitrate released from soil organic matter during mineralization (Attiwill and Adams, 1993; Jackson and Caldwell, 1993). Nitrate availability in soils is thought to fluctuate rapidly due to variation in rates of mineralization and the high mobility of nitrate ions in soil (Jackson and Caldwell, 1993; Robinson, 1994; Jackson and Caldwell, 1996). To cope with this heterogeneity, plants respond both developmentally and physiologically to local increases in nitrate availability. Developmental responses include root proliferation (Hodge et al., 1999) and changes in specific root length (Robinson and Rorison, 1983), while physiological responses include up-regulation of nitrate transporters and enzymes involved in nitrate assimilation (Krapp et al., 2002). Because physiological responses can occur much more quickly than responses that require the production of new roots, they allow roots to respond rapidly to short-term changes in the root environment.
The supply of mineral nutrients, especially nitrate, is known to influence rates of transpiration and leaf expansion. Physiological studies beginning in the 1980s suggested that this was mediated, at least in part, by an effect of nitrate availability on root hydraulic properties (Fiscus et al., 1983; Radin and Boyer, 1982; Radin and Eidenbock, 1984). In general, root hydraulic resistance increased when nitrate availability was low and decreased when nitrate supply was high (Carvajal et al., 1996; Clarkson et al., 2000). These changes, however, were observed over a period of several days, or at least several hours, and usually preceded the response of stomata or any changes in growth (for review see Clarkson et al., 2000). Whether sudden changes in nitrate availability can lead to rapid changes in root hydraulic properties, possibly through the modification of the number or activity of plasma membrane aquaporins, is not known (Carvajal et al., 1996; Henzler et al., 1999). In this paper, the temporal dynamics with which root hydraulic properties respond to alterations in external nitrate availability are examined and the basic mechanisms connecting nitrate availability with changes in root hydraulic properties are discussed.
Materials and methods
Cultivation of plants
Sunflower (Helianthus annuus cv. Holiday) plants were grown hydroponically in modified Hoagland's solution with 2.0 mM or 0.2 mM nitrate as the sole source of nitrogen, resulting in high (HN) and low nitrate (LN) treatments. The HN nutrient solution contained 603 μM Ca(NO3)2, 795 μM KNO3, 190 μM KH2PO4, 270 μM MgSO4, 2 μM MnSO4, 0.85 μM ZnSO4, 0.15 μM CuSO4, 20 μM H3BO3, 0.25 μM Na2MoO4, and 40.5 μM FeNa-EDTA, while the composition of the LN nutrient solution was 60.3 μM Ca(NO3)2, 79.5 μM KNO3, 360 μM K2SO4, 540 μM CaCl2, 190 μM KH2PO4, 270 μM MgSO4, 2 μM MnSO4, 0.85 μM ZnSO4, 0.15 μM CuSO4, 20 μM H3BO3, 0.25 μM Na2MoO4, and 40.5 μM FeNa-EDTA. Approximately 20 plants were grown in 30 l containers in which the well-aerated nutrient solution was renewed once or twice weekly depending on the size of plants. As plants increased in size, the number per container decreased due to several plants per day being removed for measurements. Natural (greenhouse) illumination was supplemented with metal halide lamps, providing a minimum intensity of 450 μmol m−2 s−1 PAR and a constant photoperiod of 16 h. Mean day/night temperatures were 25/16 °C. All plants were grown in the HN solution for 4 weeks, prior to the initiation of the LN treatment. Measurements took place 6–9 weeks after germination, at which time the plants had 6–10 leaves and a total dry mass 2–4 g.
Determination of root hydraulic resistance
Two methods were used to quantify the effect of nutrient availability on root hydraulic properties: a modified root pressure chamber described by Stirzaker and Passioura (1996) was used to determine changes in hydraulic resistance in vivo, while a standard hydraulic apparatus was used to quantify water flux through excised root systems.
The root pressure chamber technique allowed us to monitor instantaneous changes in the hydraulic resistance of intact, transpiring plants. Resistance was calculated as the pressure gradient from the root chamber to the shoot divided by the transpiration rate. An active feedback system controlled the pressure applied to roots such that the pressure in the shoot xylem was held constant (at atmospheric pressure). Pressure in the shoot xylem was monitored after removing the lamina of a leaf located in the middle of the stem and attaching a pressure transducer (PX26 Omega Engineering Inc. Stamford, CT, USA). The transducer was connected to the petiole using a short piece of thick-wall tubing that was sealed on the petiole using Parafilm (Pechinez Plastic Packaging, Menasha, WI, USA).
Plants were sealed in the root pressure chamber using a flexible dental impression material (Regular type Exaflex, GC America Inc., Alsip, IL, USA) to form a gasket around the base of the stem. Within the pressure chamber, the container containing the roots+nutrient solution of desired composition was continuously aerated using a battery-operated pump. Access ports allowed us to amend or exchange the hydroponic solution while maintaining the pressure in the root chamber. The gas composition supplied to the root chamber was controlled so as to keep the partial pressure of oxygen equal to that of the air. Transpiration rates were determined from changes in the weight of the pressure chamber, which was placed on an electronic balance (±0.01 g; Model 3808, Sartorius, Germany). Changes in mass due to changes in gas concentration within the root pressure chamber were taken into account when calculating transpiration rate.
A hydraulic apparatus, in which the pressure gradient driving xylem flow was created by compressed air, was used to quantify changes in the hydraulic properties of excised root systems in response to nitrate availability and anoxia. Flow-through detached root systems, immersed in a well-aerated and stirred nutrient solution, was driven by a pressure difference of 175 kPa. Under this delivery pressure the hydraulic component of the driving force dominates (Fig. 1), allowing changes in root hydraulic properties to be quantified independent of any changes in solute accumulation rates (root pressure). Flow rates were measured by directing the outflow from the root systems to an analytical balance (±0.00001 g Model 3200BX, Sartorius, Germany) using thick-walled tygon tubing. Pressure in the system was continuously monitored using a pressure transducer (PX01K1-050GV, Omega, Stamford, CT, USA) and recorded by a computer. The nutrient solutions used in these measurements were identical to those used in cultivation, except for the addition of 10 mM 2-(N-morpholino) ethanesulphonic acid (MES) set to pH 6.1 by KOH. The dry mass of root systems used for all measurements ranged from 0.3–0.5 g.
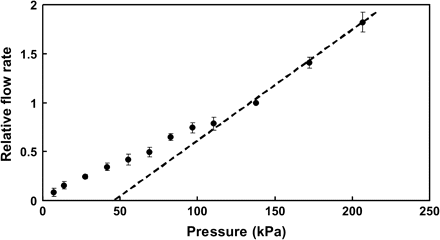
Relationship between the externally applied pressure and resulting flow rate of xylem sap through detached sunflower root systems. Each point represents the mean of six independent replicates, error bars represent 2 SE. At pressures above 100 kPa the pressure:flow relationship was linear, indicating that the osmotic component of the driving force (i.e. flow due to root pressure) was negligible. All further measurements on detached root systems were conducted using an externally supplied pressure of 175 kPa.
Experimental treatments
Changes in nitrate concentration were carried out by adding sodium nitrate (stock solution) to the root medium or exchanging a HN solution for a LN one. Anoxia was elicited by switching the aeration system intake to a nitrogen tank. Inhibition of nitrate reductase was induced by adding 0.15 mM sodium tungstate (Deng et al., 1989; Barthes et al., 1996) to the LN nutrient solution 20 h prior to any measurements. This treatment was shown to reduce NR activity by more than 50% compared with control plants (Barthes et al., 1996).
Sunflower, like many plants (Clarkson et al., 2000; Uehlein and Kaldenhoff, 2006), exhibits significant diurnal changes in root hydraulic permeability under control (constant) conditions. To account for this natural diurnal variation, experimental treatments were imposed at the same time of day and the experimental data compared against control plants monitored over the same time period. Data describing the response of hydraulic properties of root systems were calculated as the mean of flow rates measured on three to four different root systems normalized to the flow rate at the time when nitrate was applied.
Nitrate content
Nitrate content of roots was determined for oven-dried tissue. Roots were finely ground, extracted in hot demineralized water, and measured colorimetrically (Cataldo et al. 1975).
Results
Addition of nitrate to plants grown in low nitrate (LN) conditions resulted in a fast decrease in root hydraulic resistance: about 20% in the first hour, with the resistance continuing to fall over a period of several hours (Fig. 2). The initial response to the addition of 2 mM and 6 mM of nitrate was similar, however, the effect elicited by the higher nitrate level persisted for a longer period of time, eventually leading to much lower root hydraulic resistance. The resistance of the control plants (no addition of nitrate) increased throughout the experiment, reflecting an underlying diurnal trend of increasing resistance during the morning into mid-afternoon (data not shown). Interestingly, the response of LN plants to a subsequent nitrate withdrawal from 6 mM to 0.2 mM concentration was much faster than their response to nitrate addition. Furthermore, hydraulic resistance following this decrease in nitrate levels did not return to control values suggesting that some of the hydraulic modifications induced by exposure to high nitrate remained in place (Fig. 3). Nitrate addition did not induce any significant changes in transpiration rate of intact plants (data not shown), nor did plants with severed roots respond to nitrate addition suggesting that the entire response occurs within the roots.
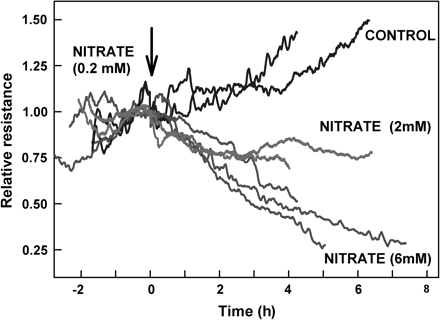
Changes in the relative hydraulic resistance of LN-grown intact sunflower plants in response to changes in nitrate concentration. Hydraulic resistance was measured using the root pressure chamber technique; values are scaled relative to the value at the time of when the experimental treatments were imposed. Concentrated stock solution was added to the hydroponic solution (at time=0) so that the concentration of nitrate supplied to the roots increased from 0.2 mM to 2 mM or 6 mM. Separate runs of plants with no addition of nitrate served as control.
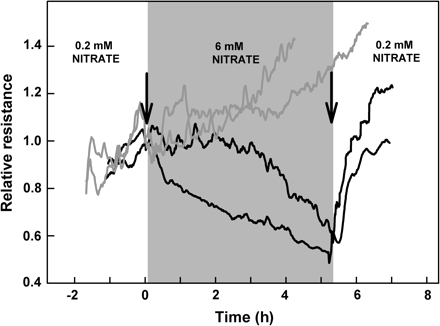
Hydraulic resistance in response to both increases and decreases in nitrate levels (arrows) for two sunflower plants (black lines). Hydraulic resistance decreased following an increase in nitrate concentrations from 0.2 mM to 6 mM and then recovered quickly upon return to a 0.2 mM nitrate solution. Hydraulic resistance was measured in vivo using the root pressure chamber technique; values are scaled relative to the value at the time of when the first experimental treatment was imposed. To aid comparison, data from two control plants (grey lines) are reprinted from Fig. 2.
Dramatic changes in root hydraulic properties in response to nitrate addition or its subsequent withdrawal were limited to LN-grown plants. Plants grown in high nitrate (HN) conditions (2 mM) were not responsive either to further increases in nitrate levels (6 mM) or to reduced nitrate availability (0.2 mM). This lack of response was presumably due to high NO3− content in HN plants (47 mg nitrate g−1 of dry root matter) as opposed to 5.5 mg g−1 in roots of LN plants. This difference might reflect the storage capacity of the vacuoles and the potential for stored nitrate to influence root hydraulic properties.
Flow rate through the roots of decapitated LN plants was related to the nitrate concentration in the root solution (Figs 2, 4). Increasing nitrate concentrations (0.5 mM to 5 mM) resulted in higher flow rates, corresponding to lower root hydraulic resistances.
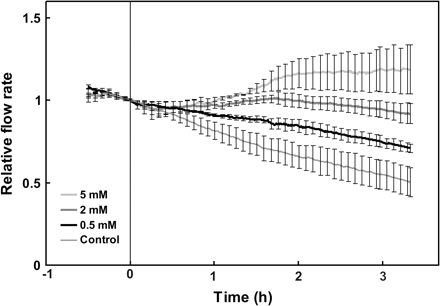
Relative flow rate of water through detached sunflower roots in response to increases in nitrate concentration. Each point represents the mean of three independent measurements per treatment; error bars represent 2 SE. An externally applied hydrostatic pressure of 175 kPa was used to generate the measured flow. Data were normalized relative to the flow rate at the time of nitrate addition (at time zero) for each measurement.
Treatment with sodium tungstate eliminated the effect of nitrate additions on the root hydraulic properties of LN-grown plants both in vivo (Fig. 5A) and with detached root systems (Fig. 5B). Reductions in oxygen levels resulted in a significant increase in root hydraulic resistance (Fig. 6; seen as drop in flow rate). The inhibitory effect of anoxia was much greater in root systems previously exposed to 5 mM nitrate than in control plants (Fig. 6).
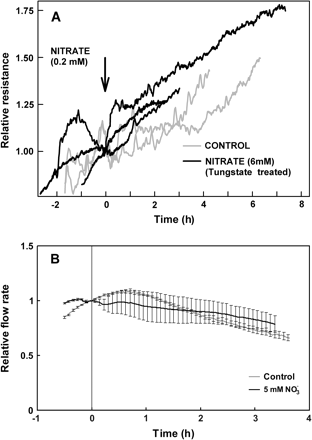
Response of root hydraulic properties of plants previously exposed for 20 h to sodium tungstate, an inhibitor of nitrate reductase, to increases in nitrate concentration supplied to the roots. (A) Changes in the relative hydraulic resistance of intact sunflower plants in response to changes in nitrate concentration as measured by the root pressurization technique. Root hydraulic resistance of tungstate-treated plants was unaffected by an increase of nitrate concentration from 0.2 mM to 6 mM (addition indicated by arrow). Values are scaled relative to the value at the time of nitrate addition (arrow); to aid comparison, the time-course of two control plants are reprinted from Fig. 2. (B) Relative flow rate of water through detached roots of sunflower plants in response to an external (175 kPa) pressure gradient. Data were normalized relative to the flow rate measured at the time of nitrate addition (time zero). Shown are means of three plants; error bars represent 2 SE.
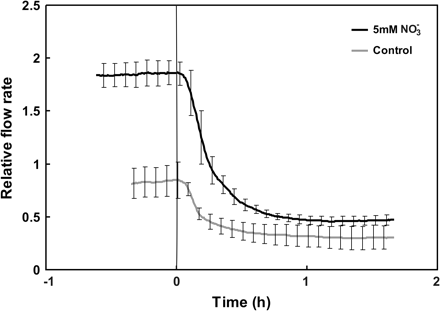
Relative flow rate of water through detached roots of sunflower plants when challenged with anoxia. One set of plants received 5 mM nitrate several hours before the anoxic treatment, while the second group of plants served as control. An externally applied hydrostatic pressure of 175 kPa was used to generate the measured flow. Data were normalized relative to the flow rate at the time of nitrate application. Each line represents the mean of three plants; error bars represent 2 SE.
Discussion
The ability to monitor, essentially continuously, the hydraulic properties of intact, transpiring plants allowed the interaction between nitrate supply and root properties to be probed in considerable detail. The results indicate shorter response times to changes in external nitrate availability than previously reported (Radin and Matthews, 1989; Clarkson et al., 2000). For example, experiments with Lotus japonicus showed that significant changes in root hydraulic properties requires, at a minimum, several hours following nitrate re-supply (Clarkson et al., 2000; Prosser et al., 2006). These results suggest that roots become more permeable to water within 20–30 min after the application of nitrate and that this increase in permeability continues over time with the maximum enhancement being dependent on nitrate concentration (Fig. 2). Such a fast response requires not only rapid signal transduction but also an equally rapid mechanism leading to changes in root hydraulic properties.
Mean hydraulic resistance calculated for excised roots of LN plants before the start of the experimental treatments was 0.0013±0.0002 MPa g−1 FW h−1 μl−1 (mean ±SE, that equals hydraulic conductivity 840±108 μl h−1 g−1 FW MPa−1), similar to values previously reported for root systems of sunflowers grown in hydroponics (Quintero et al., 1999). Because the addition of nitrate changes the osmotic properties of the solution surrounding the roots, it is worth asking to what extent the changes reported here could be the direct result of osmotic effects. Increased osmotic pressure of the solution, as well as the potential build-up of unstirred layers in the root cortex, would slow the flux of water. This suggests that the magnitude of changes that are presented here may be underestimated. On the other hand, rapid uptake of nitrate and its transport to xylem could build up an osmotic gradient into the xylem, stimulating water flux across the root cortex. Nitrate concentration in the xylem of sunflower plants can vary by an order of magnitude in the milimolar range, depending on the external nitrate supply (Nikolic and Romheld, 2003). This study's measurements of changes in osmolality of xylem sap after nitrate addition revealed that the osmotic potential of xylem sap decreased from 0.02 MPa to 0.015 MPa within the first 30 min after addition and remained constant over the next 120 min. This suggests that in these experiments, accumulation of nitrate and other ions in the root xylem did not contribute to the observed increase in water flow.
Similar to intact plants, the hydraulic resistance of root systems from decapitated plants also decreased in response to increased nitrate supply (Fig. 4). Furthermore, although the decrease in hydraulic resistance after the addition of nitrate to LN plants was rapid, the increase in resistance after subsequent nitrate withdrawal was even faster (Fig. 3). Differences in the dynamics of the response to nitrate addition versus nitrate withdrawal suggest that the mechanisms underlying these changes in root hydraulic properties may differ. In particular, the response to nitrate withdrawal was so abrupt that it may indicate the closure of a water pathway through the root, whereas the steady increases in permeability with greater nitrate supply suggest a process by which the water transport capacity of the root is enhanced. Similar dynamics after nitrate withdrawal and re-supply were reported for roots of Lotus japonicus (Prosser et al., 2006).
Nitrate assimilation represents a potentially important step in the signal transduction chain (Forde and Clarkson, 1999; Prosser et al., 2006). LN-grown sunflower plants exposed for 20 h to sodium tungstate no longer altered their hydraulic properties when supplied with nitrate. This suggests that it is not nitrate itself but the downstream products of its assimilation that are required to induce hydraulic changes in the root. Results of Barthes et al. (1996) indicate that urea, a source of NH4+, can reverse the negative effect of tungstate treatment. The rate of xylem exudation in detopped maize seedlings after tungstate treatment matched the rate of control plants when urea was the sole nitrogen source. These results also suggest that a product of nitrate assimilation is involved in the signal transduction pathway responsible for changes in root hydraulic properties (Barthes et al., 1996).
Nitrate uptake and assimilation involves a net consumption of protons, raising the possibility of a direct feedback between nitrate assimilation and the regulation of aquaporins. Despite the existence of biochemical and biophysical mechanisms for pH homeostasis (Marschner, 1995), Espen et al. (2004) showed that blocking nitrate reductase can lead to a measurable change in cytosolic pH. Decreases in cytosolic pH have also been shown reversibly to alter root hydraulic properties due to the protonation of a tyrosine residue on the cytosolic side of majority PIPs, resulting in a dramatic increase of root hydraulic resistance (Tournaire-Roux et al., 2003). This gating mechanism raises the possibility that changes in cytosolic pH due to nitrate assimilation could be involved in triggering nitrate-induced changes in the permeability of roots to water.
Aquaporins are responsible for a major part of the permeability of root cell membranes to water (Javot and Maurel, 2002). A number of studies have demonstrated that changes in aquaporin expression are correlated with changes in root hydraulic properties (Steudle and Henzler, 1995; Maurel, 1997; Henzler et al., 1999; Martre et al., 2002; Uehlein and Kaldenhoff, 2006). Anoxia was applied as a test for the engagement of aquaporins in the examined response (Tournaire-Roux et al., 2003). Although anoxia is not a selective blocker of aquaporins, it causes decreased activity of plasma membrane H+-ATPases and, therefore, rapid decreases in cytoplasmic pH. The increase in resistance (presented here as a decrease in the measured flow rate) was greater for plants resupplied with nitrate, presumably because more aquaporins were present (Fig. 6). Anoxia never entirely blocked water transport through roots, suggesting the presence of an apoplastic pathway (Steudle and Peterson, 1998) or the existence of intrinsic (i.e. non-aquaporin-mediated) membrane permeability to water.
The proposed mechanism of fast and reliable changes of root hydraulic properties to nitrate availability may play a critical role in the plant's overall strategy for acquiring resources from a highly dynamic source. Nitrate concentrations in soil solution frequently fluctuate within days or even hours due to environmental factors affecting mineralization rates and bacterial activity in the rhizosphere (Attiwill and Adams, 1993; Jackson and Caldwell, 1993). It is proposed that the ability to increase water uptake by roots in nitrate-rich soil domains, while at the same time reducing water uptake from nitrate-poor regions represents an important mechanism by which plants overcome their sessile life form, dynamically utilizing available resources without long-term developmental modifications. An increase in water uptake rates will cause highly mobile nitrate ions to be brought via convection flow to the root surface, which significantly contributes to greater nitrate uptake (Buysse et al., 1996). Hence, a rapid decrease in root hydraulic resistance in the presence of increased nitrate availability could enhance a plant's ability to compete for nitrate in the soil and thus would be an important trait for non-nitrogen-fixing plants. It is worth noting that our experiments examined only plants grown in hydroponics. The root systems of hydroponically grown plants typically differ in their morphology (longer, thinner roots with less branching and greater water content) and, due to their less robust structure, generally have greater permeability for water (Steudle and Peterson, 1998) than roots grown in soil. Therefore, the magnitude and dynamics of root response to nitrate availability may differ from what occurs in natural conditions.
This paper lays the groundwork for more detailed explorations of the underlying mechanism(s) of in the interaction between root hydraulic properties and external nitrate availability. Future research should examine the role of changes in cytoplasmic pH upon nitrate uptake and assimilation and investigate the relationship between external nitrate availability and expression of aquaporins in root cells. Of equal importance will be studies on the physiological significance of this interaction, emphasizing how local changes in root physiology enhance a global strategy for acquiring nitrogen from the highly dynamic soil environment.
We thank Anna Zwieniecka and Olda Jůza for technical assistance, Anna Górska for valuable help with some experiments and stimulating discussions. This work was financially supported by the Czech Ministry of Education, Youth and Sport by grant no. 1P05ME793, US Department of Agriculture grant NRI-2005-35100-16057, National Science Foundation grants DEB-0415938 and DGE-0312142 and the Andrew W. Mellon Foundation.




Comments