-
PDF
- Split View
-
Views
-
Cite
Cite
Agnese Comelli, Davide Mangioni, Lucia Scaramella, Anna Maraschini, Chiara Gaudino, Christian Folli, Ferruccio Ceriotti, Fabio Triulzi, Ciro Canetta, Andrea Gori, Alessandra Bandera, Strongyloides stercoralis central nervous system dissemination in a migrant misdiagnosed with eosinophilic granulomatosis with polyangiitis, Journal of Travel Medicine, Volume 29, Issue 1, January 2022, taab177, https://doi.org/10.1093/jtm/taab177
Close - Share Icon Share
A 67-year-old Brazilian woman living in Italy since 1993 had last travelled to Brazil 8 years ago.
In her past medical history, she reported a diagnosis of eosinophilic granulomatosis with polyangiitis (formerly known as Churg Strauss syndrome) treated with systemic steroids (around 0.1 mg/kg/day of prednisone) since 2016. Azathioprine, anti-IgE (omalizumab) and anti-IL5 monoclonal antibodies (mepolizumab) were sequentially added with no benefit. As complication, she developed metasteroid diabetes.
The patient has a long history of hospital admission because of COPD exacerbation. In September 2020, after one of the last hospitalizations, she had not followed the steroid de-escalation prescribed at the time of discharge, continuing to take a dosage equal to 0.8–1 mg/kg/day of prednisone. Later, in November 2020, she got through a severe bacterial meningitis (CSF with neutrophilic pleocytosis, hypoglycorrhachia, lactate 7.9 mmol/l and elevated protein) without microbiologic isolation. At that time, cerebral MRI performed was without abnormalities.
Two months later, she was admitted to the acute medical ward of Foundation IRCCS Ca′ Granda Ospedale Maggiore Policlinico, in Milan, Northern Italy, because of malaise, weight loss, anorexia, mild ideomotor, slowing and worsening of lower limbs neuropathy and all those symptoms were observed at admission.
She underwent cerebral MRI to exclude embolic events and, conversely, it showed several small T2-FLAIR hyperintense and DWI-restricted lesions consistent with infective aetiology (Figure 1).
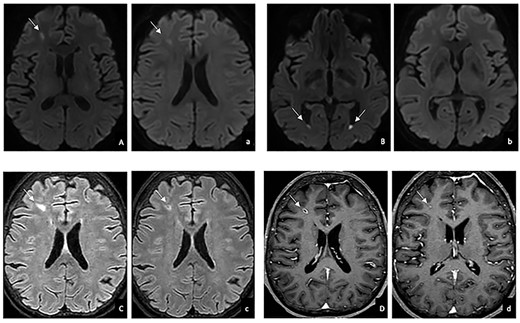
Brain MRI before (uppercase) and after (lowercase) antiparasitic treatment in DWI (A, B, a, b), T2-FLAIR weighted (C, c) and T1 weighted (D,d) images: reduction in DWI restriction (A,a), size (C,c) and contrast enhancement (D, d); resolution of the fluid levels in the lateral ventricles (B,b).
Later on, she underwent ID consultation because of a Citrobacter braakii bacteriaemia and to further investigate the cerebral lesions. Considering the several clues (Enterobacteriaceae spp. bacteraemia, epidemiological risk factor, history of transient urticaria and hypereosinophilia with recurrent bronchitis/asthma), she was screened for Strongyloides stercoralis infection and serology (Strongyloides ratti ELISA from Bordier Affinity Products, Crissier, Switzerland) resulted positive.
Eosinophil count was normal (always <200/μl). Rhabditiform larvae were detected in faeces, in sputum and in gastric ulcer biopsy (performed because of an episode of severe hematemesis). HIV 1-2 and HTLV-1 serology were negative.
Specific serology on CSF was negative while identification of S. stercoralis DNA through PCR was not available. On CSF white blood cells were absent, glucose and proteins were normal and lactate was 1.9 mmol/l. Differential diagnoses tested on CSF were excluded: no bacterial isolation in CSF culture, research of acid-alcohol resistant bacilli was negative by microscopy and culture, Mycobacterium tuberculosis DNA was negative, Cryptococcus neoformans antigen, herpetic virus DNA and T. gondii DNA were negative.
Antibiotic was discontinued after 7 days.
No lung lesions were detected on X-ray and the basic laboratory work-up (blood test count, kidney and liver test) was normal.
We concluded for an S. stercoralis hyperinfection with a high suspicion of CNS (central nervous system) dissemination.
Monoclonal antibodies were stopped, steroids tapered and the patient started treatment with ivermectin 200 mcg/kg/day, which was continued for 14 days after the first negative stool microscopic exam.
After discharge, she showed improved clinical status, stool microscopic exam and serology were negative at 6 months and MRI at 3 months after treatment showed a reduction in size and DWI hyperintensity of the brain lesions.
S. stercoralis is a soil-transmitted helminth distributed widely in tropical and subtropical areas and Brazil is defined as hyperendemic region.1
Although this patient had already spent more than 20 years in Italy, the unique ability of Strongyloides spp. to autoinfect explains why it can persist lifelong if not adequately treated.2
Immunomodulatory and immunosuppressive agents, HTLV-1 and steroids use (equivalent to 20 mg/day of prednisone for more than 2 weeks) are the main risk factors for dissemination, hyperinfection and death.2,3
CNS involvement other than bacterial meningitis is possible in such context with primary invasion of CNS by S. stercoralis larvae that is a rare finding but some case reports were published.4
In published cases, the most common immunosuppressive treatment was steroid and six out of seven patients died.4
By contrast, our patient has recovered by respiratory and neurologic symptoms, and CNS lesions at MRI have shown a significant improvement.
In conclusion, the present case highlights two main points.
First, screening programs tailored on the patients who are planning to receive corticosteroids or other immune suppressive medications are needed to prevent complicated form of S. stercoralis infection.
Second, high-income countries are more and more inhabited by migrants coming from different epidemiologic settings and there is an urgent need for a wider knowledge of neglected tropical diseases among all specialists in order to enrich the differential diagnosis process and avoid to misdiagnose deadly infectious condition.5
Author Contributions
A.M. and F.C. performed the parasitological examinations. A.C., L.S., C.F. and D.M. provided direct care for the patient. C.G. and F.T. performed MRI exams, A.C. did the literature research, interpreted the findings and wrote the first version of the manuscript. C.C., A.G. and A.B. supervised methodologic procedures and manuscript writing. All authors critically reviewed and approved the final version of the manuscript.
Acknowledgments
We thank all the participants who contributed to this study. Conflict of interest: The authors declare no conflict of interest.
Financial Support
None.
Initially submitted 03 October 2021; accepted for publication 26 October 2021.



