-
PDF
- Split View
-
Views
-
Cite
Cite
Elien Lecomte, Guy Laureys, Frederick Verbeke, Cristina Domingo Carrasco, Marjan Van Esbroeck, Ralph Huits, A clinician’s perspective on yellow fever vaccine-associated neurotropic disease, Journal of Travel Medicine, Volume 27, Issue 7, October 2020, taaa172, https://doi.org/10.1093/jtm/taaa172
Close - Share Icon Share
Abstract
Yellow fever (YF) causes high fever, liver dysfunction, renal failure, hypercoagulopathy and platelet dysfunction and can lead to shock and death with a case-fatality ratio of 20–50%. YF vaccination results in long-lasting protective immunity. Serious adverse events (SAEs), such as YF vaccine-associated neurotropic disease (YEL-AND) are rare. We present a case of a 56-year-old Caucasian man with fever, headache, cognitive problems at the emergency department. He received a primary YF vaccination 4 weeks prior to symptom onset. Cerebrospinal fluid tested positive (POS) for YF virus by reverse transcriptase polymerase chain reaction and confirmed diagnosis of YEL-AND. The patient recovered with symptomatic treatment. We reviewed published clinical reports on YEL-AND indexed for MEDLINE. We identified and analyzed 53 case reports. Forty-five patients were male and eight were female. Twenty-nine cases met criteria for definite YEL-AND and twenty-four for suspected YEL-AND according to YF Vaccine Safety Working Group. We applied the Brighton Collaboration diagnostic criteria to assess the diagnostic accuracy of the clinical diagnoses and found meningoencephalitis in 38 reported YEL-AND cases, Guillain Barré Syndrome (GBS) in seven, Acute Disseminated Encephalomyelitis (ADEM) in six and myelitis in five. Thirty-five patients recovered or improved; however, not all cases had a complete follow-up. The prognosis of YEL-AND presenting with GBS, ADEM or myelitis was poor. Fourteen patients received therapy (corticosteroids, intravenous immunoglobulins and/or plasmapheresis). In conclusion, YF vaccine-associated neurotropic disease is a very rare but SAE after YF vaccination. We described a case of YEL-AND and propose a standardized clinical workup of this condition based on a review of the literature. Centralized registration of complications of YF vaccination is encouraged.
Background
Yellow fever (YF) causes high fever, liver dysfunction, renal failure, hypercoagulopathy and platelet dysfunction and can lead to shock and death with a case-fatality rate of 20–50%. In-hospital fatality rates of YF as high as 67% have been reported.2 The causative agent is the yellow fever virus (YFV), a member of the Flaviridae family, which is transmitted through infectious bites of Aedes species mosquitoes. Nine hundred million people in South America and Sub-Saharan Africa are at risk of infection during recurring sylvatic and urban YF outbreaks.1,3
Specific antiviral therapy is not available but effective live-attenuated vaccines against YFV have been available since the 1930s.4 Vaccination results in long-lasting protective immunity and neutralizing antibodies can be detected in >75% of individuals at 10 days, and in over 99% at 28 days post-vaccination.1
Side effects are typically mild and include short-lived self-limiting injection site reactions, myalgia, low fever and headache.1 Only rarely does YF vaccination lead to serious complications, such as YF vaccine-associated viscerotropic disease (YEL-AVD) or YF vaccine-associated neurotropic disease (YEL-AND).5 We describe a case of YEL-AND and present a review of the literature focusing on the clinical presentation of this condition.
Results
Case Presentation
In June 2018, a 56-year-old immunocompetent Caucasian man with arterial hypertension but otherwise unremarkable medical history was admitted to the Ghent University Hospital because of fever, headache and difficulties with short-term memory had started 7 days before. He also had anorexia and nausea with vomiting.
Four weeks prior to the onset of these symptoms, he was vaccinated for the first time against YF virus, by subcutaneous administration of the 17D-204 YF vaccine strain (Stamaril, Sanofi Pasteur) because of intended travel to Tanzania. He also received a hepatitis A vaccine (Havrix 1440, GlaxoSmithKline). Three days after the vaccination, he developed a flu-like illness that resolved in three days but he remained fatigued.
Recent travel included a business trip to Japan, 3 months before the current illness and he had spent a week hiking in Southern Germany 1 month before. He did not report any tick or mosquito bites (see Figure 1). The patient was never vaccinated against other Flaviviridae, e.g. tick-borne-encephalitis virus (TBEV) or Japanese encephalitis virus (JEV).
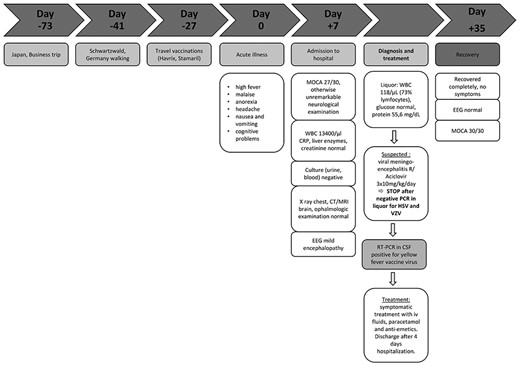
Timeline: Day 0 is the day of the acute illness. Summary of clinical and diagnostic features, treatment and follow-up.
Upon physical examination, the patient had a fever (38.3°C), blood pressure of 140/95 mmHg and a heart rate of 94 bpm. He did not show meningeal symptoms, photophobia, motor or sensory deficits on neurological examination. Tendon reflexes were normal. No retinal abnormalities were detected on funduscopic examination. He manifested delayed recall but did not show a significant cognitive impairment [Montreal-Cognitive-Assessment (MOCA) was 27/30].
The blood count showed a leucocyte count of 13 400/μl (upper limit reference interval 9300/μl), but no elevation of C-reactive protein (CRP). The liver enzymes and serum creatinine concentrations were all within the normal range. There were no signs of organ failure. Urine and blood cultures remained negative (NEG). A chest radiography showed no infiltrates. Computed tomography (CT) of the brain showed no space-occupying lesions. Magnetic resonance imaging (MRI) of the brain showed moderate cerebral atrophy, predominantly frontal, but there were no white matter lesions nor contrast-enhancing lesions.
Opening pressure at lumbar puncture was 16 cm H2O. Examination of cerebrospinal fluid (CSF) showed an elevated white blood cell (WBC) count (118/μl; 73% lymphocytes), elevated protein (55.6 mg/dl) and normal glucose and lactate concentrations.
An electroencephalogram (EEG) showed a posterior dominant background rhythm of 10 Hz with intermittent theta-activity, consistent with mild encephalopathy.
Parental administration of acyclovir (3 × 10 mg/kg daily) was started for a presumptive diagnosis of herpetic meningoencephalitis (ME). The treatment was discontinued within 24 h, when a NEG polymerase chain reaction (PCR) result for herpes simplex virus and varicella-zoster virus in CSF returned from the laboratory.
We detected anti-flavivirus antibodies in the patient’s serum, most likely resulting from seroconversion after YFV vaccination (Table 1). Neutralizing antibodies against YF virus were detected in serum that was obtained 7 days after onset of the acute illness (PRNT90 assay titer 1:131) but not in CSF.
| Antibodies . | IgM . | IgG . | NA . |
|---|---|---|---|
| WNV | NEG | POS/1/1000 | |
| DENV | NEG | POS/1/1000 | |
| JEV | WEAK POS | POS/1/10000 | |
| YFV | POS | POS/1/1000 | POS (1:131) |
| TBEV | – | POS/1/1000 |
| Antibodies . | IgM . | IgG . | NA . |
|---|---|---|---|
| WNV | NEG | POS/1/1000 | |
| DENV | NEG | POS/1/1000 | |
| JEV | WEAK POS | POS/1/10000 | |
| YFV | POS | POS/1/1000 | POS (1:131) |
| TBEV | – | POS/1/1000 |
This table shows extensive cross-reactivity between antibodies against antigenically related flaviviruses in the serum of our patient, 33 days after YF vaccination. An indirect immunofluorescence assay was used for the detection of IgM and IgG (EUROIMMUN, ref FI 2665–1010 G and M). PRNT90 assay titer against YFV in a porcine stable kidney cell line (>1:10 is considered protective). WNV, West Nile virus; DENV, dengue virus.
| Antibodies . | IgM . | IgG . | NA . |
|---|---|---|---|
| WNV | NEG | POS/1/1000 | |
| DENV | NEG | POS/1/1000 | |
| JEV | WEAK POS | POS/1/10000 | |
| YFV | POS | POS/1/1000 | POS (1:131) |
| TBEV | – | POS/1/1000 |
| Antibodies . | IgM . | IgG . | NA . |
|---|---|---|---|
| WNV | NEG | POS/1/1000 | |
| DENV | NEG | POS/1/1000 | |
| JEV | WEAK POS | POS/1/10000 | |
| YFV | POS | POS/1/1000 | POS (1:131) |
| TBEV | – | POS/1/1000 |
This table shows extensive cross-reactivity between antibodies against antigenically related flaviviruses in the serum of our patient, 33 days after YF vaccination. An indirect immunofluorescence assay was used for the detection of IgM and IgG (EUROIMMUN, ref FI 2665–1010 G and M). PRNT90 assay titer against YFV in a porcine stable kidney cell line (>1:10 is considered protective). WNV, West Nile virus; DENV, dengue virus.
YF vaccine-associated neurotropic disease (YEL-AND) was confirmed by detection of YFV RNA by real-time reverse transcription PCR (RT-PCR) in CSF, sampled 33 days after the vaccination (repeated three times; cycle threshold values 41.1, 39.0 and 41.4).6
Treatment was aimed at symptom relief and the patient’s condition gradually improved. At a follow-up visit 1 month after diagnosis, all clinical symptoms had disappeared and the modified Rankin Scale (mRS) score was zero. The MOCA score was now 30/30, with a normal EEG.
Literature Overview
YEL-AND is a rare neurological complication caused by YF vaccination.
In active surveillance and randomized controlled trials, no cases of YEL-AND were detected.7,8 Based on pharmacovigilance database searches, the incidence is estimated at 3.9 per million administered vaccine doses (range 0.16–14.6 per million).5,9–15
The CDC’s YF Vaccine Safety Working Group (YFVS) case definitions for YEL-AND classify cases as suspect when 1 to 30 days after YF vaccination level 1 evidence (i.e. one or more of fever and headache (>24 h); focal neurological dysfunction; mental status change; new-onset seizure or recurrence of previously controlled seizures; CSF pleiocytosis (>5 WBC/μl); elevated CSF protein (>1.5 times normal limit)) AND level 2 evidence (i.e. neuroimaging consistent with inflammation and/or EEG finding consistent with encephalopathy) are present, in absence of an alternate diagnosis. Probable cases are suspect cases that have vaccine-type YF virus (VT-YFV) isolated from blood >7 days post-vaccination or serum concentrations of VT-YFV that exceed 3 log10 pfu/ml on any day. Definite cases are suspect cases with YFV-specific IgM detectable in CSF and/or isolation or amplification of VT-YFV from CSF.1
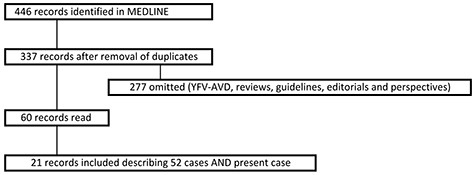
To investigate the clinical presentation and diagnostic features of YEL-AND, we searched MEDLINE for clinical case reports, using the following search strategy: YF vaccine AND neurologic (52 results) OR neurotropic (43) OR meningitis (66) OR ocular (2) OR encephalitis (224) OR myelitis (40) OR encephalomyelitis (19); limits were set for species (human) and publication dates (12 May 1940, 2020), not for language.
We identified a total of 52 cases describing YEL-AND between 1965 and 2018,3,14,16–34 and after including our patient, we analyzed 53 (Figure 2, Table 2).
| Patient characteristics . | Totals . | . | Missinga . |
|---|---|---|---|
| Gender ratio (M:F) | 45:8 | – | |
| Age (median, range); (years) | 38.5 | (0.1–78) | 1 |
| Medical history | 37 | 16 | |
| Healthy | 31 | (83.8%) | |
| Cardiovascular history | 4 | (10.8%) | |
| Other (end stage renal disease, alcoholic liver disease and undiagnosed HIV) | 2 | (5.4%) | |
| Time from vaccination to symptom onset (median, range);(days) | 15.5 | (2–50) | 1 |
| MRI/CT abnormalities (percentage) | 16/22 | (72.7%) | 31 |
| EEG abnormalities (percentage) | 8/9 | (88.9%) | 44 |
| Lumbar puncture (percentage) | 50/53 | (94.3%) | |
| CSF findings | |||
| WBC > 5/μl | 28/38 | (73.7%) | 15 |
| Protein > 50 mg/dl | 29/38 | (76.3%) | 15 |
| YFV RT-PCR in CSF | 2/9 | (22.2%) | 44 |
| Anti-YFV IgM in CSF | 26/31 | (81.2%) | 22 |
| Meningoencephalitis (as reported) | 38 | (73.1%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 25 | (65.8%) | |
| Brighton criteria level 3 | 8 | (21.1%) | |
| Misclassification | 1 | (21.1%) | |
| Insufficient data | 4 | (10.5%) | |
| GBS (as reported) | 7 | (13.7%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 6 | (85.7%) | |
| Brighton criteria level 3 | – | – | |
| Misclassification | 1 | (14.3%) | |
| ADEM (as reported) | 6 | (11.8%) | |
| Brighton criteria level 1 | 5 | (83.3%) | |
| Brighton criteria level 2 | 1 | (16.6%) | |
| Brighton criteria level 3 | – | – | |
| Myelitis (as reported)b | 5 | (9.4) | |
| Brighton criteria level 2 | 5 | (100%) | |
| Good outcome (for cases with reported outcomes) (percentage) | 35/51 | (68.6%) | 2 |
| Meningoencephalitis | 29/38 | (76.3%) | 2 |
| ADEM | 2/6 | (33.3%) | |
| GBS | 2/7 | (28.6%) | |
| Myelitisb | 2/5 | (40%) | |
| Age in good outcome group (median, IQR (years)) | 32.5 | (18–55) | |
| Age in poor outcome group (median, IQR (years)) | 53 | (21–59) | |
| Therapy (percentage) | 14/53 | (26.4%) | |
| Patient characteristics . | Totals . | . | Missinga . |
|---|---|---|---|
| Gender ratio (M:F) | 45:8 | – | |
| Age (median, range); (years) | 38.5 | (0.1–78) | 1 |
| Medical history | 37 | 16 | |
| Healthy | 31 | (83.8%) | |
| Cardiovascular history | 4 | (10.8%) | |
| Other (end stage renal disease, alcoholic liver disease and undiagnosed HIV) | 2 | (5.4%) | |
| Time from vaccination to symptom onset (median, range);(days) | 15.5 | (2–50) | 1 |
| MRI/CT abnormalities (percentage) | 16/22 | (72.7%) | 31 |
| EEG abnormalities (percentage) | 8/9 | (88.9%) | 44 |
| Lumbar puncture (percentage) | 50/53 | (94.3%) | |
| CSF findings | |||
| WBC > 5/μl | 28/38 | (73.7%) | 15 |
| Protein > 50 mg/dl | 29/38 | (76.3%) | 15 |
| YFV RT-PCR in CSF | 2/9 | (22.2%) | 44 |
| Anti-YFV IgM in CSF | 26/31 | (81.2%) | 22 |
| Meningoencephalitis (as reported) | 38 | (73.1%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 25 | (65.8%) | |
| Brighton criteria level 3 | 8 | (21.1%) | |
| Misclassification | 1 | (21.1%) | |
| Insufficient data | 4 | (10.5%) | |
| GBS (as reported) | 7 | (13.7%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 6 | (85.7%) | |
| Brighton criteria level 3 | – | – | |
| Misclassification | 1 | (14.3%) | |
| ADEM (as reported) | 6 | (11.8%) | |
| Brighton criteria level 1 | 5 | (83.3%) | |
| Brighton criteria level 2 | 1 | (16.6%) | |
| Brighton criteria level 3 | – | – | |
| Myelitis (as reported)b | 5 | (9.4) | |
| Brighton criteria level 2 | 5 | (100%) | |
| Good outcome (for cases with reported outcomes) (percentage) | 35/51 | (68.6%) | 2 |
| Meningoencephalitis | 29/38 | (76.3%) | 2 |
| ADEM | 2/6 | (33.3%) | |
| GBS | 2/7 | (28.6%) | |
| Myelitisb | 2/5 | (40%) | |
| Age in good outcome group (median, IQR (years)) | 32.5 | (18–55) | |
| Age in poor outcome group (median, IQR (years)) | 53 | (21–59) | |
| Therapy (percentage) | 14/53 | (26.4%) | |
Summary of most important findings of case reports founded in literature.
aMissing refers to the number of cases where the variable was not reported. M, male; F, female; RT-PCR, reverse transcriptase polymerase chain reaction.
bMyelitis was diagnosed in five cases (9.4%), three of whom had concurrent meningoencephalitis.
| Patient characteristics . | Totals . | . | Missinga . |
|---|---|---|---|
| Gender ratio (M:F) | 45:8 | – | |
| Age (median, range); (years) | 38.5 | (0.1–78) | 1 |
| Medical history | 37 | 16 | |
| Healthy | 31 | (83.8%) | |
| Cardiovascular history | 4 | (10.8%) | |
| Other (end stage renal disease, alcoholic liver disease and undiagnosed HIV) | 2 | (5.4%) | |
| Time from vaccination to symptom onset (median, range);(days) | 15.5 | (2–50) | 1 |
| MRI/CT abnormalities (percentage) | 16/22 | (72.7%) | 31 |
| EEG abnormalities (percentage) | 8/9 | (88.9%) | 44 |
| Lumbar puncture (percentage) | 50/53 | (94.3%) | |
| CSF findings | |||
| WBC > 5/μl | 28/38 | (73.7%) | 15 |
| Protein > 50 mg/dl | 29/38 | (76.3%) | 15 |
| YFV RT-PCR in CSF | 2/9 | (22.2%) | 44 |
| Anti-YFV IgM in CSF | 26/31 | (81.2%) | 22 |
| Meningoencephalitis (as reported) | 38 | (73.1%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 25 | (65.8%) | |
| Brighton criteria level 3 | 8 | (21.1%) | |
| Misclassification | 1 | (21.1%) | |
| Insufficient data | 4 | (10.5%) | |
| GBS (as reported) | 7 | (13.7%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 6 | (85.7%) | |
| Brighton criteria level 3 | – | – | |
| Misclassification | 1 | (14.3%) | |
| ADEM (as reported) | 6 | (11.8%) | |
| Brighton criteria level 1 | 5 | (83.3%) | |
| Brighton criteria level 2 | 1 | (16.6%) | |
| Brighton criteria level 3 | – | – | |
| Myelitis (as reported)b | 5 | (9.4) | |
| Brighton criteria level 2 | 5 | (100%) | |
| Good outcome (for cases with reported outcomes) (percentage) | 35/51 | (68.6%) | 2 |
| Meningoencephalitis | 29/38 | (76.3%) | 2 |
| ADEM | 2/6 | (33.3%) | |
| GBS | 2/7 | (28.6%) | |
| Myelitisb | 2/5 | (40%) | |
| Age in good outcome group (median, IQR (years)) | 32.5 | (18–55) | |
| Age in poor outcome group (median, IQR (years)) | 53 | (21–59) | |
| Therapy (percentage) | 14/53 | (26.4%) | |
| Patient characteristics . | Totals . | . | Missinga . |
|---|---|---|---|
| Gender ratio (M:F) | 45:8 | – | |
| Age (median, range); (years) | 38.5 | (0.1–78) | 1 |
| Medical history | 37 | 16 | |
| Healthy | 31 | (83.8%) | |
| Cardiovascular history | 4 | (10.8%) | |
| Other (end stage renal disease, alcoholic liver disease and undiagnosed HIV) | 2 | (5.4%) | |
| Time from vaccination to symptom onset (median, range);(days) | 15.5 | (2–50) | 1 |
| MRI/CT abnormalities (percentage) | 16/22 | (72.7%) | 31 |
| EEG abnormalities (percentage) | 8/9 | (88.9%) | 44 |
| Lumbar puncture (percentage) | 50/53 | (94.3%) | |
| CSF findings | |||
| WBC > 5/μl | 28/38 | (73.7%) | 15 |
| Protein > 50 mg/dl | 29/38 | (76.3%) | 15 |
| YFV RT-PCR in CSF | 2/9 | (22.2%) | 44 |
| Anti-YFV IgM in CSF | 26/31 | (81.2%) | 22 |
| Meningoencephalitis (as reported) | 38 | (73.1%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 25 | (65.8%) | |
| Brighton criteria level 3 | 8 | (21.1%) | |
| Misclassification | 1 | (21.1%) | |
| Insufficient data | 4 | (10.5%) | |
| GBS (as reported) | 7 | (13.7%) | |
| Brighton criteria level 1 | – | – | |
| Brighton criteria level 2 | 6 | (85.7%) | |
| Brighton criteria level 3 | – | – | |
| Misclassification | 1 | (14.3%) | |
| ADEM (as reported) | 6 | (11.8%) | |
| Brighton criteria level 1 | 5 | (83.3%) | |
| Brighton criteria level 2 | 1 | (16.6%) | |
| Brighton criteria level 3 | – | – | |
| Myelitis (as reported)b | 5 | (9.4) | |
| Brighton criteria level 2 | 5 | (100%) | |
| Good outcome (for cases with reported outcomes) (percentage) | 35/51 | (68.6%) | 2 |
| Meningoencephalitis | 29/38 | (76.3%) | 2 |
| ADEM | 2/6 | (33.3%) | |
| GBS | 2/7 | (28.6%) | |
| Myelitisb | 2/5 | (40%) | |
| Age in good outcome group (median, IQR (years)) | 32.5 | (18–55) | |
| Age in poor outcome group (median, IQR (years)) | 53 | (21–59) | |
| Therapy (percentage) | 14/53 | (26.4%) | |
Summary of most important findings of case reports founded in literature.
aMissing refers to the number of cases where the variable was not reported. M, male; F, female; RT-PCR, reverse transcriptase polymerase chain reaction.
bMyelitis was diagnosed in five cases (9.4%), three of whom had concurrent meningoencephalitis.
We identified 29 of 53 (54.7%) definite cases of YEL-AND (YFV IgM antibodies and/or RT-PCR POS in CSF) and 24 (45.3%) who met the criteria for suspected YEL-AND (YFV-specific IgM or RT-PCR in CSF NEG/not determined). Note that three had symptom onset >30 days after vaccination, yet all three of them had IgM antibodies in CSF.14,20,22 Following YFVS case definitions, we classified these as definite YEL-AND. All patients were vaccinated with the 17D (17DD or 17D-204) strain.
Forty-five patients (85%) were male and eight were female (15%). The median age was 38.5 years [interquartile range (IQR) 19.8–58.3], with 12 cases (22.6%) older than 60. We identified three cases involving infants of 9 months or younger. Four patients had a cardiovascular history, two had other medical histories (end-stage renal disease, alcoholic liver disease). The patient with liver disease was found to have a previously undiagnosed HIV infection with advanced immunosuppression (CD4-cell count 108/μl). Thirty-one (83,8%) were previously healthy. Of 16 patients, past medical history was not available.
We used the Brighton Collaboration diagnostic criteria to assess the diagnostic accuracy of YEL-AND case reports identified through our search.35,36 The reported clinical presentation was ME in 38 (73.1%), Guillain Barré Syndrome (GBS) in seven (13.2%), Acute Disseminated Encephalomyelitis (ADEM) in six (11.3%) and myelitis in five (9.4%), three of whom had concurrent ME. However, according to the Brighton criteria that rank the diagnostic certainty from high (level 1) to low (level 3), ME patients were classified as level 2 in 25 of 38, level 3 in eight, one patient did not meet the diagnostic criteria and in four the provided information was insufficient to confirm the diagnosis. For ADEM, five out of six patients met level 1 and one patient met level 2. Six patients with GBS met level 2 and one did not meet the criteria for lack of weakness. All myelitis patients met level 2 of the Brighton criteria (Table 2).
The median time from vaccination until symptom onset was 15.5 days (range 2–50 days). The median time to onset was similar for patients with ME, GBS and ADEM (16, 16 and 13 days, respectively), but it was much longer for myelitis (45 days).
In 22 cases, CT or MRI was performed. Imaging abnormalities were reported in 16 out of 22 cases (72.7%) and included meningeal enhancement, white matter hyperintensities and demyelination.
EEG was abnormal in eight out of nine published cases (88.9%) and readings reported were compatible with encephalopathy or epileptic discharges.
Lumbar puncture was performed in 50 out of 53 diagnostic workups. WBC counts and protein levels were reported in 38 cases. CSF pleiocytosis was observed in 28 patients (73.7%), range 10–465/μl; protein levels were elevated >50 mg/dl in 29 patients (76.3%), ranging from 55.5–300 mg/dl.
RT-PCR for YFV was performed in only nine cases; two cases (including our patient) had detectable YFV RNA and seven had a NEG RT-PCR result in CSF. Twenty-six patients had anti-flavivirus IgM antibodies in CSF and seven did not.
A good outcome was defined as mRS score of 0, 1 or 2; a bad outcome as mRS ≥ 3.37–39 Outcomes were reported for 51 cases. Thirty-five patients (68.6%) recovered or improved; however, not all cases had a complete follow-up, defined as complete recovery or mRS score at 3 months or longer after vaccination.37–39 Thirty-one cases had a complete follow-up. Prognosis of GBS, ADEM and myelitis was poor (5/7, 4/6 and 2/5, respectively). Five cases of GBS, ADEM and myelitis had a complete follow-up, eight had an incomplete follow-up and in five cases period of follow-up was not mentioned. Clinical recovery was complete for 29/38 ME cases with reported outcomes. We retrieved two cases of infants (38 days and 5 weeks old) with YEL-AND after VT-YFV exposure through breast milk following maternal vaccination.28,29 Both made a full recovery.28,29 Two fatalities have been documented. The first one was a 3-year-old girl who died 12 days after 17D vaccination in 1966.32 The second was the 53-year-old Thai man with alcoholic liver disease and newly diagnosed HIV-infection with low CD4 counts, who developed encephalomyelitis 3 days after vaccination. The patient died 10 days after vaccination.33
The median age was 32.5 years in the good outcome group, and 53 years in the poor outcome group.
Fourteen patients (26.4%) received therapy. Most patients receiving therapy had a clinical presentation of GBS (n = 6) and ADEM (n = 4), followed by ME (n = 1) and a combination of myelitis with ME (n = 3). Five patients received intravenous immunoglobulin (IVIG), six received corticosteroids, one patient had plasmapheresis, one had a combination of IVIG and plasmapheresis and one received a combination of corticosteroids and plasmapheresis (Table 3).3,14,19,23,27 Six of the treated patients had a good outcome, seven remained with sequelae (four with GBS, two with ADEM and one with a combination of myelitis and ME) and one died (a combination of myelitis and ME).
| Treatment/presentation . | GBS . | ADEM . | ME . | ME + myelitis . |
|---|---|---|---|---|
| IVIG | 3 | 1 | 1 | |
| Corticosteroids | 1 | 3 | 2 | |
| Plasmapheresis | 1 | |||
| IVIG + plasmapheresis | 1 | |||
| corticosteroids + plasmapheresis | 1 |
| Treatment/presentation . | GBS . | ADEM . | ME . | ME + myelitis . |
|---|---|---|---|---|
| IVIG | 3 | 1 | 1 | |
| Corticosteroids | 1 | 3 | 2 | |
| Plasmapheresis | 1 | |||
| IVIG + plasmapheresis | 1 | |||
| corticosteroids + plasmapheresis | 1 |
Summary of therapies given to patients with YEL-AND per clinical presentation.
| Treatment/presentation . | GBS . | ADEM . | ME . | ME + myelitis . |
|---|---|---|---|---|
| IVIG | 3 | 1 | 1 | |
| Corticosteroids | 1 | 3 | 2 | |
| Plasmapheresis | 1 | |||
| IVIG + plasmapheresis | 1 | |||
| corticosteroids + plasmapheresis | 1 |
| Treatment/presentation . | GBS . | ADEM . | ME . | ME + myelitis . |
|---|---|---|---|---|
| IVIG | 3 | 1 | 1 | |
| Corticosteroids | 1 | 3 | 2 | |
| Plasmapheresis | 1 | |||
| IVIG + plasmapheresis | 1 | |||
| corticosteroids + plasmapheresis | 1 |
Summary of therapies given to patients with YEL-AND per clinical presentation.
Discussion
The mechanisms of neurotropism of YFV vaccines are poorly understood. The incidence of YEL-AND was high until the seed-lot standardization process was implemented, which led to a uniform composition of the vaccine in 1945.10,40 This suggested that neurotropism of YF vaccine strains was caused by population variation between strains.10,40 In the 1930s, two live attenuated YF vaccines were developed, the French neurotropic vaccine (also known as ‘Mayali strain’) and the 17D vaccine strain. Multiple passages of both strains in the mouse brain resulted in lower viscerotropism and less systemic illness but in higher neurotropism.4 Administration of French neurotropic vaccine (FNV) was associated with encephalitis (3–4 per 1000 vaccines), that had a case fatality rate of 38% in Nigeria in 1950–1951.41 The 17D strain that resulted from extensive passaging of the Asibi strain (named after the African patient it was obtained from) in nervous tissue-deprived chicken embryos, eventually lost its neurovirulence by a chance mutation.4 The 17D vaccine genome was shown to be genetically highly stable, whereas the FNV strain genome frequently accumulated nucleotide substitutions mutations.42 The French strain never lost its neurotropism despite similar attempts.4 From 1983 onward, WHO prequalified 17D YF vaccine is produced by four manufacturers; 17DD is used in South America and 17D-204 elsewhere.1,40,43
Surveillance-based estimates of 17D YEL-AND incidence vary from 0.16 to 14.6 per million doses (mean 3.9 per million). These variations are attributable to different definitions used for diagnosing YEL-AND, heterogeneity of the participants and co-administration of other vaccines.5,7,9,11–15 YEL-AND is almost only observed following the primary vaccination.11
Serious adverse events (SAEs) like YEL-AND are more frequently seen in men, neonates (<6 months) and above 60 years of age.5,44,45 Male predominance was also clearly demonstrated in this review. Age is an important risk factor for developing SAE after YFV vaccination. Young infants (<6 months) have an immature blood–brain barrier that leads to a higher susceptibility to neuro-invasion and YEL-AND.46 Patients over 60 years old developed a slower antibody response and had higher viremia levels after primary vaccination with 17D YF vaccine, compared to younger vaccines. In addition, only half of them had antibodies ten days after immunization, compared to >75% in people under 60 years of age. This finding is likely to reflect the acquired impairment of innate and adaptive immune responses in elderly people.46,47 New UK Guidance states that vaccination for persons of 60 years or older is contraindicated when travelling to areas with low potential for YFV exposure.48,49
Immunodeficiency is another risk factor for SAE, although larger studies are required to establish the safety and efficacy of YF vaccination in immunosuppressed patients. Vaccination is contraindicated in immunodeficient patients and patients with thymus disorders that are associated with abnormal immune cell function.5,44,45 A recent prospective observational study by Bühler et al. compared the safety and immunogenicity of primary YF vaccination in patients on a low dose (≤20 mg/week) methotrexate and controls. The frequency of local and systemic reactions in 32 patients and controls was similar and no SAE occurred.50 However, because of the small sample size rare SAE such as YEL-AND could have been missed. In patients with methotrexate, it may take longer to develop a protective immune response after vaccination, but all participants had protective antibody titers 28 days post-vaccination.50 YF vaccination of patients with low-active autoimmune diseases was safe after the withdrawal of immunomodulating therapy, but it led to a lower seropositivity rate (78% versus 96%) at day 28 after vaccination.51
There is a paucity of data on the safety of YF vaccination in severely immunocompromised HIV-POS patients. Because of the single case of fatal encephalomyelitis after YF vaccination in a man with previously undiagnosed HIV,33 most guidelines recommend against YF vaccination in HIV-POS patients with CD4 counts < 200 cells/μl.52,53 Although YF vaccination generally induces lower neutralizing antibody titres that decline more rapidly than in HIV-NEG individuals, the long-term immune response to YFV (up to 10 years) in patients taking combination antiretroviral therapy and who had suppressed HIV viral loads at the time of vaccination, is comparable to HIV-NEG subjects.53,54
Vaccination of lactating and pregnant women requires special consideration. Vaccination of lactating women should be postponed for the duration of breastfeeding because of the risk of VT-YFV transmission via breast milk to their infants.1,28,29,52 However, when traveling to endemic YF areas cannot be avoided, these women should be vaccinated. Some experts have suggested to suspend breastfeeding for at least 2 weeks after YF vaccination.52 Observational studies in vaccinated pregnant women do not show safety issues. Mortality rates of YF infection among pregnant women are probably much higher than in a non-pregnant population. When travelling to a high-transmission country is unavoidable and the risk of YFV exposure is prolonged, the benefits outweigh the theoretical risks and vaccination should be considered.55 Checklists can assist physicians in evaluating individual travelers’ risks of YFV vaccination.48
Physicians should strongly suspect YEL-AND in patients who develop symptoms of ME, myelitis, GBS or ADEM after YFV vaccination. We found three reports of patients with onset of symptoms started at 45- and 50-days post-vaccination (two with myelitis and one with GBS). Therefore, the criterion of symptom onset within 30 days in CDC case definitions and WHO recommendations for YEL-AND should be re-evaluated.1,56
A standardized work-up (blood and urine examination, microbiological cultures and chest X-ray) should be performed to rule out other etiology. Lumbar puncture with WBC counts and chemistry of CSF is indicated. In most cases, patients had a pleiocytosis and/or elevated protein levels in liquor. PCR, antibodies and microbiological cultures in CSF must be performed to exclude other relevant causative agents. Serum antibodies against YFV are of limited use in diagnosing YEL-AND. Vaccine-induced seroconversion is expected and does not distinguish YFV-associated adverse events from vaccination. In addition, because of the well-known phenomenon of extensive cross-reactivity between antibodies against antigenically related flaviviruses (see Table 1), serologic diagnosis of YFV should be based on virus neutralization assays.57
In the US Vaccine Adverse Event Reporting System database 17 cases of YEL-AND were summarized, nine (=53%) of which had a POS YFV RT-PCR or IgM antibodies in liquor meeting the criteria for definite YEL-AND.5 To increase the diagnostic accuracy, a standardized work-up that includes both YFV-specific RT-PCR and IgM in CSF is mandatory. In endemic areas, distinguishing wild type YFV from 17D YF vaccine virus could be important to discriminate infection from adverse reactions to vaccination. Two real-time RT-PCR assays have been described for this purpose.58,59
Our search results indicate that MRI of the brain and spinal cord frequently detected abnormalities and that EEG-readings may support the diagnosis, although radiologic and clinical neurophysiological investigations findings are often non-specific for YEL-AND. Reporting on additional studies was frequently incomplete (see Table 2).
Most patients will recover with symptomatic treatment, which is the only treatment available at this point. In the analysis of 67 neurological cases in Brazil, 7.5% had sequelae after discharge.11 Lethal cases were very rare (<1.0%). Neuropsychiatric sequelae are thought to occur infrequently but systematic long-term follow-up studies are lacking.32,46 In our review, YEL-AND had a higher risk of neurologic sequelae when presenting with myelitis, ADEM or GBS. However, the lower proportion of patients with a favorable outcome (68.6%) may have been caused by incomplete reporting of follow-up. Some cases improved with the use of IVIG, corticosteroids and/or plasmapheresis. We would consider these therapies in patients with severe neurological deficits, neurological deterioration and patients presenting with myelitis, GBS or ADEM, but given the paucity of data, strong recommendations cannot be made.
This review is the first to summarize all case reports of YEL-AND indexed in MEDLINE to date and to analyze the clinical evaluation of individual patients. We used a clearly defined search strategy. However, cases may have been excluded from our analysis for lack of standardization in reporting YEL-AND. A particular strength of our review is the use of Brighton Collaboration diagnostic criteria to assess the diagnostic accuracy; this should enable future comparison of case reports of this rare SAE.
Conclusion
Our review aims to contribute to a better understanding of YEL-AND and to facilitate the diagnosis of this rare complication of YFV vaccination by clinicians. Our analysis indicates that YEL-AND occurred across all age groups but also that in reported cases with poor outcomes, the median age was higher than in cases with good outcomes (53 years versus 32.5 years, respectively). YF vaccine should not be administered to infants under 6 months of age, and caution should be exercised when vaccinating children between 6 and 9 months of age and with primovaccination of persons older than 60 years. Although we did not identify case reports involving vaccination of pregnant women or patients with known immunosuppression, the benefits and potential harm of YF immunization should be considered carefully in these groups, and vaccination of lactating women should be delayed because of the risk of transmission of VT-YFV to their infants.
We caution against the strict application of the YFVS time criterion as YEL-AND may occur later than 30 days after vaccination. YEL-AND frequently manifests as ME, but may also present as myelitis, GBS or ADEM. While YEL-AND ME has a good prognosis, the outcomes of GBS, ADEM and myelitis are less favorable.
When suspecting YEL-AND, we recommend a standardized diagnostic workup that should include lumbar puncture with a YFV-specific IgM detection assay and RT-PCR, as well as EEG and MRI of the central nervous system.
To ascertain diagnostic accuracy, we recommend using the Brighton Collaboration diagnostic criteria for reporting encephalitis, ADEM, GBS and myelitis. Standardized reporting should enable classification of YEL-AND cases as suspected, probable or definite. We also recommend documentation of the length of follow-up and standardization of reporting functional outcomes of YEL-AND (e.g. mRS score at 3 months). Central registration of complications of YF vaccination—(e.g. by European Medicines Agency in Europe)—is encouraged to permit better estimates of YEL-AND occurrence.60
Take Home Messages
(i) Clinical presentation, additional investigations, therapies and outcome measures should be reported in a standardized manner. (ii) The diagnostic workup for suspected YEL-AND should include lumbar puncture with both YFV-specific IgM antibody detection and RT-PCR in CSF, EEG and MRI. (iii) The time interval between vaccination and onset of YEL-AND symptoms can exceed 30 days. (iv) We suggest using the Brighton Collaboration criteria when reporting on YFV adverse events to ensure diagnostic accuracy. (v) Outcomes should be reported with mRS score at 3 months. (vi) Fatalities of YEL-AND are rare but cases presenting with GBS, ADEM and myelitis have poor outcomes compared to cases of ME. (vii) Treatment with IVIG, plasmapheresis and corticosteroids can be considered in patients with severe neurological deficits; however, the level of evidence for these therapeutic interventions in YEL-AND is low.
Consent to participate
Informed consent of patient was obtained according to the Declaration of Helsinki.
Consent for publication
All authors have read the final draft and agreed to the publication of the manuscript.
Sources of funding
None.
Author’s contributions
Lecomte, Elien: Conceptualization, Methodology, Formal Analysis, Investigation, Writing- Original Draft, Visualization. Laureys, Guy: Writing-Review and Editing. Verbeke, Frederick: Resources, Writing-Review and Editing. Domingo, Cristina: Resources, Writing-Review and Editing. Van Esbroeck, Marjan: Resources, Writing-Review and Editing. Huits, Ralph: Conceptualization, Methodology, Resources, Writing-Review and Editing, Supervision, Funding Acquisition.
Acknowledgements
We thank the patient who gave us permission to publish his case history. We also would like to thank the Robert Koch Institute in Berlin, Germany, and the Tropical Institute in Antwerp, Belgium, for the laboratory analysis of our patient’s samples.
Conflict of Interest: None declared.
References
Medicines and Healthcare products Regulatory Agency.



