-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel Bierbrier, Emilie Javelle, Francesca F Norman, Lin Hwei Chen, Emmanuel Bottieau, Eli Schwartz, Karin Leder, Kristina M Angelo, Rhett J Stoney, Michael Libman, Davidson H Hamer, Ralph Huits, Bradley A Connor, Fabrice Simon, Sapha Barkati, for the GeoSentinel Network, Chikungunya infection in returned travellers: results from the GeoSentinel network, 2005–2020, Journal of Travel Medicine, Volume 31, Issue 2, March 2024, taae005, https://doi.org/10.1093/jtm/taae005
Close - Share Icon Share
Abstract
Chikungunya is an important travel-related disease because of its rapid geographical expansion and potential for prolonged morbidity. Improved understanding of the epidemiology of travel-related chikungunya infections may influence prevention strategies including education and vaccination.
We analysed data from travellers with confirmed or probable chikungunya reported to GeoSentinel sites from 2005 to 2020. Confirmed chikungunya was defined as a compatible clinical history plus either virus isolation, positive nucleic acid test or seroconversion/rising titre in paired sera. Probable chikungunya was defined as a compatible clinical history with a single positive serology result.
1202 travellers (896 confirmed and 306 probable) with chikungunya were included. The median age was 43 years (range 0–91; interquartile range [IQR]: 31–55); 707 (58.8%) travellers were female. Most infections were acquired in the Caribbean (28.8%), Southeast Asia (22.8%), South Central Asia (14.2%) and South America (14.2%). The highest numbers of chikungunya cases reported to GeoSentinel were in 2014 (28.3%), 2015 (14.3%) and 2019 (11.9%). The most frequent reasons for travel were tourism (n = 592; 49.3%) and visiting friends or relatives (n = 334; 27.7%). The median time to presentation to a GeoSentinel site was 23 days (IQR: 7–52) after symptom onset. In travellers with confirmed chikungunya and no other reported illnesses, the most frequently reported symptoms included musculoskeletal symptoms (98.8%), fever/chills/sweats (68.7%) and dermatologic symptoms (35.5%). Among 917 travellers with information available, 296 (32.3%) had a pretravel consultation.
Chikungunya was acquired by international travellers in almost 100 destinations globally. Vector precautions and vaccination where recommended should be integrated into pretravel visits for travellers going to areas with chikungunya or areas with the potential for transmission. Continued surveillance of travel-related chikungunya may help public health officials and clinicians limit the transmission of this potentially debilitating disease by defining regions where protective measures (e.g. pretravel vaccination) should be strongly considered.
Introduction
Chikungunya is a vector-borne viral disease transmitted primarily by Aedes aegypti and Aedes albopictus mosquitoes.1Infection is clinically characterized by acute onset of fever, rash and joint pains with resolution in 1–3 weeks. However, in one-third of the patients, the arthralgias and arthritis may persist and cause prolonged morbidity for months to years.2 Rare and more severe manifestations include encephalitis, severe sepsis, and Guillain-Barré syndrome.3
Chikungunya virus (CHIKV) is an alphavirus first identified in Tanzania in 1953.4 Outbreaks were originally confined to Africa and Asia and most travel-associated cases were epidemiologically linked to these destinations.5 In 2004, the spread of a new CHIKV sublineage (Indian Ocean lineage [IOL]) in coastal Kenya led to the emergence of epidemics in previously unaffected countries in the Indian Ocean and India. While the IOL were spreading, the Asian lineage were also circulating in Indonesia and the South Pacific and moved to the Americas. Autochthonous transmission of CHIKV was first reported in the Americas in 2013 in the Caribbean, spreading through the continent, and travel-associated chikungunya reported from this area were frequent.6 The risk of contracting chikungunya infection is most significant for travellers heading to countries currently experiencing ongoing chikungunya outbreaks in the Americas, parts of Africa and Asia. Travel-associated outbreaks have also led to transmission in non-endemic parts of Europe (e.g. Italy in 2007 and 2017) and rarely in the United States, where competent vectors are present.2
Returning travellers diagnosed with chikungunya are important sentinels for the identification of outbreaks.7 Sentinel infections have been reported among travellers from Thailand, Maldives, Myanmar, Bali, Martinique and Guadeloupe in recent years.8–12
Chikungunya is an important travel-related disease because of its geographical expansion, potential for prolonged morbidity, and our limited understanding of factors associated with ongoing outbreaks. The geographical range of Aedes mosquitoes is continually expanding, in part as a result of climate changes.2 A recent study demonstrated that substantial changes detected in vectorial system lead to temperature-specific adjustments to achieve arboviral transmission; as a result, A. albopictus was able to transmit CHIKV as efficiently at 20 and 28°C.13
The epidemiology of chikungunya among international travellers can serve as a model for understanding the dynamics of newly emerging mosquito-borne diseases and their global dissemination. This analysis provides valuable insights into where CHIKV is acquired by international travellers, which may contribute to the implementation of prevention strategies, including education about personal protective measures and vaccination. The objective of this report is to describe the epidemiology of chikungunya in travellers reported to GeoSentinel from 2005 through 2020.
Methods
Data source: GeoSentinel global surveillance system
GeoSentinel, a global surveillance and research network founded in 1995 to monitor travel-related disease among international travellers and migrants, comprises 71 sites in 29 countries and is a collaboration between the Centers for Disease Control and Prevention (CDC) and the International Society of Travel (ISTM).14 Sites are experienced in evaluating and treating patients with travel-related infectious diseases and contribute anonymous clinician-based surveillance data on ill persons who recently crossed an international border. A confirmed diagnosis of chikungunya in GeoSentinel is defined as a compatible clinical history plus either virus isolation, positive nucleic acid test, or seroconversion/rising titre in paired sera. A probable diagnosis of chikungunya is defined as a compatible clinical history with a single positive serology result. GeoSentinel’s surveillance data collection protocol has been reviewed by a human subject advisor at CDC’s National Center for Emerging and Zoonotic Infectious Diseases and has been classified as public health surveillance and not human subjects research. Additional ethics clearance was obtained by GeoSentinel member sites as required by their local institutional review boards.
Data inclusion and exclusion
Travellers with a probable or confirmed diagnosis of chikungunya seen from 1 January 2005 to 31 December 2020 were eligible for inclusion in the analysis. Travellers before 2018 with more than one diagnosis were excluded because sites were unable to list which country of exposure and type of travel was related to each diagnosis. The region of exposure was categorized using an algorithm based on the travellers’ reported travel destinations and place of exposure as assigned by the treating clinician, when known, and was classified using modified UNICEF-defined regions.15 Regions included Caribbean, Central America, Oceania, South America, South Central Asia, Southeast Asia and sub-Saharan Africa. Travellers without a region of exposure were removed, as in the authors’ experience, which indicates misclassification within the database.
Data management and analysis
Data were extracted from the GeoSentinel database on traveller demographics (e.g. sex, age, resident country), travel details (e.g. travel dates and duration, reason for travel, country/region of exposure, pretravel consultation) and clinical information (e.g. date of illness onset, presenting symptoms, date of presentation to GeoSentinel site, confirmed or probable chikungunya classification, hospitalization). Data on traveller symptoms was recorded starting in January 2008 and data on diagnostic testing performed began in October 2015.
In April 2013, GeoSentinel started recording the date of illness onset. Travellers were grouped into the following categories reflecting the time elapsed between illness onset and GeoSentinel site visit date: ≤ 21, 21–90 and > 90 days.
All analyses were descriptive. Frequencies were performed using Microsoft Excel for Mac version 16.7.
Results
A total of 1202 travellers with chikungunya (74.5% confirmed, 25.5% probable) were included (Table 1). The most frequent countries of residence were France (14.8%), the Netherlands (13.7%), Germany (13.6%) and Canada (10.9%).
Characteristics of travellers with chikungunya reported to GeoSentinel from 2005–2020 (N = 1202)
| Characteristic . | Number . | % . |
|---|---|---|
| Chikungunya case definition (n = 1202) | ||
| Probable | 306 | 25.5 |
| Confirmed | 896 | 74.5 |
| Age (n = 1200) | ||
| Median age in years (interquartile range) | 43 (31–55) | |
| <18 years | 29 | 2.4 |
| 18–39 | 493 | 41.0 |
| 40–65 | 576 | 48.0 |
| >65 | 102 | 8.5 |
| Sex (n = 1202) | ||
| Female | 707 | 58.8 |
| Male | 495 | 41.2 |
| Clinical setting (n = 1198) | ||
| Seen after travel | 1101 | 91.9 |
| Seen during travel | 94 | 7.9 |
| Migration travel | 3 | 0.3 |
| Travel reason (n = 1202) | ||
| Tourism (vacation) | 597 | 49.7 |
| Visiting friends or relatives (VFR) | 329 | 27.4 |
| Business | 172 | 14.3 |
| Missionary/volunteer/researcher/aid work | 74 | 6.2 |
| Education | 9 | 0.7 |
| Migration | 8 | 0.7 |
| Military | 7 | 0.6 |
| Planned medical care | 4 | 0.3 |
| Conference | 2 | 0.2 |
| Pretravel consultation (n = 917) | ||
| Yes | 296 | 32.3 |
| No | 621 | 67.7 |
| Reported symptoms (n = 840) | ||
| Rheumatic and musculoskeletal (including arthralgia, myalgia, arthritis) | 830 | 98.8 |
| Constitutional symptoms (fever/sweats/chills) | 577 | 68.7 |
| Dermatological (including diffuse or focal rash, itch, lesion) | 298 | 35.5 |
| Other1 | 525 | 62.5 |
| Number of recorded symptoms per traveller (n = 840) | ||
| 1 | 191 | 22.7 |
| 2 | 227 | 27.0 |
| 3 | 227 | 27.0 |
| 4 | 111 | 13.2 |
| 5+ | 84 | 10.0 |
| Characteristic . | Number . | % . |
|---|---|---|
| Chikungunya case definition (n = 1202) | ||
| Probable | 306 | 25.5 |
| Confirmed | 896 | 74.5 |
| Age (n = 1200) | ||
| Median age in years (interquartile range) | 43 (31–55) | |
| <18 years | 29 | 2.4 |
| 18–39 | 493 | 41.0 |
| 40–65 | 576 | 48.0 |
| >65 | 102 | 8.5 |
| Sex (n = 1202) | ||
| Female | 707 | 58.8 |
| Male | 495 | 41.2 |
| Clinical setting (n = 1198) | ||
| Seen after travel | 1101 | 91.9 |
| Seen during travel | 94 | 7.9 |
| Migration travel | 3 | 0.3 |
| Travel reason (n = 1202) | ||
| Tourism (vacation) | 597 | 49.7 |
| Visiting friends or relatives (VFR) | 329 | 27.4 |
| Business | 172 | 14.3 |
| Missionary/volunteer/researcher/aid work | 74 | 6.2 |
| Education | 9 | 0.7 |
| Migration | 8 | 0.7 |
| Military | 7 | 0.6 |
| Planned medical care | 4 | 0.3 |
| Conference | 2 | 0.2 |
| Pretravel consultation (n = 917) | ||
| Yes | 296 | 32.3 |
| No | 621 | 67.7 |
| Reported symptoms (n = 840) | ||
| Rheumatic and musculoskeletal (including arthralgia, myalgia, arthritis) | 830 | 98.8 |
| Constitutional symptoms (fever/sweats/chills) | 577 | 68.7 |
| Dermatological (including diffuse or focal rash, itch, lesion) | 298 | 35.5 |
| Other1 | 525 | 62.5 |
| Number of recorded symptoms per traveller (n = 840) | ||
| 1 | 191 | 22.7 |
| 2 | 227 | 27.0 |
| 3 | 227 | 27.0 |
| 4 | 111 | 13.2 |
| 5+ | 84 | 10.0 |
1Includes: Fatigue, headache, gastrointestinal symptoms (including nausea, vomiting, abdominal pain, acute and chronic diarrhoea, anorexia), neurologic symptoms (including psychologic symptoms, neck stiffness, dizziness), cardiac symptoms, respiratory symptoms, ocular symptoms and throat or nose symptoms.
Characteristics of travellers with chikungunya reported to GeoSentinel from 2005–2020 (N = 1202)
| Characteristic . | Number . | % . |
|---|---|---|
| Chikungunya case definition (n = 1202) | ||
| Probable | 306 | 25.5 |
| Confirmed | 896 | 74.5 |
| Age (n = 1200) | ||
| Median age in years (interquartile range) | 43 (31–55) | |
| <18 years | 29 | 2.4 |
| 18–39 | 493 | 41.0 |
| 40–65 | 576 | 48.0 |
| >65 | 102 | 8.5 |
| Sex (n = 1202) | ||
| Female | 707 | 58.8 |
| Male | 495 | 41.2 |
| Clinical setting (n = 1198) | ||
| Seen after travel | 1101 | 91.9 |
| Seen during travel | 94 | 7.9 |
| Migration travel | 3 | 0.3 |
| Travel reason (n = 1202) | ||
| Tourism (vacation) | 597 | 49.7 |
| Visiting friends or relatives (VFR) | 329 | 27.4 |
| Business | 172 | 14.3 |
| Missionary/volunteer/researcher/aid work | 74 | 6.2 |
| Education | 9 | 0.7 |
| Migration | 8 | 0.7 |
| Military | 7 | 0.6 |
| Planned medical care | 4 | 0.3 |
| Conference | 2 | 0.2 |
| Pretravel consultation (n = 917) | ||
| Yes | 296 | 32.3 |
| No | 621 | 67.7 |
| Reported symptoms (n = 840) | ||
| Rheumatic and musculoskeletal (including arthralgia, myalgia, arthritis) | 830 | 98.8 |
| Constitutional symptoms (fever/sweats/chills) | 577 | 68.7 |
| Dermatological (including diffuse or focal rash, itch, lesion) | 298 | 35.5 |
| Other1 | 525 | 62.5 |
| Number of recorded symptoms per traveller (n = 840) | ||
| 1 | 191 | 22.7 |
| 2 | 227 | 27.0 |
| 3 | 227 | 27.0 |
| 4 | 111 | 13.2 |
| 5+ | 84 | 10.0 |
| Characteristic . | Number . | % . |
|---|---|---|
| Chikungunya case definition (n = 1202) | ||
| Probable | 306 | 25.5 |
| Confirmed | 896 | 74.5 |
| Age (n = 1200) | ||
| Median age in years (interquartile range) | 43 (31–55) | |
| <18 years | 29 | 2.4 |
| 18–39 | 493 | 41.0 |
| 40–65 | 576 | 48.0 |
| >65 | 102 | 8.5 |
| Sex (n = 1202) | ||
| Female | 707 | 58.8 |
| Male | 495 | 41.2 |
| Clinical setting (n = 1198) | ||
| Seen after travel | 1101 | 91.9 |
| Seen during travel | 94 | 7.9 |
| Migration travel | 3 | 0.3 |
| Travel reason (n = 1202) | ||
| Tourism (vacation) | 597 | 49.7 |
| Visiting friends or relatives (VFR) | 329 | 27.4 |
| Business | 172 | 14.3 |
| Missionary/volunteer/researcher/aid work | 74 | 6.2 |
| Education | 9 | 0.7 |
| Migration | 8 | 0.7 |
| Military | 7 | 0.6 |
| Planned medical care | 4 | 0.3 |
| Conference | 2 | 0.2 |
| Pretravel consultation (n = 917) | ||
| Yes | 296 | 32.3 |
| No | 621 | 67.7 |
| Reported symptoms (n = 840) | ||
| Rheumatic and musculoskeletal (including arthralgia, myalgia, arthritis) | 830 | 98.8 |
| Constitutional symptoms (fever/sweats/chills) | 577 | 68.7 |
| Dermatological (including diffuse or focal rash, itch, lesion) | 298 | 35.5 |
| Other1 | 525 | 62.5 |
| Number of recorded symptoms per traveller (n = 840) | ||
| 1 | 191 | 22.7 |
| 2 | 227 | 27.0 |
| 3 | 227 | 27.0 |
| 4 | 111 | 13.2 |
| 5+ | 84 | 10.0 |
1Includes: Fatigue, headache, gastrointestinal symptoms (including nausea, vomiting, abdominal pain, acute and chronic diarrhoea, anorexia), neurologic symptoms (including psychologic symptoms, neck stiffness, dizziness), cardiac symptoms, respiratory symptoms, ocular symptoms and throat or nose symptoms.
The two most common regions of exposure were the Caribbean (n = 347, 28.9%) and South-East Asia (n = 274, 22.8%) (Figure 1). The most frequent countries of exposure are described in Table 2. Among 917 travellers with information available, approximately one-third (n = 296, 32.3%) obtained a pretravel consultation prior to departure. Among 891 travellers who went to a single destination, the median duration of travel was 23 days (IQR 15–43).
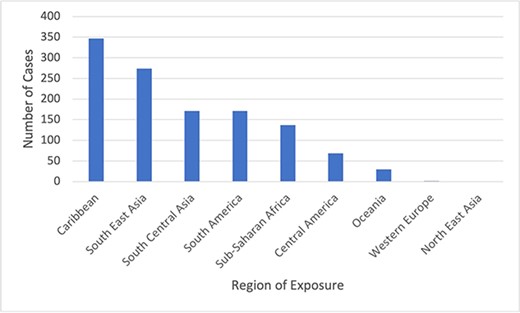
Regions of chikungunya exposure among travellers reported to GeoSentinel, 2005–2020 (N = 1202)
Countries of chikungunya exposure among travellers reported to GeoSentinel, 2005–2020 (N = 1202)
| Country . | Number (%) . |
|---|---|
| India | 130 (10.8) |
| Dominican Republic | 89 (7.4) |
| Thailand | 70 (5.8) |
| Indonesia | 57 (4.7) |
| Martinique | 56 (4.7) |
| Singapore | 56 (4.7) |
| Suriname | 50 (4.2) |
| Netherlands Antilles | 41 (3.4) |
| Brazil | 40 (3.3) |
| Guadeloupe | 40 (3.3) |
| Colombia | 36 (3.0) |
| Haiti | 35 (2.9) |
| Myanmar | 31 (2.5) |
| Jamaica | 27 (2.3) |
| Nicaragua | 23 (1.9) |
| Reunion | 20 (1.9) |
| French Polynesia | 18 (1.7) |
| Philippines | 16 (1.3) |
| Maldives | 15 (1.3) |
| Puerto Rico | 15 (1.3) |
| Costa Rica | 13 (1.1) |
| Kenya | 12 (1.0) |
| Mauritius | 12 (1.0) |
| Mexico | 12 (1.0) |
| Sri Lanka | 12 (1.0) |
| Democratic Republic of the Congo | 11 (0.9) |
| Honduras | 11 (0.9) |
| Venezuela | 10 (0.8) |
| Cuba | 10 (0.8) |
| Ecuador | 9 (0.8) |
| Madagascar | 9 (0.8) |
| Bolivia | 9 (0.8) |
| Tonga | 8 (0.7) |
| Barbados | 8 (0.7) |
| French Guiana | 7 (0.6) |
| Guatemala | 7 (0.6) |
| Trinidad and Tobago | 7 (0.6) |
| Equatorial Guinea | 6 (0.5) |
| Saint Martin | 6 (0.5) |
| Angola | 6 (0.5) |
| Cambodia | 5 (0.4) |
| Republic of Congo | 5 (0.4) |
| Djibouti | 5 (0.4) |
| El Salvador | 5 (0.4) |
| Somalia | 5 (0.4) |
| Bangladesh | 5 (0.4) |
| Guyana | 4 (0.3) |
| Saint Vincent and The Grenadines | 4 (0.3) |
| Senegal | 4 (0.3) |
| United Republic of Tanzania | 4 (0.3) |
| Timor-Leste | 4 (0.3) |
| Benin | 4 (0.3) |
| Cameroon | 3 (0.3) |
| Eritrea | 3 (0.3) |
| Ethiopia | 3 (0.3) |
| Gabon | 3 (0.3) |
| Ghana | 3 (0.3) |
| Nepal | 3 (0.3) |
| Nigeria | 3 (0.3) |
| Pakistan | 3 (0.3) |
| Panama | 3 (0.3) |
| Saint Lucia | 3 (0.3) |
| American Samoa | 3 (0.3) |
| Aruba | 2 (0.2) |
| Comoros | 2 (0.2) |
| Dominica | 2 (0.2) |
| Seychelles | 2 (0.2) |
| South Africa | 2 (0.2) |
| Viet Nam | 2 (0.2) |
| The Bahamas | 2 (0.2) |
| Burundi | 1 (0.1) |
| Central African Republic | 1 (0.1) |
| Fiji | 1 (0.1) |
| France | 1 (0.1) |
| Grenada | 1 (0.1) |
| Guinea | 1 (0.1) |
| Kiribati | 1 (0.1) |
| Laos | 1 (0.1) |
| Mali | 1 (0.1) |
| Mozambique | 1 (0.1) |
| Namibia | 1 (0.1) |
| Paraguay | 1 (0.1) |
| Peru | 1 (0.1) |
| Portugal | 1 (0.1) |
| Rwanda | 1 (0.1) |
| Saint Kitts and Nevis | 1 (0.1) |
| Samoa | 1 (0.1) |
| South Sudan | 1 (0.1) |
| Taiwan Province, People’s Republic of China | 1 (0.1) |
| Uganda | 1 (0.1) |
| Uruguay | 1 (0.1) |
| Vanuatu | 1 (0.1) |
| Virgin Islands, U.S. | 1 (0.1) |
| Cote d’Ivoire | 1 (0.1) |
| Missing | 27 (2.2) |
| Country . | Number (%) . |
|---|---|
| India | 130 (10.8) |
| Dominican Republic | 89 (7.4) |
| Thailand | 70 (5.8) |
| Indonesia | 57 (4.7) |
| Martinique | 56 (4.7) |
| Singapore | 56 (4.7) |
| Suriname | 50 (4.2) |
| Netherlands Antilles | 41 (3.4) |
| Brazil | 40 (3.3) |
| Guadeloupe | 40 (3.3) |
| Colombia | 36 (3.0) |
| Haiti | 35 (2.9) |
| Myanmar | 31 (2.5) |
| Jamaica | 27 (2.3) |
| Nicaragua | 23 (1.9) |
| Reunion | 20 (1.9) |
| French Polynesia | 18 (1.7) |
| Philippines | 16 (1.3) |
| Maldives | 15 (1.3) |
| Puerto Rico | 15 (1.3) |
| Costa Rica | 13 (1.1) |
| Kenya | 12 (1.0) |
| Mauritius | 12 (1.0) |
| Mexico | 12 (1.0) |
| Sri Lanka | 12 (1.0) |
| Democratic Republic of the Congo | 11 (0.9) |
| Honduras | 11 (0.9) |
| Venezuela | 10 (0.8) |
| Cuba | 10 (0.8) |
| Ecuador | 9 (0.8) |
| Madagascar | 9 (0.8) |
| Bolivia | 9 (0.8) |
| Tonga | 8 (0.7) |
| Barbados | 8 (0.7) |
| French Guiana | 7 (0.6) |
| Guatemala | 7 (0.6) |
| Trinidad and Tobago | 7 (0.6) |
| Equatorial Guinea | 6 (0.5) |
| Saint Martin | 6 (0.5) |
| Angola | 6 (0.5) |
| Cambodia | 5 (0.4) |
| Republic of Congo | 5 (0.4) |
| Djibouti | 5 (0.4) |
| El Salvador | 5 (0.4) |
| Somalia | 5 (0.4) |
| Bangladesh | 5 (0.4) |
| Guyana | 4 (0.3) |
| Saint Vincent and The Grenadines | 4 (0.3) |
| Senegal | 4 (0.3) |
| United Republic of Tanzania | 4 (0.3) |
| Timor-Leste | 4 (0.3) |
| Benin | 4 (0.3) |
| Cameroon | 3 (0.3) |
| Eritrea | 3 (0.3) |
| Ethiopia | 3 (0.3) |
| Gabon | 3 (0.3) |
| Ghana | 3 (0.3) |
| Nepal | 3 (0.3) |
| Nigeria | 3 (0.3) |
| Pakistan | 3 (0.3) |
| Panama | 3 (0.3) |
| Saint Lucia | 3 (0.3) |
| American Samoa | 3 (0.3) |
| Aruba | 2 (0.2) |
| Comoros | 2 (0.2) |
| Dominica | 2 (0.2) |
| Seychelles | 2 (0.2) |
| South Africa | 2 (0.2) |
| Viet Nam | 2 (0.2) |
| The Bahamas | 2 (0.2) |
| Burundi | 1 (0.1) |
| Central African Republic | 1 (0.1) |
| Fiji | 1 (0.1) |
| France | 1 (0.1) |
| Grenada | 1 (0.1) |
| Guinea | 1 (0.1) |
| Kiribati | 1 (0.1) |
| Laos | 1 (0.1) |
| Mali | 1 (0.1) |
| Mozambique | 1 (0.1) |
| Namibia | 1 (0.1) |
| Paraguay | 1 (0.1) |
| Peru | 1 (0.1) |
| Portugal | 1 (0.1) |
| Rwanda | 1 (0.1) |
| Saint Kitts and Nevis | 1 (0.1) |
| Samoa | 1 (0.1) |
| South Sudan | 1 (0.1) |
| Taiwan Province, People’s Republic of China | 1 (0.1) |
| Uganda | 1 (0.1) |
| Uruguay | 1 (0.1) |
| Vanuatu | 1 (0.1) |
| Virgin Islands, U.S. | 1 (0.1) |
| Cote d’Ivoire | 1 (0.1) |
| Missing | 27 (2.2) |
Countries of chikungunya exposure among travellers reported to GeoSentinel, 2005–2020 (N = 1202)
| Country . | Number (%) . |
|---|---|
| India | 130 (10.8) |
| Dominican Republic | 89 (7.4) |
| Thailand | 70 (5.8) |
| Indonesia | 57 (4.7) |
| Martinique | 56 (4.7) |
| Singapore | 56 (4.7) |
| Suriname | 50 (4.2) |
| Netherlands Antilles | 41 (3.4) |
| Brazil | 40 (3.3) |
| Guadeloupe | 40 (3.3) |
| Colombia | 36 (3.0) |
| Haiti | 35 (2.9) |
| Myanmar | 31 (2.5) |
| Jamaica | 27 (2.3) |
| Nicaragua | 23 (1.9) |
| Reunion | 20 (1.9) |
| French Polynesia | 18 (1.7) |
| Philippines | 16 (1.3) |
| Maldives | 15 (1.3) |
| Puerto Rico | 15 (1.3) |
| Costa Rica | 13 (1.1) |
| Kenya | 12 (1.0) |
| Mauritius | 12 (1.0) |
| Mexico | 12 (1.0) |
| Sri Lanka | 12 (1.0) |
| Democratic Republic of the Congo | 11 (0.9) |
| Honduras | 11 (0.9) |
| Venezuela | 10 (0.8) |
| Cuba | 10 (0.8) |
| Ecuador | 9 (0.8) |
| Madagascar | 9 (0.8) |
| Bolivia | 9 (0.8) |
| Tonga | 8 (0.7) |
| Barbados | 8 (0.7) |
| French Guiana | 7 (0.6) |
| Guatemala | 7 (0.6) |
| Trinidad and Tobago | 7 (0.6) |
| Equatorial Guinea | 6 (0.5) |
| Saint Martin | 6 (0.5) |
| Angola | 6 (0.5) |
| Cambodia | 5 (0.4) |
| Republic of Congo | 5 (0.4) |
| Djibouti | 5 (0.4) |
| El Salvador | 5 (0.4) |
| Somalia | 5 (0.4) |
| Bangladesh | 5 (0.4) |
| Guyana | 4 (0.3) |
| Saint Vincent and The Grenadines | 4 (0.3) |
| Senegal | 4 (0.3) |
| United Republic of Tanzania | 4 (0.3) |
| Timor-Leste | 4 (0.3) |
| Benin | 4 (0.3) |
| Cameroon | 3 (0.3) |
| Eritrea | 3 (0.3) |
| Ethiopia | 3 (0.3) |
| Gabon | 3 (0.3) |
| Ghana | 3 (0.3) |
| Nepal | 3 (0.3) |
| Nigeria | 3 (0.3) |
| Pakistan | 3 (0.3) |
| Panama | 3 (0.3) |
| Saint Lucia | 3 (0.3) |
| American Samoa | 3 (0.3) |
| Aruba | 2 (0.2) |
| Comoros | 2 (0.2) |
| Dominica | 2 (0.2) |
| Seychelles | 2 (0.2) |
| South Africa | 2 (0.2) |
| Viet Nam | 2 (0.2) |
| The Bahamas | 2 (0.2) |
| Burundi | 1 (0.1) |
| Central African Republic | 1 (0.1) |
| Fiji | 1 (0.1) |
| France | 1 (0.1) |
| Grenada | 1 (0.1) |
| Guinea | 1 (0.1) |
| Kiribati | 1 (0.1) |
| Laos | 1 (0.1) |
| Mali | 1 (0.1) |
| Mozambique | 1 (0.1) |
| Namibia | 1 (0.1) |
| Paraguay | 1 (0.1) |
| Peru | 1 (0.1) |
| Portugal | 1 (0.1) |
| Rwanda | 1 (0.1) |
| Saint Kitts and Nevis | 1 (0.1) |
| Samoa | 1 (0.1) |
| South Sudan | 1 (0.1) |
| Taiwan Province, People’s Republic of China | 1 (0.1) |
| Uganda | 1 (0.1) |
| Uruguay | 1 (0.1) |
| Vanuatu | 1 (0.1) |
| Virgin Islands, U.S. | 1 (0.1) |
| Cote d’Ivoire | 1 (0.1) |
| Missing | 27 (2.2) |
| Country . | Number (%) . |
|---|---|
| India | 130 (10.8) |
| Dominican Republic | 89 (7.4) |
| Thailand | 70 (5.8) |
| Indonesia | 57 (4.7) |
| Martinique | 56 (4.7) |
| Singapore | 56 (4.7) |
| Suriname | 50 (4.2) |
| Netherlands Antilles | 41 (3.4) |
| Brazil | 40 (3.3) |
| Guadeloupe | 40 (3.3) |
| Colombia | 36 (3.0) |
| Haiti | 35 (2.9) |
| Myanmar | 31 (2.5) |
| Jamaica | 27 (2.3) |
| Nicaragua | 23 (1.9) |
| Reunion | 20 (1.9) |
| French Polynesia | 18 (1.7) |
| Philippines | 16 (1.3) |
| Maldives | 15 (1.3) |
| Puerto Rico | 15 (1.3) |
| Costa Rica | 13 (1.1) |
| Kenya | 12 (1.0) |
| Mauritius | 12 (1.0) |
| Mexico | 12 (1.0) |
| Sri Lanka | 12 (1.0) |
| Democratic Republic of the Congo | 11 (0.9) |
| Honduras | 11 (0.9) |
| Venezuela | 10 (0.8) |
| Cuba | 10 (0.8) |
| Ecuador | 9 (0.8) |
| Madagascar | 9 (0.8) |
| Bolivia | 9 (0.8) |
| Tonga | 8 (0.7) |
| Barbados | 8 (0.7) |
| French Guiana | 7 (0.6) |
| Guatemala | 7 (0.6) |
| Trinidad and Tobago | 7 (0.6) |
| Equatorial Guinea | 6 (0.5) |
| Saint Martin | 6 (0.5) |
| Angola | 6 (0.5) |
| Cambodia | 5 (0.4) |
| Republic of Congo | 5 (0.4) |
| Djibouti | 5 (0.4) |
| El Salvador | 5 (0.4) |
| Somalia | 5 (0.4) |
| Bangladesh | 5 (0.4) |
| Guyana | 4 (0.3) |
| Saint Vincent and The Grenadines | 4 (0.3) |
| Senegal | 4 (0.3) |
| United Republic of Tanzania | 4 (0.3) |
| Timor-Leste | 4 (0.3) |
| Benin | 4 (0.3) |
| Cameroon | 3 (0.3) |
| Eritrea | 3 (0.3) |
| Ethiopia | 3 (0.3) |
| Gabon | 3 (0.3) |
| Ghana | 3 (0.3) |
| Nepal | 3 (0.3) |
| Nigeria | 3 (0.3) |
| Pakistan | 3 (0.3) |
| Panama | 3 (0.3) |
| Saint Lucia | 3 (0.3) |
| American Samoa | 3 (0.3) |
| Aruba | 2 (0.2) |
| Comoros | 2 (0.2) |
| Dominica | 2 (0.2) |
| Seychelles | 2 (0.2) |
| South Africa | 2 (0.2) |
| Viet Nam | 2 (0.2) |
| The Bahamas | 2 (0.2) |
| Burundi | 1 (0.1) |
| Central African Republic | 1 (0.1) |
| Fiji | 1 (0.1) |
| France | 1 (0.1) |
| Grenada | 1 (0.1) |
| Guinea | 1 (0.1) |
| Kiribati | 1 (0.1) |
| Laos | 1 (0.1) |
| Mali | 1 (0.1) |
| Mozambique | 1 (0.1) |
| Namibia | 1 (0.1) |
| Paraguay | 1 (0.1) |
| Peru | 1 (0.1) |
| Portugal | 1 (0.1) |
| Rwanda | 1 (0.1) |
| Saint Kitts and Nevis | 1 (0.1) |
| Samoa | 1 (0.1) |
| South Sudan | 1 (0.1) |
| Taiwan Province, People’s Republic of China | 1 (0.1) |
| Uganda | 1 (0.1) |
| Uruguay | 1 (0.1) |
| Vanuatu | 1 (0.1) |
| Virgin Islands, U.S. | 1 (0.1) |
| Cote d’Ivoire | 1 (0.1) |
| Missing | 27 (2.2) |
Data on chikungunya-associated sign and symptoms were available for 840 travellers with confirmed chikungunya and no other concurrent diagnosis and are described in Table 1. The most common recorded signs and symptoms are rheumatic and musculoskeletal (n = 830, 98.8%).
For 851 travellers with date of illness onset available, the median time to presentation was 23 days (IQR: 7–52). Of these, 390 travellers (45.8%) presented to a GeoSentinel site < 21 days after illness onset, 352 (41.4%) presented 21–90 days after illness onset, and 109 (12.8%) presented > 90 days after illness onset. Among 1198 travellers with information available, 94 (7.8%) were seen at a GeoSentinel site during travel. Of 862 travellers with available data, 773 (89.7%) were managed as outpatients and 89 (10.3%) were hospitalized. There were no reported deaths.
Years with the highest numbers of reported chikungunya cases were 2014 (28.9%), 2015 (14.3%) and 2019 (11.9%). In 2014 and 2015, the most common regions of travel were the Caribbean (59.7%) and South America (19.4%) (Figure 2). In 2019, the most common regions of travel were Southeast Asia (n = 72, 50.4%), sub-Saharan Africa (n = 27, 18.9%) and South Central Asia (n = 23, 16.8%).
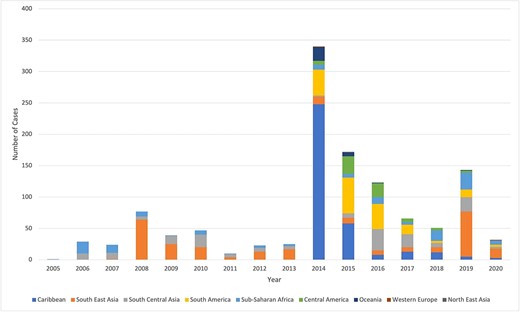
Annual number of chikungunya infections in travellers reported to GeoSentinel by region, 2005–2020 (N = 1202)
Diagnostic tests performed (n = 206) among 195 patients with confirmed chikungunya are in Table 3. Nucleic acid amplification testing was most frequent (n = 79; 38.4%).
Diagnostic tests performed among travellers with confirmed chikungunya, Geosentinel, 2015–2022 (n = 206 tests among 195 travellers with information available)*
| Diagnostic test . | n . | % . |
|---|---|---|
| Nucleic acid amplification test (e.g. RT-PCR, LAMP) | 79 | 38.4 |
| Paired serology: seroconversion/≥4-fold rise in titre | 65 | 31.6 |
| Antibody (serology)1 | 52 | 25.2 |
| Antigen | 9 | 4.4 |
| Culture | 1 | 0.5 |
| Diagnostic test . | n . | % . |
|---|---|---|
| Nucleic acid amplification test (e.g. RT-PCR, LAMP) | 79 | 38.4 |
| Paired serology: seroconversion/≥4-fold rise in titre | 65 | 31.6 |
| Antibody (serology)1 | 52 | 25.2 |
| Antigen | 9 | 4.4 |
| Culture | 1 | 0.5 |
RT-PCR: reverse transcriptase polymerse chain reaction
LAMP: loop-mediated isothermal amplification
1Cases from 2015 through 2019 had only this option available to document antibody testing; in 2019 this option was changed to ‘paired serology: seroconversion/≥4-fold rise in titre.’
Diagnostic tests performed among travellers with confirmed chikungunya, Geosentinel, 2015–2022 (n = 206 tests among 195 travellers with information available)*
| Diagnostic test . | n . | % . |
|---|---|---|
| Nucleic acid amplification test (e.g. RT-PCR, LAMP) | 79 | 38.4 |
| Paired serology: seroconversion/≥4-fold rise in titre | 65 | 31.6 |
| Antibody (serology)1 | 52 | 25.2 |
| Antigen | 9 | 4.4 |
| Culture | 1 | 0.5 |
| Diagnostic test . | n . | % . |
|---|---|---|
| Nucleic acid amplification test (e.g. RT-PCR, LAMP) | 79 | 38.4 |
| Paired serology: seroconversion/≥4-fold rise in titre | 65 | 31.6 |
| Antibody (serology)1 | 52 | 25.2 |
| Antigen | 9 | 4.4 |
| Culture | 1 | 0.5 |
RT-PCR: reverse transcriptase polymerse chain reaction
LAMP: loop-mediated isothermal amplification
1Cases from 2015 through 2019 had only this option available to document antibody testing; in 2019 this option was changed to ‘paired serology: seroconversion/≥4-fold rise in titre.’
Discussion
We describe the epidemiology of chikungunya in travellers presenting to GeoSentinel sites over a 15-year period. These data represent one of the largest collections of travel-associated chikungunya infections published to date. More than half of the travellers sought medical attention after 21 days from the onset of symptoms, indicating our ability to identify post-acute and chronic cases and emphasizing the importance of monitoring post-acute symptoms. Furthermore, this accounts for the lower proportion of dermatological issues and the significant prevalence of rheumatic and musculoskeletal presentations.
Our data captures the documented chikungunya outbreak in 2013–2014 in the Caribbean, which was the most frequently reported region of acquisition overall, with the number of cases reported to GeoSentinel surging from the Caribbean in 2014.16 Vasquez and colleagues established a temporal connection between the emergence of newly diagnosed imported cases of chikungunya and the outbreaks that occurred in Martinique and Guadeloupe in 2013–2014, highlighting the role of travellers as epidemiological sentinels.12 The first case of chikungunya in the Caribbean documented by GeoSentinel was in January 2014; the traveller had a reported illness onset date of November 2013 and acquired their illness in Martinique. This timing aligns with the first documented chikungunya cases in residents of Saint Martin, which were in the same geographic region (Caribbean).17 Phylogenetic analysis of CHIKV strains from another study linked the Caribbean CHIKV strains to the Asian genotype, which had been circulating in Southeast Asia and was introduced into the Western Hemisphere via the Western and South Pacific.18 The outbreak of Asian genotype CHIKV in the Caribbean was linked to human travel rather than re-emergence of a previously unrecognized endemic strain.19,20 Data have since demonstrated that the source of chikungunya in Aruba during the outbreak was from two distinct lineages, rather than a single source.21 The emergence of chikungunya in the Americas remains a public health concern due to the millions of travellers (predominantly tourists) visiting this popular vacation destination annually.22
In 2019, there was another peak in chikungunya among travellers reported to GeoSentinel returning predominantly from Southeast and Central Asia, most frequently to Thailand and Myanmar; this peak in 2019 corresponds to the outbreak of chikungunya in Thailand, Myanmar and Maldives during the same time period.11,23,24 This further reinforces the importance of travellers in the identification and monitoring of chikungunya outbreaks. 25
The number of travel-associated chikungunya cases reported by both the CDC and European Centre for Disease Prevention and Control (ECDC) has significantly decreased since 2019.26,27 This decline can be primarily attributed to the reduction in travel resulting from the COVID-19 pandemic-related restrictions. Nonetheless, this decline in cases occurs within the context of diminishing major outbreaks, which is probably attributed to a level of herd immunity that restricts the occurrence of significant epidemics until a new birth cohort provide additional amplifying host. 28
In the early 2000s, chikungunya was not as well known to clinicians in non-endemic areas and CHIKV diagnostic tests were less readily available. We did not identify a surge in cases during the 2006 Indian Ocean outbreak, likely a result of under-reporting and misdiagnosis of chikungunya cases early in the outbreak from a lack of readily available diagnostics; fewer sites were part of GeoSentinel in earlier years. Furthermore, it’s conceivable that the island of Réunion, Seychelles, Mauritius and Madagascar may not have been as frequently visited by travellers. Now, with increased availability of CHIKV diagnostic testing, chikungunya must remain on the differential diagnosis of a traveller returning from an endemic area with rheumatic and musculoskeletal, constitutional, or dermatologic symptoms.
In our analysis, only one-third of travellers for whom there were data received pretravel advice. This is likely due to the large number of tourist travellers to the Caribbean, which may not be considered a region with many infectious disease threats. However, CDC recommends pretravel consultations for all travellers regardless of destination. 29 In a recent European study investigating the etiologies of fever in returning travellers, arboviruses emerged as the leading causes of acute undifferentiated febrile illnesses. 30 Vector precautions should be integrated into pretravel visits for travellers going to areas with mosquito-borne (or other arthropod-borne) diseases or areas with the potential for transmission. Travellers should be counselled on the use of physical barriers to prevent mosquitoes from entering the environment (e.g. screens, mosquito nets) and personal protective measures (e.g. permethrin-impregnated clothing, insect repellent). 2,16
Providing chikungunya prevention recommendations to travellers is important because although chikungunya is usually not fatal, there may be significant associated morbidity; rheumatologic symptoms of chikungunya may persist for months to years following acute infection. 2 In this analysis, nearly 13% of travellers presented > 90 days after illness symptom onset, which may be a reflection of the longer term morbidity of the disease. A systematic review and meta-analysis including 34 studies found that 43% (95% CI, 35–52%) of patient did not recover fully after 3 months while after a 12-month follow-up, the overall no-recovery rate was 21% (95% CI, 19–22%).31 Hospitalization due to chikungunya is not uncommon, as seen in this analysis where 89 individuals (10.3%) required hospitalization. In recently published case series involving travellers, Javelle et al. reported hospitalization for four out of nine patients (44%), while Dudouet et al. and Tozan et al. noted two out of eight cases (25%) and one out of eight cases (12.5%) requiring hospital care, respectively. 9,11,32 In addition to persistence of symptoms and hospitalization, travellers with chikungunya may incur significant economic costs associated with their illness with a median lost income of USD $2400 (IQR 1200–3600). 32 On 9 November 2023, the US Food and Drug Administration approved Ixchiq (Valneva Austria GmbH), which is the first commercially available chikungunya vaccine. This single-dose live attenuated vaccine is intended for individuals aged 18 and above who have an increased risk of CHIKV exposure. 33 The vaccine is currently under review by the American Advisory Committee on Immunization Practices. Multiple additional chikungunya vaccines are in various stages of development. 34
Despite vector control measures, propagation of Aedes mosquitoes to regions once uninhabited is expected because of climate change, accelerating the vector’s potential to transmit CHIKV. 35 For example, Brazil and Paraguay reported large numbers of chikungunya cases in 2022 and 2023.36,37 In the absence of vaccination campaigns in endemic areas, even with effective public health measures, there is a threat of spread to neighbouring unaffected countries with viable Aedes vectors. 38,39 Also, given that almost half of travellers in this analysis presented < 21 days after illness onset, it is important to stress mosquito bite avoidance measures if living in areas with Aedes vectors and deferring blood donation during this time. In addition to traveller data, national surveillance systems for early detection of chikungunya outbreaks are of critical importance so travellers and clinicians may remain informed of high-risk areas.
Limitations
This analysis has several limitations. GeoSentinel sites only capture data from travellers who seek care at a participating site and therefore do not represent all chikungunya cases in travellers between 2005 and 2020. These sites may also serve as travel and tropical medicine referral centers, leading to a prolonged time to presentation at the GeoSentinel site, which may not be reflective of timing or duration of symptoms. These data may also overrepresent travellers originating from certain countries, where more GeoSentinel sites exist. The low number of travellers seen during travel is likely a reflection of a paucity of GeoSentinel sites in the tropical areas of the Americas, Africa and Asia and may be in part due to lack of diagnostic tools in these regions. Further, GeoSentinel does not have data on total number of travellers, and thus there is no denominator to calculate incidence rates or hazard ratios. 14 GeoSentinel does not routinely collect data on complications or clinical outcomes; therefore, follow-up data on duration of symptoms are not available. Furthermore, the symptoms and signs reported lack detailed descriptions in the database. Before 2015, data on diagnostic methods were not collected (and antibody options were changed during the collection period), so the confirmed classification of cases could not be verified during analysis. Additionally, we were unable to account for possible cross-reactivity from other alphaviruses by serology. Despite these limitations, GeoSentinel provides important data on tropical and travel related disease and remains a vital resource for identifying sentinel events for epidemic diseases, such as chikungunya.
Conclusions
Chikungunya was acquired by international travellers in almost 100 destinations globally. Pretravel consultations for individuals travelling to regions with chikungunya or areas at risk of transmission should include discussions about vector precautions and risks. Additionally, it is important to incorporate discussions about chikungunya vaccination, a novel preventive tool, and review the recommendations provided by national vaccination expert groups with travellers. Continued surveillance of travel-related chikungunya may help public health officials and clinicians limit the transmission of this potentially debilitating disease.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Disclosures
None—RB, SB, KMA, KL, EJ, FFN, LHC, EB, ES, RS, ML, RH, FS.
Takeda and Valneva advisory board; Bavarian Nordic speaker’s bureau—DHH.
Valneva advisory board—BAC.
Funding
This project was funded through a Cooperative Agreement between the US Centers for Disease Control and Prevention and the International Society of Travel Medicine (ISTM) (Federal Award Number: 1 U01CK000632-01-00). Public Health Agency of Canada also provides a grant to ISTM.
Author contributions
Rachel Bierbrier (Conceptualization-Equal, Data curation-Supporting, Formal analysis-Equal, Investigation-Equal, Methodology-Equal, Writing—original draft-Lead, Writing—review & editing-Equal), Emilie Javelle (CRediT contribution not specified), Francesca Norman (CRediT contribution not specified), Lin Chen (CRediT contribution not specified), Emmanuel Bottieau (CRediT contribution not specified), Eli Schwartz (CRediT contribution not specified), Karin Leder (CRediT contribution not specified), Kristina Angelo (CRediT contribution not specified), Rhett Stoney (CRediT contribution not specified), Michael Libman (CRediT contribution not specified), Davidson Hamer (CRediT contribution not specified), Ralph Huits (CRediT contribution not specified), Bradley Connor (CRediT contribution not specified), Fabrice Simon (CRediT contribution not specified), Sapha Barkati (Conceptualization-Equal, Data curation-Supporting, Formal analysis-Lead, Investigation-Equal, Methodology-Lead, Supervision-Lead, Validation-Lead, Visualization-Lead, Writing—original draft-Lead, Writing—review & editing-Lead).
Acknowledgements
Rhett J. Stoney, MPH, Calvin Patimeteeporn, MPH, Douglas Esposito, MD, MPH, GeoSentinel Foundation.



