-
PDF
- Split View
-
Views
-
Cite
Cite
Chaturong Putaporntip, Chew Weng Cheng, Rattanaporn Rojrung, Napaporn Kuamsab, Somchai Jongwutiwes, Non-human primate malaria in travellers, Journal of Travel Medicine, Volume 30, Issue 8, December 2023, taad135, https://doi.org/10.1093/jtm/taad135
Close - Share Icon Share
Each year >125 million international travellers visit malaria endemic countries. Of these, ~10 000 tourists have malaria after returning home.1 Although travel-related malaria is mainly caused by infections with Plasmodium vivax and Plasmodium falciparum, ecotourists to Southeast Asian countries may occasionally expose to zoonotic malaria. Between 2010 and 2017, at least six German tourists and a French traveller acquired Plasmodium knowlesi infections from Southern Thailand when the prevalence of zoonotic malaria in this country was <1% of all malaria cases.2–4 Importantly, the emergence of simian malaria in humans has posed a diagnostic challenge.5,6
With integrative control measures, the total number of malaria patients in Thailand has declined from 32 480 cases in 2010 to 2426 cases in 2021 while the ratios of P. falciparum to P. vivax were 1:1.4 and 1:43.6, respectively.7 During this period, the number of malaria patients infected with P. knowlesi had a rising trend from 1 patient in 2016 to 72 patients in 2021. Interestingly, 68 of 72 P. knowlesi-infected patients in 2021 were from Southern Thailand.7 Meanwhile, various non-human primate malarias in this region are transmitted by the same mosquito species.8 Therefore, this study aimed to investigate whether other simian malaria species could plausibly be the causative agents when knowlesi malaria patients increased. The study included recent blood samples from malaria patients in Yala, an endemic province of knowlesi malaria in Southern Thailand.7
In 2021, a total of 178 symptomatic malaria patients were reported from Yala.7 Of these, newly collected blood samples spotted onto filter paper from 33 patients were available for analysis. Analysis was performed by species-specific PCR targeting malarial mitochondrial cytochrome oxidase subunit I (mtcoi) and targeted amplicon deep sequencing (TADS) of the same locus (Supplementary Data).9 Blood samples were from both genders with male-to-female ratio of 1.75:1, age range from 9 to 61 years (mean ± S.D., 31.91 ± 14.03 years) and parasitemia from 680 to 49 320 (median 3640 parasites/μL). These patients resided in close proximity to rubber plantations or forest fringes where wild and domesticated macaques were present. Microscopy detected P. vivax (n = 27), P. falciparum (n = 3) and Plasmodium malariae/P. knowlesi (n = 3). Meanwhile, species-specific nested PCR diagnosed 29 single infections comprising P. vivax (n = 24), P. knowlesi (n = 4) and P. falciparum (n = 1) whereas co-infections occurred in 4 isolates consisting of P. vivax and Plasmodium inui (n = 2), P. falciparum and Plasmodium cynomolgi (n = 1), and P. knowlesi, P. vivax and P. falciparum (n = 1).
The templates for TADS were prepared from malarial mtcoi-PCR products spanning 300 bp fragment containing >200 ng for each isolate. The purified amplicons of each isolate were pooled from ≥5 independent amplification reactions. Analysis was performed by using Illumina NovaSeq 6000, PE150 platform. To determine Plasmodium species, known mitochondrial genomes of human and zoonotic malaria species were used as references for classification (Supplementary Data). Each isolate yielded >32 million classified reads and <66 000 unclassified reads. Malaria species could be assigned to >98.093% of the classified reads whereas unassigned reads ranged from 0.072 to 1.907%. Comparing with PCR, TADS gave concordant results in 27 isolates (81.82% of samples) (Table 1). In total, TADS detected 10 isolates with mixed species infections which included P. vivax and P. knowlesi (n = 4), P. vivax and P. inui (n = 3), P. vivax and P. falciparum (n = 1), P. falciparum and P. cynomolgi (n = 1), and P. vivax, P. falciparum and P. knowlesi (n = 1) (Figure 1).
Comparative detection of Plasmodium species by species-specific PCR and TADS
| Targeted amplicon deep sequencing . | Species-specific nested PCR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| V . | F . | K . | F + V . | V + I . | V + K . | F + C . | F + V + K . | Total . | |
| V | 22 | 22 | |||||||
| F | 1 | 1 | |||||||
| K | 0 | ||||||||
| F + V | 1 | 1 | |||||||
| V + I | 1 | 2 | 3 | ||||||
| V + K | 4 | 4 | |||||||
| F + C | 1 | 1 | |||||||
| F + V + K | 1 | 1 | |||||||
| Total | 24 | 1 | 4 | 0 | 2 | 0 | 1 | 1 | 33 |
| Targeted amplicon deep sequencing . | Species-specific nested PCR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| V . | F . | K . | F + V . | V + I . | V + K . | F + C . | F + V + K . | Total . | |
| V | 22 | 22 | |||||||
| F | 1 | 1 | |||||||
| K | 0 | ||||||||
| F + V | 1 | 1 | |||||||
| V + I | 1 | 2 | 3 | ||||||
| V + K | 4 | 4 | |||||||
| F + C | 1 | 1 | |||||||
| F + V + K | 1 | 1 | |||||||
| Total | 24 | 1 | 4 | 0 | 2 | 0 | 1 | 1 | 33 |
V, P. vivax; F, P. falciparum; K, P. knowlesi; I, P. inui and C, P. cynomolgi.
Comparative detection of Plasmodium species by species-specific PCR and TADS
| Targeted amplicon deep sequencing . | Species-specific nested PCR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| V . | F . | K . | F + V . | V + I . | V + K . | F + C . | F + V + K . | Total . | |
| V | 22 | 22 | |||||||
| F | 1 | 1 | |||||||
| K | 0 | ||||||||
| F + V | 1 | 1 | |||||||
| V + I | 1 | 2 | 3 | ||||||
| V + K | 4 | 4 | |||||||
| F + C | 1 | 1 | |||||||
| F + V + K | 1 | 1 | |||||||
| Total | 24 | 1 | 4 | 0 | 2 | 0 | 1 | 1 | 33 |
| Targeted amplicon deep sequencing . | Species-specific nested PCR . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| V . | F . | K . | F + V . | V + I . | V + K . | F + C . | F + V + K . | Total . | |
| V | 22 | 22 | |||||||
| F | 1 | 1 | |||||||
| K | 0 | ||||||||
| F + V | 1 | 1 | |||||||
| V + I | 1 | 2 | 3 | ||||||
| V + K | 4 | 4 | |||||||
| F + C | 1 | 1 | |||||||
| F + V + K | 1 | 1 | |||||||
| Total | 24 | 1 | 4 | 0 | 2 | 0 | 1 | 1 | 33 |
V, P. vivax; F, P. falciparum; K, P. knowlesi; I, P. inui and C, P. cynomolgi.
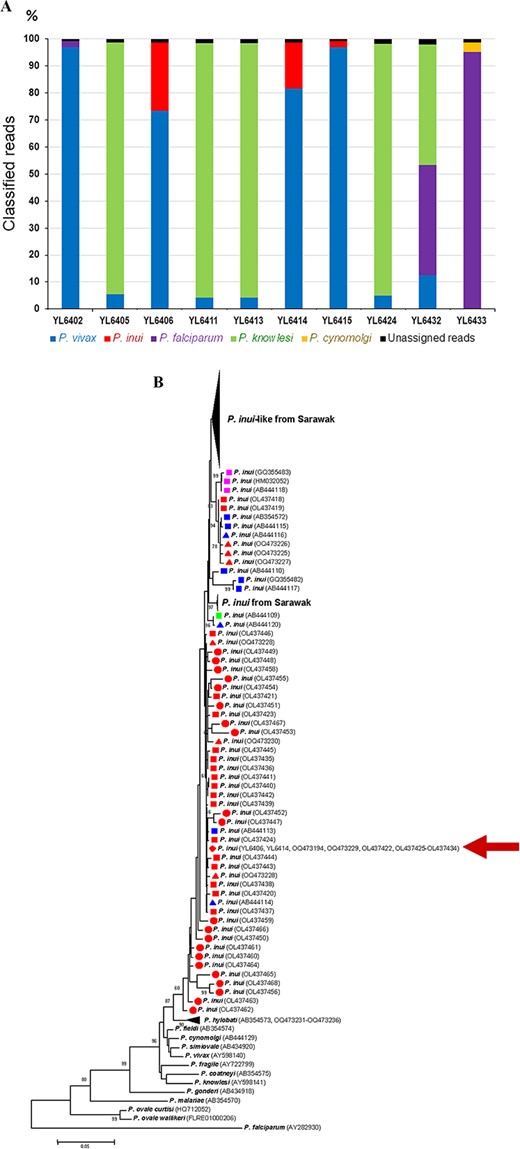
(A) Distribution of Plasmodium species among classified reads of Plasmodium mtcoi-targeted deep amplicon sequencing of isolates containing mixed-species infections. (B) Maximum likelihood tree inferred from the Plasmodium mitochondrial cytochrome oxidase subunit 1 gene spanning 1319 bp with emphasis on P. inui isolates from humans (circles), macaques (squares) and Anopheles (triangles). Isolates/clones are from Thailand (red), Peninsular Malaysia (blue), South China (purple) and Sulawesi (green). The corresponding GenBank accession numbers are shown in parentheses. Bootstrap values >60% are indicated along the branches. Scale bar represents number of nucleotide substitutions per site. Red arrow denotes identical sequences among two human isolates in this study, two isolates from Anopheles introlatus and 11 isolates from macaques in Narathiwat Province.8
The results from species-specific PCR assay have enabled identification of P. inui, P. knowlesi and P. cynomolgi among 33 recent blood samples of malaria patients in Yala where both macaque reservoirs and Anopheles vectors have been recently reported from Narathiwat, a nearby province.8 Nevertheless, some isolates containing very low levels of co-infecting species seemed to escape detection by PCR, probably due to stochastic distribution of the minor populations in the blood samples and inadequate sampling from small volumes of specimens. The DNA template derived from pooled amplicons for TADS seemed to be superior to conventional PCR to detect minor parasite populations. It is noteworthy that all six isolates with mixed infections that failed to be diagnosed by PCR contained ≤5% classified reads by TADS. Taken together, TADS detected 2.5 times more mixed species infections than PCR (10 vs 4 isolates).
Previous analyses of the mitochondrial genomes have revealed that P. inui comprised diverse lineages.10 The sequences of P. inui mtcoi spanning 1.319 kb could be determined by direct sequencing of the PCR-amplified products from isolates YL6406 and YL6414 which showed identical sequences. Interestingly, both isolates shared identical sequences across 1.319 kb fragment of mtcoi with 11 isolates from macaques and two isolates from Anopheles introlatus in Narathiwat while these isolates differed from other previously characterized human isolates.8,9 A phylogenetic tree inferred from the 1.319-kb mtcoi fragment has suggested that geographic isolation could have influenced evolution and diversification of P. inui because all Thai isolates formed distinct lineages from those belonging to P. inui and P. inui-like parasites from Malaysian Borneo (Figure 1B).10
It is noteworthy that various strains of P. inui have been previously identified among patients from diverse regions of Thailand.9 The presence of multiple strains of P. inui capable of infecting humans akin to those observed in P. knowlesi could suggest that cross-species transmission between monkeys and humans might not be recently established. Importantly, P. inui was the most prevalent species in macaques and poses a risk of cross-species transmission from nonhuman primates to humans akin to P. knowlesi and other simian malaria species. Microscopic diagnosis of P. inui has been compromised by the morphological similarity with other human and simian malaria parasites in thick blood smears.9 The relatively low parasitemia of P. inui, probably due to the slow growth rate, requiring 72-hour asexual erythrocytic cycle, could further impede the identification in thin blood films. Therefore, molecular detections seemed to be indispensable for the diagnosis of simian malaria.
The emergence of zoonotic malaria species other than P. knowlesi amid a decline in malaria prevalence in this study could be a diagnostic concern for travellers to malaria endemic areas that overlap with the habitats of macaque natural hosts. Although most zoonotic malaria may be benign and well responsive to chloroquine, it remains unknown whether antimalarial chemoprophylaxis provides complete protection. Awareness of emerging simian Plasmodium species in certain areas may assist in an early diagnosis with proper diagnostic tools, leading to a prompt treatment for travellers returning to non-endemic countries.
Acknowledgements
The authors are grateful to all patients who donated their blood samples. They also thank the staff of the Bureau of Vector Borne Disease, Department of Disease Control, Ministry of Public Health, Thailand, for assistance in field work.
Funding
This study was funded by Ratchadapiseksompotch Fund for Health Science, Chulalongkorn University (Grant No. RCU_H_64_015_30 to S.J. and C.P.). C.W.C. is supported by British Heart Foundation—Mautner Career Development fellowship.
Author contributions
Chaturong Putaporntip (Conceptualization-Equal, Data curation-Equal, Formal analysis-Equal, Funding acquisition-Supporting, Investigation-Lead, Methodology-Equal, Project administration-Equal, Resources-Supporting, Software-Supporting, Supervision-Equal, Validation-Equal, Writing – original draft-Equal, Writing – review & editing-Equal, Chew Weng Cheng (Data curation-Supporting, Formal analysis-Equal, Methodology-Equal, Software-Lead, Validation-Equal, Writing – review & editing-Supporting, Rattanaporn Rojrung (Investigation-Supporting), Napaporn Kuamsab (Investigation-Supporting, and Somchai Jongwutiwes (Conceptualization-Equal, Data curation-Equal, Formal analysis-Equal, Funding acquisition-Lead, Methodology-Equal, Project administration-Equal, Resources-Lead, Software-Supporting, Supervision-Equal, Validation-Equal, Visualization-Lead, Writing – original draft-Equal, Writing – review & editing-Equal)
Conflict of interest
None declared.
Ethical approval statement
This study was reviewed and approved by the Institutional Review Board in Human Research of Faculty of Medicine, Chulalongkorn University, Thailand (IRB No. 322/59 and COA No. 041/2016). All procedures were performed in accordance to the relevant guidelines and regulations.
Data availability statement
Data are available on request. The data underlying this article will be shared on reasonable request to the corresponding author.



