-
PDF
- Split View
-
Views
-
Cite
Cite
Guillaume Thizy, Eric Caumes, Joffrey Molher, Frederic Ariey, Olivier Lortholary, Pierre Buffet, Cléa Melenotte, Disseminated mucocutaneous leishmaniasis in a traveller with idiopathic CD4 lymphocytopenia, Journal of Travel Medicine, Volume 30, Issue 8, December 2023, taad063, https://doi.org/10.1093/jtm/taad063
Close - Share Icon Share
A 48-year-old man, without any pre-existing medical condition except recurrent genital herpes, sought medical attention for disseminated cutaneous leishmaniasis (DCL). He had been living in Dakar, Senegal, for 20 years, but he had travelled to over 80 countries during his life. In June 2021, he had been hiking for 10 days in the Amazonian forest of Bolivia and reported numerous insect bites (Figure 1A). A few of these lesions progressed to ulceration within a month without improvement after treatment with amoxicillin-clavulanate (Figure 1B–D). In Dakar, direct examination of the skin biopsy identified histocytes parasitized by Leishmania amastigotes. He received two lines of intramuscular meglumine antimoniate (20 and 60 mg/kg/day) for >3 weeks each. All lesions, except the one on the forehead, disappeared. Four months later, all lesions reappeared.
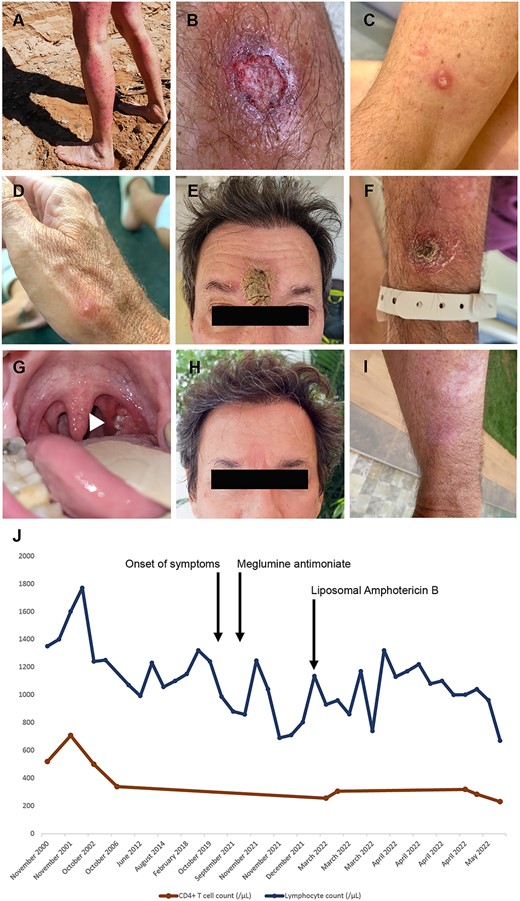
Clinico-biological presentation: (A) numerous local reactions to insect bites on the patient’s legs in Bolivia; (B–G) 15 lesions were distributed all over the body on the face, ear, upper and lower limbs in addition to an ulcerated lesion of the left tonsil; (B–D) papular and pustular and ulcerated skin lesion before treatment; (E and F) ulcerated skin lesions on initial presentation after failure of the first line of therapy; (G) ulcerated lesion of the right tonsil (arrow head); (H and I) healed lesions on the forehead (6 cm) after completion of the treatment with LAB; (J) evolution of total lymphocyte count (blue) and CD4+ T cell count (red) before and after completion of the treatment with LAB.
In 2022, he consulted at the Necker Pasteur Center for Infectious Diseases and Tropical Medicine, Paris, France. Fifteen ulcero-crusted cutaneous lesions, distributed all over his body, were observed (Figure 1E–G). Nasofibroscopic examination showed three ulcerated lesions in the nasal septum, middle conchae and tonsil. Skin and mucosal biopsies were PCR- and culture-positive for Leishmania braziliensis. Isolated CD4 lymphopenia was identified (256/μl, 35%) at diagnosis and had been present since 2006 (Figure 1J). Common causes of CD4 lymphopenia were absent. A 2-cm cervical lymphadenopathy was identified on CT scan. He was treated with liposomal amphotericin B (LAB) (cumulative dose 51 mg/kg) over 4 weeks. He had no drug-related adverse events, and 30 days after the last LAB perfusion, cutaneous and mucosal ulcerations were completely reepithelialized. The patient was relapse-free at 12 months of follow up (Figure 1H and I). CD4 lymphocytopenia persisted after completion of treatment (Figure 1J).
Cutaneous leishmaniasis (CL) is a neglected tropical disease caused by Leishmania, a protozoan parasite transmitted by sandflies.1 The L. braziliensis is a CL-causing species endemic in the Amazonian region of Bolivia and is associated, in 1–20% of cases, with disfiguring and potentially life-threatening mucosal lesions known as mucosal leishmaniasis. DCL (>10 lesions distributed over more than two non-contiguous parts of the body), is rare, seen in 0.2–3.9% and 0.5–1% of tegumentary leishmania cases in Brazil and in European travellers, respectively.1
Systemic treatments for ML and DCL include LAB, pentavalent antimonial (SbV) compounds or oral miltefosine.2 IDSA guidelines propose LAB cumulative dosage ranging from 18 to 21 mg/kg for CL and from 20 to 60 mg/kg for ML.2 Cure rate with a median cumulative dose of 20 mg/kg LAB varied from 44 to 85% and reached 70% with sodium stibogluconate.3 Cumulative dose of LAB ≤ 30 mg/kg was associated with a higher failure rate than cumulative dose of LAB > 30 mg/kg (42 vs 25%).4
A target cumulative dose of LAB > 30 mg/kg increases the chance of a positive outcome when treating DCL.4 Severe, disseminated and relapsing CL and ML have been reported in immunocompromised patients with T-cell-impaired immunity.5 In case of relapse, secondary prophylaxis with oral miltefosine will be considered following a second curative course.
Acknowledgements
None.
Funding
Cléa Melenotte is currently funded by the ANRS, Agence nationale de recherches sur le sida et les hépatites virales.
Authors’ contributions
G.T. and CM wrote the article; E.C., P.B. and C.M. followed the patient; E.C. and O.L. proposed a careful review of the paper; J.M. performed nasofibroscopy; F.A. performed the parasitological diagnosis; P.B. and C.M. and supervised the work.
CRediT author statement
Guillaume Thizy (Conceptualization [supporting], Data curation [lead], Investigation [equal], Resources [lead], Writing—original draft [equal]), Eric Caumes (CRediT contribution not specified), Joffrey Molher (Data curation [equal], Methodology [equal], Validation [equal]), Frederic Ariey (Investigation [equal], Methodology [equal], Validation [equal], Visualization [equal]), Olivier Lortholary (Investigation [equal], Supervision [equal], Validation [equal]), Pierre Buffet (Conceptualization [equal], Supervision [equal], Validation [equal]), and Cléa Melenotte (Conceptualization [supporting], Data curation [lead], Investigation [equal], Resources [lead], Writing—original draft [equal])).
Conflict of interest
None declared.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Informed and written consent was obtained from the patient.



