-
PDF
- Split View
-
Views
-
Cite
Cite
Domenico Ponticelli, Ippazio C Antonazzo, Grazia Caci, Andrea Vitale, Giovanni Della Ragione, Maria L Romano, Mario Borrelli, Beniamino Schiavone, Riccardo Polosa, Pietro Ferrara, Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months, Journal of Travel Medicine, Volume 28, Issue 8, December 2021, taab173, https://doi.org/10.1093/jtm/taab173
Close - Share Icon Share
Research letter
Emerging evidence is offering significant insights into the effectiveness and safety of the vaccination against the Coronavirus Disease 2019 (COVID-19), but another crucial aspect of the current global vaccination campaign is the time trend of the antibody response to COVID-19 vaccines over a longer period and the resulting duration of the protection offered.1 Here, we present data on the dynamics of antibodies that bind SARS-CoV-2 spike (S) protein receptor binding domain (RBD)—the most critical target for SARS-CoV-2-specific antibodies within the S1 sub-unit2—after 6 months from the administration with BNT162b2 vaccine.
This analysis, built as a longitudinal observational design, is part of the VASCO project (‘Monitoraggio della risposta al Vaccino Anti-SARS-CoV-2/COVID-19 negli operatori sanitari del Pineta Grande Hospital’), which defines an ongoing broad study on the response to BNT162b2 mRNA COVID-19 vaccine in a sample of healthcare workers (HCWs) of the Pineta Grande Hospital (Castel Volturno, Caserta, Italy), investigating effectiveness, immunogenicity and safety of the vaccination. Complete methods of the VASCO project have been presented elsewhere.3
In this survey, HCWs who were administered the two-dose BNT162b2 mRNA vaccine 21 days apart between December 2020 and January 2021 were invited to undertake a quantitative serology test for the research of SARS-CoV-2 S-RBD-specific immunoglobulins G (IgG). Seroconversion, defined as the development of any detectable SARS-CoV-2 S-RBD-specific IgG in serum sample, was evaluated through Snibe—Maglumi® SARS-CoV-2 S-RBD IgG chemiluminescent immunoassay (CLIA).4 Reactivity was intended as an antibody level equal to or greater than 1.0 AU/ml. According to the manufacturer’s recommendations, the Maglumi® SARS-CoV-2 S-RBD IgG CLIA presented sensitivity of 100% (95% confidence interval [CI], 99.9–100%) and specificity of 99.6% (95% CI, 98.7–100%) after the 15th day from symptom onset.4 HCWs underwent six longitudinal serological assays every 30 days, the first of which was performed within 1 month after completing the vaccination cycle. If a HCW had had a previous infection with SARS-CoV-2 6 months prior to the vaccination or if he/she had contracted the infection after the administration of the first vaccine dose, the cycle was considered complete with a unique dose, as per Italian Ministry of Health guidelines and according to literature findings.5,6 CLIA results were expressed as median IgG value and interquartile range (IQR). Differences between medians were assessed through Mann–Whitney U test; multivariate regression analyses were built to investigate the association between the level of the vaccine-elicited antibodies and potential predictors, such as sex, age, previous SARS-CoV-2 infection and post-first dose infection. A P-value of 0.05 was set as significance level.
Overall, we analyzed the sera of 162 subjects, being mostly women (58.0%) with a mean age of 42.5 years (±11.9 SD). Twenty-eight HCWs had a history of previous SARS-CoV-2 infection. At the first serum sample, the median anti-S-RBD IgG reached 540.0 AU/ml (IQR 64.5–1102.0). In the following tests, a progressive decay of antibodies was seen, up to the value of 55.7 AU/ml (IQR 26.2–84.7) at the 6-month follow-up (Figure 1). No significant associations were found according to vaccinees’ sex and age. Within 1 month from the vaccination, there was a significant higher S-RBD-reactive antibody response in those subjects with previous SARS-CoV-2 infection (medians: 1534.9 [IQR 1142.0–2000.0] vs. 407.7 [IQR 60.0–846.6]; P = 0.001) and the significance remained after adjusting for age and sex (β = 1762.2; 95% CI 1022.9–2501.6; P < 0.001; R2adj = 0.29). Differences in IgG titres between those with previous SARS-CoV-2 infection and those without were no longer significant at the following serological surveys. Three vaccinees tested positive at the RT-PCR assay for qualitative detection of SARS-CoV-2 nucleic acid on nasopharyngeal swabs before the administration of the second vaccine dose; no statistical association was detected between the infection after the first dose and humoral response. No other infections were reported in the follow-up passive surveillance described previously.3
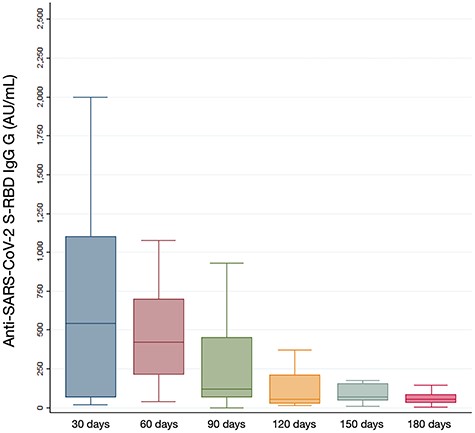
Boxplots of post-vaccination SARS-CoV-2 S-RBD immunoglobulins G (IgG) evaluated during 30-day time-points in the study population. Seroconversion was evaluated through Snibe—Maglumi® SARS-CoV-2 S-RBD IgG chemiluminescent immunoassay with reactivity cut-off set at 1.0 AU/ml.
Our findings suggest two important aspects of the use of BNT162b2 vaccine in addressing the COVID-19 pandemic. First, they confirm the persistence of anti-S-RBD neutralizing antibodies through 6 months after the vaccination. These results mirror emerging evidence and represent a crucial non-obvious positive finding,1 which confirm this duration of persistence as an added value, along with effectiveness and safety results.2 Second, similarly to other vaccines, the level of the antibodies elicited by the vaccine started to decrease from the second month after vaccination and during the entire investigated time period (Figure 1). Such a finding is also consistent with the decline of infection-induced S-antibody levels observed after infection,7 where a substantial cellular immune memory appears to be maintained,8 as confirmed by the lower vaccine-elicited IgG titre in naïve vaccinees, compared with those with previous SARS-CoV-2 infection, which serves as immune priming.5,6
In conclusion, although a decreasing antibody level is likely to keep offering an active protection through the persistence of immune memory, the decrease in anti-S-RBD IgG must be studied in continuous monitoring of vaccine-induced immunogenicity. This may allow to determine a protective antibody threshold below which the risk of break-through infections increase considerably and which could thus guide the time point when to offer a booster dose. So far, the role of cellular immunity and the clinical significance of lower antibody levels are yet to be fully understood, particularly in view of emerging viral variants. The presented data could therefore inform public health decision-makers and help to predict the future course of the vaccination campaign and efforts. This paper has strengths and weaknesses. It is worth noting that it offers interesting insights into important aspects related to COVID-19 vaccine based on real-world data, being based on specific and sensitive antibody assay, which accurately correlate with SARS-CoV-2 infection. However, the study relays on HCWs who voluntarily decided to participate and potential selection bias should be considered. Moreover, the possibility of further undetected possible confounders of the vaccine-induced humoral response cannot be completely excluded.
This real-world study documents the time trend of antibody response to mRNA BNT162b2 COVID-19 vaccine.
Persistence of anti-SARS-CoV-2 S-RBD neutralizing IgG through 6 months after the vaccination was confirmed.
The level of the antibodies elicited by the vaccine started to decrease from the second month after vaccination.
The vaccine-induced immunogenicity and protection need to be further monitored in order to maximize the current vaccination efforts.
Authors’ contributions
D.P. and P.F. conceived and designed the study. P.F. led the statistical analyses and wrote the first draft of the article. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the manuscript. All authors have read and approved the final manuscript.
Funding
This research received no external funding.
Conflict of interest
R.P. is a full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. In relation to his recent work in the area of respiratory diseases, clinical immunology, and tobacco control, RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics and Forest Laboratories. Lecture fees from a number of European EC industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vaper advocacy no-profit organizations. R.P. has also received grants from European Commission initiatives (U-BIOPRED and AIRPROM) and from the Integral Rheumatology & Immunology Specialists Network (IRIS) initiative. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl. and Health Diplomats. R.P. has served on the Medical and Scientific Advisory Board of Cordex Pharma, Inc., CV Therapeutics, Duska Therapeutics Inc, Pfizer and PharmaCielo. R.P. is also founder of the Centre for Tobacco prevention and treatment (CPCT) at the University of Catania and of the Centre of Excellence for the acceleration of Harm Reduction (CoEHAR) at the same University, which has received support from Foundation for a Smoke Free World to conduct eight independent investigator-initiated research projects on harm reduction. R.P. is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA) and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on ‘Requirements and test methods for emissions of electronic cigarettes’ (CEN/TC 437; WG4). All other authors declare no conflict of interest.
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki. The VASCO project was approved by the Institutional Review Board—Comitato Etico Campania Nord, with referral number CECN/1614/2021. All participants provided written informed consent before enrolment into the study.
References
Author notes
Riccardo Polosa and Pietro Ferrara authors contributed equally to this work.



