-
PDF
- Split View
-
Views
-
Cite
Cite
Jean-Marc Schwob, Caroline F Samer, Patrice H Lalive, Gilles A Eperon, Live vaccines and immunosuppressive monoclonal antibodies: weighing up the benefit–risk assessment for natalizumab, Journal of Travel Medicine, Volume 28, Issue 3, April 2021, taaa235, https://doi.org/10.1093/jtm/taaa235
Close - Share Icon Share
Over the last two decades, an increasing number of immunosuppressive/immunomodulating (ISIM) treatments has been approved for immune-mediated inflammatory disorders (IMID), mostly in the form of monoclonal antibodies (mAbs). While the effect of these drugs in controlling deleterious autoimmune mechanisms may be spectacular, one of the main possible adverse effects is a dysfunctional immune response against certain pathogens and dysfunctional vaccine immune response.1 For these reasons, a growing number of new ISIMs are introduced with recommendations for their compatibility with vaccination.1 These raise two general concerns. The first is immunogenicity: several studies have shown limited efficacy when immunization was performed during ISIM treatment.1 The second is the safety of vaccine administration.
Regarding the safety concern, live vaccines are contraindicated during most courses of ISIM due to a proven or suspected risk of developing a vaccine-strain infection. Most of the current recommendations are crude simplifications based on the broad immunosuppressive drugs used since the 1970s for solid organ transplantation. They merely take into account the complexity of the immune system and how pathogens interact with it the variability of IMID nor the heterogeneity of the targeted immunosuppressive effect of ISIM. While the principle of safety and precaution must always be the primary concern, finer recommendations should be considered for the specific and selective immunosuppressive treatments that have been introduced more recently. Strong recommendations should be evidence based. And all immunizations (live or inactivated), whether for safety or immunogenicity concerns should ideally always be carried out before ISIM introduction. However, a case can be made for a pharmaco-physiopathological approach made on a case-by-case basis, assessing the benefit/risk ratio between the safety concerns of live-vaccine immunization and the risk for infectious disease (e.g. people working or living in a specific infectious disease-endemic area). We would like to illustrate our approach with the example of natalizumab.
Huttner et al. recently published the largest cohort of multiple sclerosis patients who received yellow fever vaccine (YFV), a live attenuated vaccine.2 Of these vaccinated patients, 10 had been under treatment with ISIM, of which eight (all primary immunization) were on natalizumab (NZB). One of these eight patients also received measles–mumps–rubella (MMR) vaccine (as secondary vaccination). [personal communication] NZB is an mAb for which there are only expert recommendations that generally contraindicate live vaccines. To our knowledge, data from only a single patient receiving live-attenuated YFV while on NZB treatment have ever been published.3 No other published studies have specifically addressed the use of live vaccines during NZB treatment. Given this paucity of evidence and a better understanding of how new mAbs interfere with vaccination, we hereby formulate a case-by-case position regarding the use of live vaccines in patients treated by NZB.
NZB is a humanized mAb that selectively blocks the α4 subunit of the α4β1and α4β7 integrins, adhesion molecules expressed on all leukocytes except neutrophils. It, therefore, blocks their interaction with their receptors, vascular cell adhesion molecule-1 (VCAM-1), osteopontin ligand and connecting segment-1 (CS-1) for α4β1 integrin and mucosal addressin cell adhesion molecule-1 (MadCAM-1) for α4β7 integrin.4 In multiple sclerosis, this action blocks the transendothelial migration (diapedesis) of mononuclear cells (mostly T cells) to the inflammatory parenchymal tissues (mainly central nervous system), thus blocking the continuation of the inflammatory cascade and the frequency and severity of relapses.5 Compared to other ISIMs, NZB appears to have little impact on systemic immunity.4 After more than a decade of use, NZB has been associated with an increased risk (incidence 0.1–1%) of progressive multifocal leukoencephalopathy (PML) due to the reactivation of latent JC virus infection. This risk resulted in a black box warning in the USA, and a restriction of NZB use in Europe. The presence of anti-JC virus antibodies, the duration of treatment (>2 years) and the prior use of immunosuppressive agents are identified risk factors for the development of PML. A slight increase of intracerebral herpesvirus infections (VZV and HSV) has also been described.6 However, no other significant opportunistic infections have been observed.
While most experts recommend avoiding live vaccines in conjunction with NZB, others have considered exceptions after a careful individual risk–benefit analysis.7 The difference in safety between primary and secondary vaccination (assumed to be lower) with a live vaccine should furthermore be taken into account. NZB could particularly increase vaccine-associated neurological diseases such as meningoencephalitis, given its theoretical pharmacological effect and known risks for opportunistic infections in such tissues. On the other hand, when vaccination is strongly recommended in a patient known to have an IMID prone to disease progression (e.g. multiple sclerosis), being able to vaccinate under ISIM is highly desirable. Indeed the risk of exacerbation is possibly reduced by shortening the delay in introducing an ISIM.2
Measles virus replication first occurs in the respiratory tract, then, after two different peaks of viremia (days 2–3, then days 4–7), in several organs (e.g. skin). Humoral immunity appears to prevent measles infection, while protective immunity against severe measles and death involves cellular immunity (CD4+Th1 and CD8+ T-cells).8 In non-immunosuppressed patients, a risk of post-vaccinal symptoms or mild self-limited disease is frequently observed with measles vaccine. After the first MMR dose, approximately 5% of vaccinated individuals may develop a vaccine-associated mild rash and 5–15% develop fever, while 0.074% have recently been reported to present a complete picture of measles.9 The measles virus is directly involved in three of the four types of measles-related encephalitis (primary measles encephalitis, measles inclusion body encephalitis and subacute sclerosing panencephalitis), while acute post-measles encephalitis appears to be immune mediated.10 Almost all cases of measles-related encephalitis are caused by the wild-type virus; Measles inclusion body encephalitis has been linked to a mutant wild-type virus, and this severe complication has been exceptionally described as a post-vaccinal disease in a child. Subacute sclerosing encephalitis has never been associated with MMR. Measles live immunization in pediatric liver transplant recipients has been evaluated and appears safe when clear eligibility criteria are applied.11 In the absence of randomized studies, the assessment of the risk of vaccine-associated measles in NZB patients is unclear. Although no adverse events were reported by Huttner et al. (secondary vaccination),2 one case of non-severe possible vaccine-associated measles has recently been observed (no further indication of vaccination history).12
For YF virus, while there is a role of cellular immunity in the physiopathology of the virus, humoral immunity and particularly neutralizing antibodies against epitopes present in the envelope glycoprotein are believed to result in an adaptive protective immunity.13 Indeed, an initial strong innate immune response after primary vaccination seems critical to vaccine immunogenicity: the variation in the innate immune response could explain the delayed antibody response and a higher risk for the rare but fatal yellow fever vaccine-associated viscerotropic disease (YEL-AVD).13 Wild YF virus does not present neurotropism, but the live attenuated YFV type 17D may rarely be associated with neurological complications, known as YEL-AND, with low mortality (<1.0%). A minority (one-third to one-half) of cases with these complications appears to be related to an auto-immune mechanism, and the rest (one-half to two-thirds) is attributed to brain tissue invasion by the virus.13 The 17D virus present in YFV is associated with very low and transient viremia, but this is generally insufficient to cause neuro-invasion or subclinical infection. The incidence of serious adverse events is estimated to be eight per million in immune-competent adults, while in the elderly, it is up to three times higher.13 It is suspected that a longer viremic period and a delayed antibody response in elderly patients might be the reasons for an increased risk of encephalitis. It has also been hypothesized that young infants, too, were at higher risk, due to the immaturity of their blood–brain barrier, so YFV is contraindicated in infants ≤6 months of age.13 Although immunosuppression is a well-known contraindication of YFV, several studies have reported on the use of YFV during immunosuppressive treatment, and a recent systematic review seems rather unconcerned about its overall safety.14 Only two studies have reported YFV immunization with NZB, for a total of nine patients, without any severe adverse event being described.2,3 Interestingly, all these patients received YFV as primary vaccination. In the absence of larger retrospective or randomized studies, the risk assessment of YEL-AND in NZB patients remains unclear.
Given the known increased risk for herpesvirus infection in patients taking NZB and the availability of alternative (inactivated) vaccines (shingles, influenza or typhoid), the precautionary principle imposes an absolute contraindication to the use of other live vaccines.
Preferably all vaccines (especially live vaccines) should always be given before the initiation of ISIM. But in practice, many patients who are already on ISIM treatment were never or insufficiently immunized against vaccine-preventable diseases. Avoiding pathogen exposure is the first-recommended management, but this is not always an option and we consequently face the ethical problem of allowing immunosuppressed individuals to be at risk of vaccine-preventable disease. Given the wide heterogeneity of biologic agents used for ISIM today, we advocate more refined recommendations based on the specific immunosuppressive and immunogenicity effects of drugs on the one hand and the specific mechanisms whereby vaccines elicit immunity on the other hand, resulting on a case-by-case management after careful risk/benefit assessment. Moreover, every vaccination with live vaccine under immunosuppressive treatment should be published, in order to cumulate data, as no trial can be made. At last, such an assessment requires expertise in clinical pharmacology and immunology but also epidemiological and pathophysiological knowledge is needed to balance the risk and benefits of immunization with a specific live vaccine in patients receiving a specific ISIM. We summarized our proposed policy on live vaccines under NZB treatment in Figure 1.
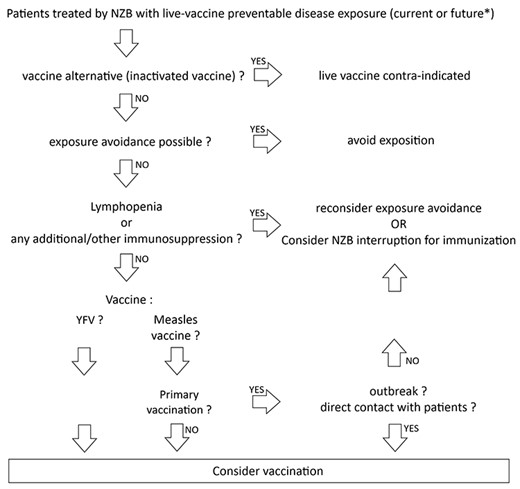
Proposed policy for the use of live vaccines in patients treated by NZB. NZB: natalizumab. YFV: yellow fever vaccine. *: for example, if modification of immunosuppressive regimen is considered and will strictly contraindicate live vaccines in the future
Author Contributions
J.M.S. studied the conceptualization and design, drafting the article. C.S. and P.L. did the manuscript revision. G.E. studied the conceptualization and design, manuscript revision.
Acknowledgments
We would like to thank Rob Hooft van Huijsduijnen for his critical reading of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of Interest: The authors have declared no conflict of interest.



