-
PDF
- Split View
-
Views
-
Cite
Cite
Rebekah Honce, Stacey Schultz-Cherry, Influenza in obese travellers: increased risk and complications, decreased vaccine effectiveness, Journal of Travel Medicine, Volume 26, Issue 3, 2019, taz020, https://doi.org/10.1093/jtm/taz020
Close - Share Icon Share
Abstract
Obesity is a worldwide epidemic and was empirically shown to increase the risk of developing severe influenza virus infection. As international travel becomes more common and obesity is now prevalent even in low- and middle-income countries, travellers may have an increased risk of contracting influenza virus especially during peak influenza season.
An analysis of the literature, centred on publications from 2014–19, was performed, with an emphasis on human epidemiological data, human studies ex vivo and studies in mouse models of obesity. Our search efforts focused on influenza disease severity, pathogenesis, evolutionary dynamics and measures of infection control in the obese and overweight host.
Obesity is associated with an increased risk of infection, as well as a greater chance for hospitalization and severe complications. Studies in mouse models of obesity have uncovered that obese hosts suffer increased viral spread, delayed viral clearance and heightened damage to the respiratory epithelium. Innate and adaptive immune responses are delayed, thus increasing morbidity and mortality. Further, infection control measures, including vaccination and antivirals, prove less effective in obese hosts. Finally, the obese microenvironment allows for increased duration and amount of viral shedding and potentially increases the chance for emergence of virulent minor variants in the viral population. Together, obese hosts are at high risk of influenza infection, as well as severe sequelae following infection.
Obese travellers should be aware of influenza activity in the regions visited, as well as take protective measures prior to travel. Vaccination is highly recommended for all travellers, but especially highly susceptible obese travellers.
Introduction
One of the biggest threats to global public health is the ever-increasing prevalence of obesity. Since 1975, the rates of obesity have tripled worldwide and currently more adults are overweight than underweight—even now becoming an issue in low- and middle-income countries.1 Obesity raises the risk for non-communicable and communicable diseases, including increasing susceptibility to infectious diseases, such as influenza virus, for travellers worldwide.2–4 A body mass index (BMI) of > 30 was recognized as a risk factor for developing severe disease during the 2009 influenza A virus (IAV) H1N1 pandemic, as it was globally associated with increased hospitalizations and mortality.5,6 The obese host harbours a unique lung microenvironment for infection due to chronic meta-inflammation that has systemic implications for pathogenesis and immunity.7
Influenza is typically a mild upper respiratory infection but can turn severe. Most seasonal human cases are caused by the H1N1 or H3N2 strains of influenza A virus, with proportionally fewer caused by influenza B viruses (IBV).8 Characterized by fever, rhinorrhea and myalgia, influenza infection typically resolves within 1 week. Severe sequelae, including infection of the lower respiratory tract and acute lung injury (ALI), can result in hospitalization, acute respiratory distress syndrome and potentially death.9 Case studies have defined extensive viral replication in the deep lung and progression to viral pneumonia in obese patients, suggesting more severe pathogenesis in this high-risk population.10–12 Recent research has identified several mechanisms behind this increased disease severity, as well as how the obese host responds to vaccination and antiviral treatment. Further, insight on how IAV responds to infection of an obese host has revealed new implications of the obesity epidemic on global public health. Together, the rising global obesity prevalence and continual circulation of IAV pose a threat to travellers worldwide and require special attention—especially for obese travellers—to prevent severe infection.
Methods
We have undertaken a review of the literature, focusing on publications from 2014–19 but extending to older literature when necessary, using combinations of the search terms `influenza virus’, `obesity’, `infection risk’, `travel and influenza infection’ and ‘influenza vaccination’.
Case reports of influenza infection in obese patients
An early autopsy case from the 2009 H1N1 `swine flu’ pandemic reported the death of a 33-year-old male 8 days post-hospital admission.12 Along with several other co-morbidities, the patient had a BMI of 38 and presented with high fever, cough, dyspnea and diarrhoea. The cause of death was identified as respiratory failure and upon autopsy, abundant viral replication in the deep lung, type I and type II pneumocytes, with less in the bronchial region and none in the trachea. The extensive viral spread was joined with severe pulmonary edema and desquamation of the respiratory epithelial cells.12 This severe pathology is reflective of other case reports, which highlight severe alveolar haemorrhaging and ALI upon infection in the obese patient.13 Other facets of infection dynamics, including host susceptibility and viral shed, may be sensitive to the obese state. Reports of avian-origin H7N9 infection in Shanghai, China, reveal all three infected patients were obese, middle-aged men.14 Finally, in a woman with a BMI of greater than 40 treated with oseltamivir for H1N1 virus during the 2009 pandemic, viral shed was detected for 19 days, with negative swabs by RT-PCR by day 31 after the onset of symptoms.10 Prolonged viral shed was also described in a man with a BMI of less than 30, but it is not stated if this patient still had a BMI in the overweight range.10 Further, epidemiological and surveillance studies have strengthened the association between obesity and IAV pathogenesis, host response and viral shed.6,15 Studies focused on IBV infection in obese humans have shown a weaker association between infection and obese status, with no association between obesity and IBV severity and length of viral shed.15,16 Mechanistic studies in mouse models, cell culture and human samples ex vivo discussed below offer potential explanations for the increased disease severity seen in the obese host.
Influenza pathogenesis in the obese host
The United States Centers for Disease Control now considers obese adults to be at high risk for influenza infection.6,17,18 Mechanistic experiments in mouse models of obesity, largely using the genetically obese, leptin deficient (OB) mouse and the high-fat diet-induced obese (DIO) mouse, have revealed increased viral spread, decreased wound repair and delayed and blunted immune responses leading to poorer infection outcomes.
Viral spread and immunopathology
Influenza virus induces increased damage and inflammation to the respiratory epithelium of obese mice. This increased damage has multiple etiologies. In both naïve and vaccinated obese mice, increased lung damage, permeability and edema as compared to wild-type (WT) mice were detected post-infection (p.i.).19–21 Further, OB and DIO mice have increased protein leakage and oxidative stress.20,22–24 Damage to the epithelial surface may also be due to higher viral titres recovered from obese mice. DIO mice inoculated with a lethal dose of PR8 mouse-adapted H1N1 virus showed higher viral titres at days 6 and 10 p.i.24 However, no difference in peak titre was recovered from OB or DIO mice inoculated with CA/09 H1N1 virus at day 3 p.i. as compared to WT.22,25 Independent of higher viral titre, by day 3 p.i., increased viral spread is evident in OB mice.25 This phenotype continues throughout infection, leading to a greater proportion of the lung in active infection and decreased area in active repair at days 7 and 8 p.i.,20 predisposing OB mice to secondary bacterial infections due to a breakdown in epithelial integrity.20,22 As with IAV, increased bacterial spread through the upper and lower respiratory tract occurs in a secondary infection model in OB mice.22 The increased susceptibility, lung damage and secondary infection risk combine to decrease survival p.i. in obese mice.20,21
Immune response
Immune responses are impaired in the obese host, with IAV-specific responses either delayed or blunted (Table 1).7 However, as is evident by the severe lung damage seen in obese mice p.i., heightened immunopathology is common.5,19,20,23,26 In studies with obese mice, increased adiposity results in baseline alterations in systemic cytokine production, with the baseline inflamed state impacting innate and adaptive immune responses.27–29 However, upon infection, OB mice show a delayed pro-inflammatory cytokine response, potentially poorly priming the innate and adaptive immune responses.20,21
| Effector . | Impact of obesity . | Reference . |
|---|---|---|
| T cells | ↓ production of naïve T cells | 35,72,73 |
| γδ | ↓ IL-2Rα | |
| CD8+ | ↓ activation markers | |
| CD4+ | ↓ activation markers | |
| B cells | ↓ IL-6 secretion | 38,47 |
| ↓ IgG producing lymphocytes post-vaccination | ||
| ↑ IgM levels | ||
| DCs | ↓ circulation and infiltration p.i. | 32,34,35 |
| ↓ Ag presentation | ||
| ↓ maturity | ||
| Macrophages | Proinflammatory phenotype | 74–76 |
| ↑ IL-6 mRNA | ||
| ↑ oxidative burst activity | ||
| ↓ PPARγ function | ||
| Natural killer cells | ↓ circulation | 34,77,78 |
| Neutrophils | ↑ numbers correlated with BMI | 78,79 |
| ↑ inflammatory free radicals upon stimulation | ||
| ↓ response to stimuli | ||
| IFN | ↓ IFN-γ from γδ T cells | 72,80,81 |
| ↓ IFNα/β in TLR-3 stimulated PBMCs | ||
| Interleukins and cytokines | ↑ baseline IL-1β, IL-6, TNFα | 47,76,78,82,83 |
| ↑ IL-5 levels in PBMCs | ||
| ↑ NF-κB activation | ||
| ↓ TNFα, IL-2, and IL-6 in stimulated PBMCs | ||
| Adipokines | ↑ leptin, ↑ leptin resistance | 82,84 |
| ↑ TGFβ in adipose tissue | ||
| ↓ adiponectin |
| Effector . | Impact of obesity . | Reference . |
|---|---|---|
| T cells | ↓ production of naïve T cells | 35,72,73 |
| γδ | ↓ IL-2Rα | |
| CD8+ | ↓ activation markers | |
| CD4+ | ↓ activation markers | |
| B cells | ↓ IL-6 secretion | 38,47 |
| ↓ IgG producing lymphocytes post-vaccination | ||
| ↑ IgM levels | ||
| DCs | ↓ circulation and infiltration p.i. | 32,34,35 |
| ↓ Ag presentation | ||
| ↓ maturity | ||
| Macrophages | Proinflammatory phenotype | 74–76 |
| ↑ IL-6 mRNA | ||
| ↑ oxidative burst activity | ||
| ↓ PPARγ function | ||
| Natural killer cells | ↓ circulation | 34,77,78 |
| Neutrophils | ↑ numbers correlated with BMI | 78,79 |
| ↑ inflammatory free radicals upon stimulation | ||
| ↓ response to stimuli | ||
| IFN | ↓ IFN-γ from γδ T cells | 72,80,81 |
| ↓ IFNα/β in TLR-3 stimulated PBMCs | ||
| Interleukins and cytokines | ↑ baseline IL-1β, IL-6, TNFα | 47,76,78,82,83 |
| ↑ IL-5 levels in PBMCs | ||
| ↑ NF-κB activation | ||
| ↓ TNFα, IL-2, and IL-6 in stimulated PBMCs | ||
| Adipokines | ↑ leptin, ↑ leptin resistance | 82,84 |
| ↑ TGFβ in adipose tissue | ||
| ↓ adiponectin |
↑, increased; ↓, decreased; Ag, antigen; IFN, interferon; PBMCs, peripheral blood mononuclear cells.
| Effector . | Impact of obesity . | Reference . |
|---|---|---|
| T cells | ↓ production of naïve T cells | 35,72,73 |
| γδ | ↓ IL-2Rα | |
| CD8+ | ↓ activation markers | |
| CD4+ | ↓ activation markers | |
| B cells | ↓ IL-6 secretion | 38,47 |
| ↓ IgG producing lymphocytes post-vaccination | ||
| ↑ IgM levels | ||
| DCs | ↓ circulation and infiltration p.i. | 32,34,35 |
| ↓ Ag presentation | ||
| ↓ maturity | ||
| Macrophages | Proinflammatory phenotype | 74–76 |
| ↑ IL-6 mRNA | ||
| ↑ oxidative burst activity | ||
| ↓ PPARγ function | ||
| Natural killer cells | ↓ circulation | 34,77,78 |
| Neutrophils | ↑ numbers correlated with BMI | 78,79 |
| ↑ inflammatory free radicals upon stimulation | ||
| ↓ response to stimuli | ||
| IFN | ↓ IFN-γ from γδ T cells | 72,80,81 |
| ↓ IFNα/β in TLR-3 stimulated PBMCs | ||
| Interleukins and cytokines | ↑ baseline IL-1β, IL-6, TNFα | 47,76,78,82,83 |
| ↑ IL-5 levels in PBMCs | ||
| ↑ NF-κB activation | ||
| ↓ TNFα, IL-2, and IL-6 in stimulated PBMCs | ||
| Adipokines | ↑ leptin, ↑ leptin resistance | 82,84 |
| ↑ TGFβ in adipose tissue | ||
| ↓ adiponectin |
| Effector . | Impact of obesity . | Reference . |
|---|---|---|
| T cells | ↓ production of naïve T cells | 35,72,73 |
| γδ | ↓ IL-2Rα | |
| CD8+ | ↓ activation markers | |
| CD4+ | ↓ activation markers | |
| B cells | ↓ IL-6 secretion | 38,47 |
| ↓ IgG producing lymphocytes post-vaccination | ||
| ↑ IgM levels | ||
| DCs | ↓ circulation and infiltration p.i. | 32,34,35 |
| ↓ Ag presentation | ||
| ↓ maturity | ||
| Macrophages | Proinflammatory phenotype | 74–76 |
| ↑ IL-6 mRNA | ||
| ↑ oxidative burst activity | ||
| ↓ PPARγ function | ||
| Natural killer cells | ↓ circulation | 34,77,78 |
| Neutrophils | ↑ numbers correlated with BMI | 78,79 |
| ↑ inflammatory free radicals upon stimulation | ||
| ↓ response to stimuli | ||
| IFN | ↓ IFN-γ from γδ T cells | 72,80,81 |
| ↓ IFNα/β in TLR-3 stimulated PBMCs | ||
| Interleukins and cytokines | ↑ baseline IL-1β, IL-6, TNFα | 47,76,78,82,83 |
| ↑ IL-5 levels in PBMCs | ||
| ↑ NF-κB activation | ||
| ↓ TNFα, IL-2, and IL-6 in stimulated PBMCs | ||
| Adipokines | ↑ leptin, ↑ leptin resistance | 82,84 |
| ↑ TGFβ in adipose tissue | ||
| ↓ adiponectin |
↑, increased; ↓, decreased; Ag, antigen; IFN, interferon; PBMCs, peripheral blood mononuclear cells.
Innate and adaptive immune cells show a pro-inflammatory phenotype at baseline but are also delayed in responding to IAV.30 Macrophages from OB mice show reduced migration to the lung, as well as reduced IFN production upon IAV infection, impairing later adaptive responses.23,25,31 Further, dendritic cells (DCs) may show reduced capability for antigen presentation, thus reducing the activation of T and B cells.32–35 Indeed, obese mice infected with CA/09 H1N1 virus show impaired T cell and B cell responses and poor clearance of IAV infected cells.36–38 Together, the poor innate and adaptive responses fail to control viral spread and lead to increased disease severity and duration observed in obese hosts.
Viral shedding and clearance
Recent studies have identified the co-morbidity of obesity during IAV infection increasing disease duration.39 In two independent studies investigating viral shedding dynamics, obesity was an independent risk factor for increased duration and amount of viral shed.15,40 Obese adults have prolonged IAV shed, with symptomatic obese adults shedding 42% longer than non-obese adults. The phenomenon was more apparent for obese adults with no or only one symptom of infection, as viral shed duration increased 104% vs non-obese adults.15 No association was found for obesity and IBV shed, yet type B viruses are typically of lesser severity in adults and infect proportionally fewer each year, potentially explaining this phenomenon.41,42 Further, viral RNA in fine and coarse aerosols in exhaled breath was positively associated with BMI.40 Together, increased shedding amount and duration may provide more time for potential transmission events.
These data are corroborated by mouse studies. While at peak infection there may be no difference in viral titre, at later timepoints OB mice show higher viral loads vs WT mice.22,25 With a potential increased length of disease, there may also be an increased time for emergence of virulent mutations. Obesity, as with other host immunocompromised states, can directly impact the viral population, potentially through reduced immune pressures and altered lung microenvironment.43,44 Upon serial passage of CA/09 H1N1 virus through OB and DIO mice, the resulting obese-passaged virus induced greater morbidity and replicated to higher titres in WT mice vs lean-passaged virus (R Honce and S Schultz-Cherry, personal communication). Continued studies of how the obese host will uniquely impact IAV population as well as the emergence of antiviral resistance and vaccine escape variants are essential for understanding how the current obesity epidemic will impact travellers and public health worldwide.
Measures of infection control
Vaccines and antivirals remain the standard of influenza virus protection and treatment.8 High-risk adults, including obese adults as well as other immunocompromised states, are urged to receive yearly vaccination.8 Travellers may face an increased risk for IAV infection as worldwide vaccine coverage rates vary (Figure 1) and can lead to higher influenza activity in the destinations visited.45 Vaccination at the start of influenza season is highly recommended for all travellers and of increased importance for obese travellers, as even an imperfect vaccine match can still provide protection against severe disease.46
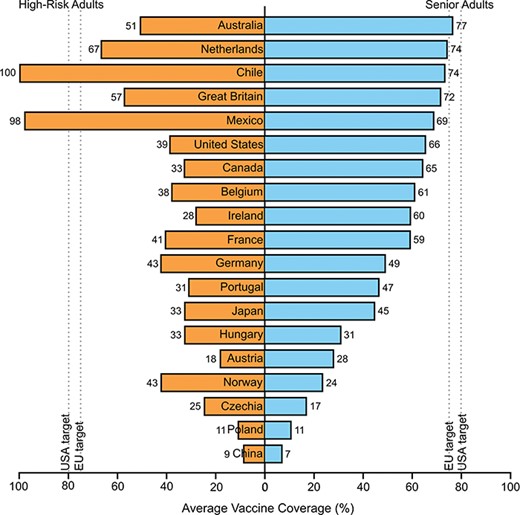
Average vaccination coverage of high-risk adults (left, orange) and senior adults (right, blue) from 2000–01 season to 2016–17 season in selected countries. Vaccination rates vary widely and can impact the level of regional influenza activity. High-risk adults were defined as having chronic illness or one or more co-morbidities, including obesity. Senior adults were defined as adults greater than 50, 55, 60 or 65, depending on country. Dashed lines represent target vaccination coverage for the European Union (75%) and for the United States (80%). Not every country had data for every influenza season. Data compiled from 85–90.
Vaccination efficacy
Comparisons of vaccine efficacy among healthy-weight, overweight and obese adults pre- and post-vaccination found that obese adults had a greater decline in influenza-specific antibody titres 1-year post-vaccination even though initial serological responses showed no significant differences.47 Protective levels of hemagglutination inhibition (HAI) titres were initially obtained from obese adults, but these measures may not be foolproof indicators of vaccine efficacy.47,48 An analysis of peripheral blood mononuclear cells (PBMCs) from overweight and obese adults stimulated with CA/09 H1N1 ex vivo reveals reduced markers of activation and function in CD4+ and CD8+ T cells.35 Some reports suggest the impairment in cellular immunity after both vaccination and natural infection extends to blunted DC maturation and poor antigen presentation and processing.34 Together, these data translate to an increased risk for influenza in the obese population—even those vaccinated—and mirror the findings from epidemiological studies. Vaccinated obese and overweight adults had a 2-fold higher risk of IAV infection vs healthy-weight adults.49 Conversely, vaccinated obese children had similar rates of protection in comparison to those observed in healthy-weight children but suffered increased symptoms upon infection.50
Mechanisms behind impaired vaccine efficacy in mouse models of obesity have found while serological responses may be equal between vaccinated obese and lean mice, OB and DIO mice have reduced survival following IAV challenge. Survival rates were not improved with vaccination, adjuvanted vaccination, increased vaccine dose or decreased viral dose.19,51 Decreased survival was concordant with increased viral titres at days 3 and 5 p.i. and reduced levels, as well as reduced breadth, of IAV-specific neutralizing antibodies.19,51,52 However, current correlates of protection being seroconversion and HAI titres may not adequately predict vaccine efficacy in human cohorts.49,51 Continued studies on vaccine dosage and formulation, and immune responses to vaccines, are required to mitigate the increased IAV infection rates seen in the obese population.53
Antiviral efficacy
Upon infection, antivirals are the current standard of treatment to ameliorate symptoms and prevent severe sequelae. Currently recommended drugs are the neuraminidase inhibitors oseltamivir (Tamiflu), zanamivir (Relenza) and peramivir (Rapivab) as well as the endonuclease inhibitor baloxavir marboxil (Xofluza), with several others in late-phase clinical trials.54 Oseltamivir clears from serum more quickly in obese patients, but not at a biologically significant rate.55–57 Interestingly, reports of increased disease severity due to obesity during the 2009 H1N1 pandemic may have spurred clinicians to prescribe antivirals earlier, therefore preventing additional burden of severe disease.58 The protective capacity of early antiviral administration is highlighted in obese mice. Antiviral administration given concurrently with IAV inoculation boosted survival from 40–80% in obese mice, suggesting that prophylactic treatment for obese travellers may be a viable option if travelling concurrent with an IAV outbreak.20,59,60 However, it is unknown how p.i. treatment with antivirals will reduce viral spread and development of severe sequelae in the obese host.
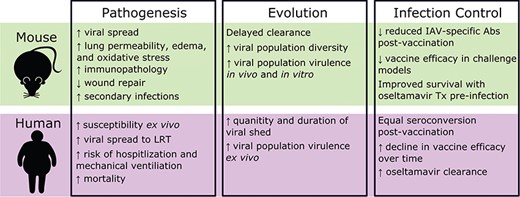
Comparison of influenza pathogenesis, evolution and infection control in mouse models of obesity and human cohorts. Tx, treatment; ↑, increase; ↓, decrease.
Obesity and risk of influenza for travellers
The obesity-induced chronic, low-grade inflammation impairs both innate and adaptive immune responses and results in increased disease severity upon influenza infection (Figure 2). Obese travellers' immune systems, already at heightened risk of encountering a pathogen due to travel, may not be adequately primed to combat influenza virus infection.61,62 Obese travellers must take appropriate precautions prior to international travel, including receiving the seasonal influenza vaccine.63 Obesity is often co-morbid with other diseases that raise risks for infection, including metabolic syndrome and diabetes mellitus, further highlighting the need to prevent infection.64 There are currently no alternative vaccinations recommended for obese individuals, such as the highly immunogenic vaccine recommended for elderly population.53 Since HAI titres wane more quickly in the obese population, using these high-dose vaccines may boost the antibody response and provide greater, longer-lasting protection.47,49 However, using higher dose and adjuvanted vaccines did not boost immunity in mouse models.51 For travellers crossing the equator, differences in circulating viruses may pose additional risks for even those vaccinated. Vaccine selections for the Northern and Southern hemispheres rely upon surveillance data from human infection in the immediately preceding season on the opposing half of the globe.65 However, these up-to-date vaccines may not be locally available to the individual travelling for example, from Europe to Australia, and their local vaccine options not be a match to their destinations' vaccine recommendations.
Influenza virus activity and circulating strains can range widely within and between countries.66 Circulation patterns of IAV subtypes H3N2 and H1N1 vary, as well as differing from IBV circulation. The vaccine uptake and natural infection history of the individual impact the immune response to subsequent infection, and travellers may not have a primed immune system for the dominant strain in their destination of choice.67 Further, cultural differences in human–animal interaction, overall hygiene practises and adventure-based travel plans pose varying risks for zoonotic transmission of animal influenza viruses to travellers visiting those countries.68–71
In summary, obese travellers should take preventive measures during the influenza season. Hand hygiene, avoiding sick people and areas where there are potentially sick animals (for example, live animal markets) and getting the annual influenza vaccine are sound measures of protection. Before travelling, consult your physician and check the status of influenza activity in the destination through the Centers of Disease Control and Prevention or World Health Organization websites. A little prevention can go a long way.
Funding
S.S-C. is supported by ALSAC and National Institutes of Allergy and Infectious Diseases contract HHSN272201400006C.
Conflict of interest: None declared.
Author Contributions
R.H. performed research, wrote original draft and edited final manuscript; S.S-C. secured funding, provided supervision and edited final manuscript.



