-
PDF
- Split View
-
Views
-
Cite
Cite
Jan A. Jacobs, Marc Van Ranst, Biometric Fingerprinting for Visa Application: Device and Procedure Are Risk Factors for Infection Transmission, Journal of Travel Medicine, Volume 15, Issue 5, 1 September 2008, Pages 335–343, https://doi.org/10.1111/j.1708-8305.2008.00232.x
Close - Share Icon Share
Abstract
Biometric fingerprint identity verification is currently introduced in visa application and entry screening at border control. The system implies physical contact between the skin and the surface of the fingerprint‐capturing and reading devices.
To assess the risk of infection transmission through fingerprinting.
The medical literature was reviewed for the potential of microorganisms to be carried on the skin of hands in the community, to be transferred from hands to inanimate surfaces, to survive on surfaces, and to be transferred in doses exceeding the infectious dose. The fingerprinting procedures as currently applied were reviewed.
Factors that favor transfer of microorganisms are large skin–surface contact between flat fingers (2 × 20 cm2) and fingerprint‐capturing device, nonporous contact surface, large overlap of contact surface and short turnaround time between successive applicants, high contact pressure, and difficulties to disinfect devices. Transmission risk exists for enteric viruses (rotavirus, norovirus, and hepatitis A virus), respiratory viruses (respiratory syncytial virus, rhinovirus, influenzavirus, etc.), and enteropathogenic bacteria with low infectious doses (Shigella dysenteriae, Enterohemorrhagic Escherichia coli, etc.). Using Monte Carlo risk analysis on US data, transmission of human rotavirus is estimated at 191 [95% credible intervals (CI) 0–289] per million fingerprint‐capturing procedures. Application of 70% isopropyl hand rub and 85% ethanol hand gel reduces the risk to 77 (95% CI 0–118) and 0.3 (95% CI 0–0.3) transmissions per million procedures, respectively.
The fingerprinting procedure as currently used is associated with a risk of infection transmission. Simple hygienic measures can considerably reduce this transmission risk.
Biometric fingerprint identification is expanding both in the public and in the private sector. One of its applications is identity verification at border control stations, and the European Union currently evaluates fingerprint‐based identity verification for visa application. 1
The procedure is as follows: as part of the interview process during visa application at the embassy, fingerprints of the 10 fingers of the applicant are captured by use of a fingerprint capturer. The image information is digitalized and assigned to the visa document. On entry in the European Union, the right index finger of the visitor is read by a small scanner (index reader), and the digitalized information is compared to the information on the visa document. This procedure verifies if the visitor really is the one who he or she claims to be.
The procedures of fingerprint capturing and reading imply physical contact between the skin and the surface of the hardware devices, and successive applicants are aligning their fingers on the same surface area. Transfer of microorganisms from environmental objects to humans has been described in both the healthcare and the community settings, and hands are known to be the main route of transfer. 2–4 Although most studies report medical devices such as thermometers and stethoscopes as the implicated objects, nonmedical objects such as keyboard covers and ball pens have also been identified as reservoirs of microbial pathogens. 5,6 By consequence, fingerprinting for visa application may be prone to transfer of microorganisms. In terms of healthcare infection transmission, transfer of microorganisms through inanimate surfaces applies to indirect contact transmission, and the objects involved are termed fomites (plural of the Latin fomes, object). 4
We presently studied the risk of transfer of microorganisms through the fingerprinting devices and procedures as currently evaluated by the European Union.
Methods
Sequence of events
The following sequence of events was formulated:
Microorganisms on hands: an applicant carries microorganisms on the skin of the hands when presenting for fingerprint capturing.
Hand–surface transfer: by application of the fingers on the optical surface area of the fingerprinting devices, microorganisms are transferred to the contact surface.
Survival of the microorganisms on the contact surface: part of these microorganisms will die after drying, and others may survive.
Surface–hand transfer: the surviving organisms are picked up by the fingers of the next applicant.
Autoinoculation: the latter applicant brings the microorganisms to the right portal of entry, the mouth, nose, or conjunctivae or occasionally the skin.
The number of organisms that reach the portal is sufficient to cause infection: it reaches the infectious dose, ie, the number of organisms required to cause infection.
Search strategies
Relevant literature was identified from the PubMed online database of the US National Library of Medicine and from infection control textbooks. Predominantly English‐language sources were reviewed. Bibliographies of all relevant papers were searched to identify additional papers. Search terms included transmission, inanimate/nonporous surface and environment, fomites, survival and persistence, hand hygiene, transmission, cross‐infection and cross‐contamination, contact transmission, autoinoculation, hand‐to‐mouth transfer, infectious and infective dose, and exposure. In addition, current editions of guidelines and consensus recommendations on healthcare hygiene from organizations were reviewed (eg, US Centers for Disease Control and Prevention, US Food and Drug Administration, International Scientific Forum on Home Hygiene). 2,4,7
Microorganisms causing person‐to‐person spread, microorganisms with high secondary attack rates, and those requiring contact isolation (and, to a lesser extent, droplet isolation) were withheld. They were evaluated for their capability of surviving on hands and fomites and for their capability and efficiency of being transferred from hand to fomites and vice versa. Further, data on their infectious dose were reviewed, as well as data on the numbers shed by patients and carriers. End point was the identification of those microorganisms that may be transferred through fingerprinting in doses sufficient to cause infection.
Studies on standards of personal hygiene in the community were reviewed to document the carriage of microorganisms on hands in the community and to assess the frequency and efficiency of autoinoculation.
Audit of the fingerprint‐capturing and reading procedures
The visa application office at the Belgian embassy in Kinshasa (RDC) and the border control point at Brussels airport were visited. The capturing and reading procedures were reviewed, and the operating staff was interviewed.
Assessment of risk and of the effect of control measures
Transmission risk combines the numbers of microorganisms transferred and the expected exposure. The exposure depends on geographic, social, and epidemiological factors. We estimated the risk of human rotavirus transmission for adults in the US setting using Monte Carlo sampling to represent the uncertainty of the estimates with 95% credible intervals (95% CI). We used AddRisk that is an add‐in to Excel (2000, v 4.0.5; Palisade Corporation, Newfield, NY, USA). The estimation was based on 1 million fingerprint‐capturing procedures, at a turnaround time of 10 minutes/applicant. Data on transmission and survival of human rotavirus were retrieved from the literature, and for the efficiency of finger–mouth transfer, data on surrogate virus were used. 2,8–11 A 50% (range 30%–60%) hand‐washing rate in the community was assumed. 12,13 Likewise, we assessed the quantitative risk reduction of hand washing (water and soap) and hygienic hand disinfection with 70% isopropyl liquid hand rub and 85% ethanol hand gel after fingerprint capturing using log reduction rates reported from laboratory experiments. 14,15
Results
The fingerprinting devices and procedure from an infection control standpoint
The fingerprint capturer (LS2 Check; Smith Heimann Biometrics, GmbH, Jena, Germany) contains an optical reader covered by an optical surface area. This area consists of a glass plate of approximately 10 × 10 cm surrounded at three sides by a plastic board that creates a U‐shaped space for application of the fingers (Figure 1A and B). Between the glass plate surface and the edges of the board, there is a split of approximately 1 mm height and 5 mm depth (Figure 1B). The index reader (Sagem MS100; Sagem, Paris, France) is a device the size of a computer mouse, with a sunken area to accommodate a single finger pad. On the index reader used at Brussels airport, leftovers of multiple fingerprints were clearly visible (Figure 1C).
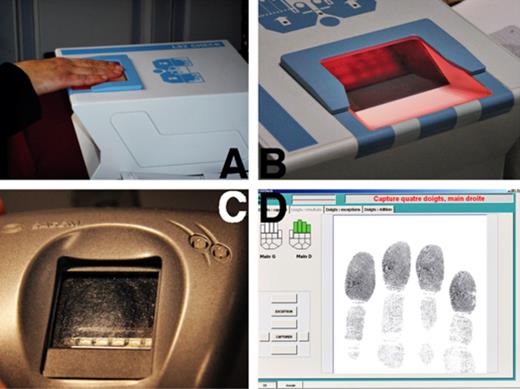
Fingerprint devices. (A) Fingerprint capturer: flat fingers of the right hand are scanned. (B) Fingerprint capturer: plastic board surrounds the fingerprint capture plate. Between the board and the plate, there is a split of approximately 1 mm height and 5 mm depth. (C) Index reader: leftovers of previous prints are clearly visible. (D) Real‐time image of (A) as displayed on the computer screen.
Fingerprint capturing involves subsequent application of the four digits of the right hand, those of the left hand, and both thumbs. The applicant places his flat fingers (finger pads as well as phalanges) on the glass plate (Figure 1A and D), resulting in a skin–surface contact area of approximately 20 cm2/hand. The overlapping contact area between the fingers of the successive applicants is very large. For capturing, a considerable pressure has to be exerted (higher than when shaking hands), and the total time of finger to surface contact for scanning 10 fingers in three capturing sets is approximately 20 seconds. Index reading takes 1 to 2 seconds, requires only a slight pressure, and involves a small contact surface (1–2 cm2).
At Brussels airport and at the embassy in Kinshasa, there was no procedure on how to deal with obvious skin defects, cuts, or abrasions. Fingers with uncovered skin defects were scanned in an identical way to fingers with intact skin. No cleaning and disinfection procedures were available, and no facilities for hand washing were present in the fingerprint‐capturing room. The operator’s manual instructions mentioned wiping off the fingerprint capturer’s glass plate once daily before use to remove latent images that are caused by leftover prints from users the day before. 16 According to the operating staff, the foreseen numbers of applicants at visa application imply at times and places a short turnaround time with delays of less than 10 minutes elapsed between two successive applicants.
Possibilities of transfer of microorganisms
Microorganisms present on visibly clean hands
Numerous studies have counted bacteria on healthcare workers’ hands, but only a few assessed bacterial loads on hands in the community. Mean log colony‐forming units (CFU) counts of 5.72 and 5.70 on a single hand were found in two recent studies of hand flora in the community. Staphylococcus aureus was present on 18.5% of hands and Gram‐negative bacteria in 75.1%. 17,18 Among primary school children, fecal contamination of fingers reached 7%, and on hands of housewives, fecal bacteria were found in densities of 300 CFU/cm2. 19,20 Most bacteria survive at least more than 30 minutes on the hands. 21
Ample epidemiological evidence of survival of viruses on hands is present for human rotavirus, hepatitis A virus, astrovirus, adenovirus, rhinovirus, respiratory syncytial virus (RSV), and influenzavirus. 9,21–23
Hand washing in the community does not meet the recommended standards of frequency and technique. Hand‐washing compliance rates after restroom use varied between 37 and 61%, with as few as 8% of observed individuals using soap and 2% washing hands for more than 10 seconds. 12,13 After changing a dirty diaper, observed childcare providers washed hands on only 42% of occasions. 24
Survival of microorganisms on visibly clean, nonporous surfaces
Most microorganisms survive long enough on fomites to be transferred through fingerprinting. 25 In general, they tend to survive better on nonporous surfaces (plastic, china, glass, and stainless steel) than on paper and fabrics (cotton, terry, and blends). 8,26,27 Survival is influenced by temperature, relative humidity, and the presence of organic material but not to an extent that is of critical importance in the short delays of the fingerprint setting. 25 Among the viruses, notably though survivors are the nonenveloped viruses (human rotavirus, hepatitis A virus, astrovirus, enteric adenovirus, and poliovirus), hepatitis A virus and human rotavirus combine long survival spans (60 d) with decay rates of only 10‐ and 100‐fold, respectively. 8,28,29 Many bacterial pathogens survive for days to weeks. 25
Transfer of microorganisms from hands to surface and vice versa
Microorganisms can be readily transferred from fomites to hands and vice versa. Compared to porous surfaces, nonporous surfaces have higher transfer efficiencies: holding a telephone receiver resulted in a 38% to 60% object‐to‐hand transfer efficiency compared to a <0.01% transfer rate from cotton to hand. 11 Residual moisture (such as after hand washing without drying) enhances hand–fomite transfer. 11
Autoinoculation
Occasionally, the microorganisms that are picked up may penetrate the skin via small cuts or abrasions. In most cases, however, the contaminated person may bring them unwittingly to the mouth (gastrointestinal infection) or the conjunctivae and nasal mucosa (respiratory tract infection). Most organisms remain long enough (at least 30 min) on the hands to allow autoinoculation. Autoinoculation may occur by eating (without prior hand washing) or by practices such as nose picking, eye rubbing, page licking, and nail biting. These practices appear to be common: one in three students rubbed their eyes during a 1‐hour lecture, 30 1 in 10 healthcare professionals licked the index finger when paging through a clinical chart, 31 approximately 45% of questioned adolescents reported to be nail biters, and nearly all adolescents surveyed admitted to picking their nose. 32,33 For bacteria as well as for viruses, autoinoculation showed transfer efficiencies of 30% to 40%. 11
Microorganisms that may be transferred through fingerprinting
Microorganisms entering by the oral route
For the adenovirus types 40 and 41, astrovirus, norovirus, and human rotavirus (all causing gastroenteritis), as well as for hepatitis A virus, strong epidemiological and laboratory evidence of transfer through fomites is available. 2,3,7–9,11,22,29,34–38 Based on a theoretical rationale (numbers of organisms shed, survival on hands and on fomites, and infectious dose) and epidemiological evidence (person‐to‐person spread), transfer of Shigella dysenteriae, Enterohemorrhagic Escherichia coli, Enteroinvasive E coli, and Salmonella typhi in numbers exceeding the infectious dose is also possible. 7,25,27,39–43 Intestinal protozoa such as Cryptosporidium spp. (oocysts), Giardia lamblia (cysts), and helminths such as Enterobius vermicularis (egg) meet the requirements for transfer through fingerprinting, and the role of fomites in their transmission has been described. 7,44
Microorganisms entering by the respiratory route
Strong epidemiological evidence in favor of transfer through fomites is present for adenovirus, herpes simplex virus type 1 (HSV1), and RSV. In addition, both experimental and laboratory data have been provided for parainfluenza virus, rhinovirus, and influenzavirus (including the H5N1 strain). 3,4,34,45–50 Severe acute respiratory syndrome–associated coronavirus is possibly transmitted through fomites: high secondary attack rate events (“super spreading events”) suggest possible fecal‐oral transmission with contamination of the environment. 51,52 A number of bacterial pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, Bordetella pertussis, and Corynebacterium diphtheriae can survive on fomites, and transmission through articles soiled with respiratory discharges has been described. However, their capacity for survival on fomites is poor, and evidence for indirect contact transmission had not been thoroughly documented. 25,34
Microorganisms entering via the skin
Finger lesions caused by HSV1 (herpetic whitlow) are mainly transmitted by direct contact, but indirect transmission is not excluded as the virus survives drying on fomites. 25
Microorganisms causing secondary infections
A number of bacteria and yeasts may cause respiratory tract, wound, and urinary tract infections, especially in hospitalized patients. They include, among others, S aureus, Enterobacter spp., E coli, Klebsiella spp., Serratia spp., Pseudomonas aeruginosa, Enterococcus spp., and Candida albicans. From a technical standpoint, their capability of being transferred through fingerprinting is excellent: they survive for extended periods on fomites (especially in the presence of organic material) and persist for at least 30 minutes on hands. 25 Laboratory experiments have demonstrated hand–fomite transfer and vice versa for C albicans and Enterococcus faecium. 53,54 Further, these microorganisms have been implicated in common source outbreaks related to medical, household, or administrative equipment. The exact role of fomites in these outbreaks is difficult to ascertain, but in a number of cases, molecular techniques proved strain identity between the infecting strain and that present on the fomite. 4,36 Although infection transmissions through fomites remain rare events in the healthcare setting, several points are of concern: (1) many of the bacterial species may develop resistance to antibiotics and (2) these multidrug‐resistant strains are spreading from hospitals to nonhospital care facilities (nursing homes and long‐term care facilities) and next to the community. 55,56
Transmission risk and health impact
The transmission risk of an infection caused by a particular microorganism combines the capability of transfer of this microorganism through fingerprinting in doses exceeding the infectious dose and the probability of exposure, ie, the probability that a person shedding the organisms will present for fingerprinting. In the absence of a particular outbreak, highest probabilities of exposure can be expected for (1) enteric viruses such as rotavirus and (2) respiratory viruses such as RSV, influenzavirus, and parainfluenzavirus. Other microorganisms occur in epidemics that are notoriously difficult to contain, eg, norovirus (gastroenteritis in hotels and cruise ships), adenovirus (conjunctivitis in the healthcare setting), and S dysenteriae (dysentery in large‐scale epidemics). 38,42
The health impact of a disease also differs according to local epidemiology. Worldwide, infections with human rotavirus causes have a case fatality rate of 0.4%, but in industrialized countries, it is as low as 0.03%. 10 Another example is hepatitis A infection: where environmental sanitation is poor, infection caused by hepatitis A is common and occurs at an early age. At this age, the disease is benign. In countries with high hygienic standards, most adults have never been infected and remain susceptible to infection, and persons aged 50 years or older have an increased risk of complications. 38
Using Monte Carlo risk analysis on US data, the mean number of transmissions of human rotavirus per million fingerprint‐capturing procedures among adult applicants was estimated as 191 (95% CI 0–289). When, after fingerprint capturing, hands are washed with soap and water, this does not result in a marked decrease of transmission (189 transmissions, 95% CI 0–277). Application of 70% isopropyl hand rub and 85% ethanol gel reduces the risk to 77 (95% CI 0–118) and 0.3 (95% CI 0–0.3) transmissions per million procedures, respectively. Due to the short contact time, small surface, and low pressure, index reading has a transmission risk that is approximately 100‐ to 1,000‐fold lower than that of fingerprint capturing.
Discussion
In this study, we demonstrated that, based on the current knowledge of indirect contact transmission, the fingerprinting procedure offers opportunities for the transfer of organisms. Microorganisms that can be transferred in quantities exceeding the infectious dose include enteric and respiratory pathogens. The transmission risk of human rotavirus in the US setting is estimated at 191 (95% CI 0–289) per million fingerprint‐capturing procedures.
Biometric identification systems are based on distinctive personal traits, such as voice, fingerprint, face, iris, and signature. Fingerprint‐based systems are known for their high discriminative capability and have a long‐standing forensic tradition. They are increasingly accepted by the general public: about 9 in 10 people who were fingerprinted for identification purposes felt it was an appropriate requirement. 57 In recent years, the systems have been technically perfected, and devices and computing power have become more economically viable. As a consequence, applications are expanding in both the public and the private sector. Most applications imply person identity verification in security infrastructures, financial transactions, and retail sales. Fingerprint verification is already part of the process of entry of asylum seekers and refugees at the European Union, and the Council Decision 2004/512 EC provides the legal framework for integrating fingerprint biometric data in all future European Union visa applications. 1,58
It was beyond the scope of this study to consider an exhaustive list of all microorganisms. Some microorganisms may become important in case of an outbreak. For instance, poliovirus is transmitted very efficiently by indirect contact but was presently not considered because of the low worldwide incidence. For some microorganisms, the numbers shed and their survival on fomites is not known, and for others, conflicting data on infectious doses are reported. 50 In parallel, there are limitations on the quantitative risk estimations we presented, eg, assumptions were made with regard to the efficiency of finger‐to‐mouth transmission of human rotavirus and the efficacy of hand rubbing by the general public. In addition, most episodes of human rotavirus infection in adults are asymptomatic or mild. On the other hand, asymptomatic rotavirus patients contribute to transmission, and host factors such as age, underlying diseases, nutrition status, and medication will worsen the clinical picture considerably. 22,59 In Western societies, approximately 20% of the population can be classified within such an “at‐risk” group. 60
Further, it should be noted that in case of an ongoing epidemic, organisms such as norovirus, S dysenteriae, and adenovirus will cause higher transmission rates than those presently estimated for human rotavirus. In addition, there is ongoing debate on lowering the age for compulsory fingerprinting from the currently regulated 12 years to 6 years or even lower. 61 Regardless of ethical and political issues, it is clear that fingerprinting of infants and children will both augment the number of transmissions (due to higher numbers of applicants shedding pathogens) and increase the severity of clinical infections (due to higher host susceptibility). Finally, it is clear that the overall transmission risk of fingerprint capturing is a compilation of the risk of all particular organisms implicated.
One might question whether the above cited studies on transfer of microorganisms are applicable to the fingerprinting devices. The laboratory transfer studies on hepatitis A virus and rhinovirus were conducted by a finger pad experiment, in which metal disks were contaminated with 10 μL viral suspensions in concentrations equal to those in affected patients. After drying, these disks were applied to finger pads, with contact surface and contact times of 1 to 3 cm2 and 5 to 10 seconds, respectively. Transmission efficiency ranged from 0.7% to 16%, and the quantities transferred were invariably higher than the infectious dose. 23,35 In terms of transmission efficiency, the conditions of fingerprint capturing (2 × 20 cm2 and 20 s, respectively) are even more favorable. The high contact pressure during fingerprinting is an additional factor: experiments showed that a high pressure (1.0 kg/cm2, as with hand shaking and opening of doors) resulted in a three times higher amount of hepatitis A virus transfer compared to low (0.2 kg/cm2) pressure. 35 In addition to these laboratory experiments, a “real‐life” study illustrated the potential of transfer through hand–fomite contact: in a flat shared by four students, a surrogate virus was applied on the door handle of the living room. It was spread by the occupiers to nearly all the important contact points in the surrounding area, including the telephone, the computer trackball, the light switches in the kitchen and the bathroom, and the handles of the refrigerator and the teapot. Within 6 hours, all occupants as well as visitors were infected. 62 Another study examined the houses of infants who were recently vaccinated for polio (during which time shedding occurs in feces): the presence of virus was demonstrated in up to 13% of bathroom, living room, and kitchen sites. Most frequently contaminated were hand contact sites such as bathroom taps, door handles, toilet flushes, liquid soap dispensers, diaper‐changing equipment, and potties. 24
In view of the ubiquitous prevalence of some of the pathogens, one might argue that the risk of transmission through fingerprinting is only similar to the risk of touching other fomites in daily life (door knobs, taps, bus rails, etc.) and that, by consequence, control measures are obsolete. In addition, the quantitative risk of infection through fingerprint capturing can be neglected in view of the risk of, for instance, travelers’ diarrhea. Although it is difficult to compare the risks, we think that in daily life, the particular conditions of fingerprinting (large contact surface, large overlap, high contact pressure, and long contact time) are not frequently met. Moreover, anecdotal evidence linking acquisition of hospital pathogens to fomites in the community raises the issue of implementation of control measures in certain community settings. For instance, carriage of multiresistant S aureus in a nurse was linked to contamination of a computer desk and joystick at home and not eliminated until the computer‐related hardware was decontaminated. 63 Finally, fingerprinting in the present setting is a mandatory act (without fingerprinting, visa application is refused) for which risks as perceived by the public are higher compared to voluntary acts. 64
Quantitative microbial risk assessment in (food) microbiology often relies on Monte Carlo analyses. 65,66 Monte Carlo analysis provides numerical expressions of risk with an indication of the uncertainties and allows assessment of the quantitative impact of control and prevention measures. One of the possible limitations is that it needs precise probability distributions for all input parameters, and it is difficult to use when rare events may have a major effect. 67
Monte Carlo analysis revealed the effect on risk reduction of alcohol‐based hand rub. In this setting, hand disinfection was considered after—and not before—the procedure because we assumed compliance by the public to be higher after the procedure and because the process of fingerprint capturing does not perform well with freshly cleaned hands. Hand washing with water and plain soap has virtually no activity against nonenveloped viruses, and antimicrobial soaps based on chlorhexidine or triclosan have only a limited activity. 21 Alcohol‐based hand rubs have superior activity and are better tolerated than antimicrobial soaps. Their use is simple: approximately 3 mL of alcohol rub is applied in the palm of the hand. Next, the hands are rubbed together covering all the surfaces of the hands, and hands are further rubbed until they are dry. There is no need for running water, sink, or drying facilities. Compared to the other alcohols such as isopropyl alcohol, ethanol in high concentrations had a superior activity on nonenveloped viruses and retains enough activity against bacterial pathogens. 21
Of course, hand disinfection is only part of a coherent prevention strategy. In that regard, we are presently studying the feasibility of other control measures including training and education of the staff, procedures in case of skin defects, and disinfection of the devices. In addition, the performance and acceptability of hand rubbing by the general public will be assessed as well as the compliance with the control measures by the operating staff. On medium‐length term, improvements in design and construction of the devices are expected. The split under the U‐shaped plastic board of the present fingerprint capturer hinders cleaning and allows the buildup of dirt and moisture, which inactivates disinfectants and allows microorganisms to survive. Newly designed devices should also withstand the corroding or decolorizing effects of disinfectants. Other possibilities should be technically explored, such as disposable transparent covers over the contact surface or a removable surface contact plate that can be detached and soaked into a disinfecting solution. Alternatively, so‐called touchless systems (which capture fingerprints without making physical contact with the optical surface) might be considered.
In conclusion, based on a literature review on indirect contact transmission, both the fingerprinting devices and the procedure allow for transfer of microorganisms in doses exceeding the infectious dose. Substantial risk reduction can be achieved by implementation of simple infection control measures.
The study has been supported financially by the Biometric Data Experimented in Visas (BIODEV) project of the European Union. The contribution was purely financial; there was no role in any of the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Declaration of interests
J. A. J. received reimbursement from BIODEV for attending the International Conference of Biometrics and Ethics, Washington, DC, November 28 to 29, 2006. Otherwise, the authors state that they have no conflicts of interest.
References
Available at: http://rivm.openrepository.com/rivm/bitstream/10029/9385/1/149106007.pdf. (Accessed 2007 Dec 16)
This study has been presented at the International Conference on Biometrics and Ethics, Washington, DC, November 28 to 29, 2006.



