-
PDF
- Split View
-
Views
-
Cite
Cite
E Abou Chawareb, S Lumbiganon, M Hammad, J Miller, O Raheem, Y Reisman, I Gruenwald, D Lischinsky, F Yafi, SAFETY AND EFFICACY ASSESSMENT OF THE VERTICA® - A RADIO FREQUENCY DEVICE FOR THE TREATMENT OF ERECTILE DYSFUNCTION (ED), The Journal of Sexual Medicine, Volume 22, Issue Supplement_2, May 2025, qdaf077.106, https://doi.org/10.1093/jsxmed/qdaf077.106
Close - Share Icon Share
Abstract
The VERTICA® is a hand-held, home use device, designed to treat “mild to moderate” erectile dysfunction (ED) using radiofrequency (RF) electrodes. These electrodes deliver sequential RF energy to the penile area, with additional electrodes on a pad applied to the perineal area to also cover the penile crus. We hypothesize that RF energy raises penile temperature, enhancing erectile response, and RF energy posits a direct structural and biochemical impact on tunical and corporal tissue, improving the tunical veno-occlusive mechanism. The purpose of the current study is to evaluate the safety and efficacy of the VERTICA® investigational device for treatment of ED.
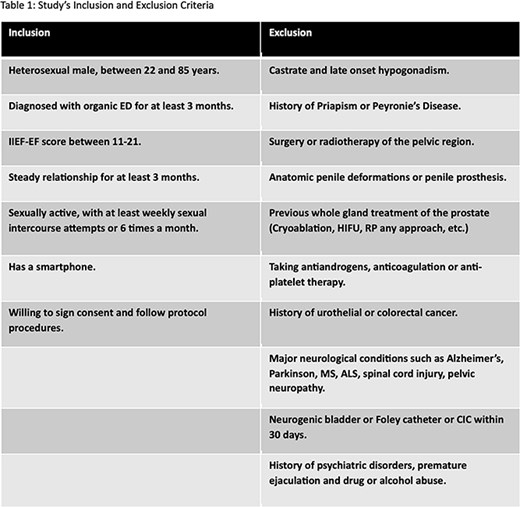
This study is a pivotal, prospective, randomized, controlled, double-blind clinical trial. Around 100, in our center 33 adult, heterosexual, and sexually active males (>21 years) with mild to moderate organic ED (IIEF EF score 11-21) will be enrolled and randomized 1:1 for Active or Sham VERTICA® treatment. Patients are instructed to use the device 3 times a week for 4 weeks, twice a week for an additional 4 weeks and then 1-2 times a week for another 4 months. Compliance data is monitored using a smartphone app. Subjects are instructed to attempt sexual activity weekly or 6 times a month and fill a Sexual Encounter Profile(SEP) form. Monthly follow-up assessments at months 1, 2, 3, and 6 include evaluating the efficacy of the treatment using the IIEF-EF, SEP, EHS, EDITS, and SHIM questionnaires. Enhancements in ED will be measured by comparing the scores of these questionnaires within both groups and, for every individual, before and after completing the study.
A pilot study with 28 subjects demonstrated the device’s safety and efficacy, with no serious adverse events reported in 144 treatment sessions.
VERTICA® device shows promise in the treatment of mild to moderate ED based on the pilot study and the ongoing trial. If the results of this trial are promising, VERTICA® could offer a novel and effective treatment option for men with mild to moderate ED, potentially improving their quality of life and sexual health.
FAY: Vertica: Research Investigator.