-
PDF
- Split View
-
Views
-
Cite
Cite
A Salonia, C W, E A Jannini, A COMPARATIVE EVALUATION OF PHOSPHODIESTERASE-5 INHIBITORS IN ERECTILE DYSFUNCTION TREATMENT: A SYSTEMATIC REVIEW AND NETWORK META-ANALYSIS OF DOUBLE-BLINDED, PLACEBO-CONTROLLED, RANDOMIZED TRIALS, The Journal of Sexual Medicine, Volume 22, Issue Supplement_2, May 2025, qdaf077.073, https://doi.org/10.1093/jsxmed/qdaf077.073
Close - Share Icon Share
Abstract
Erectile dysfunction (ED) is a multifaceted condition with underlying psychogenic, neurogenic, vascular, and hormonal factors, affecting 10–48% of global male population. The European Medicine Agency (EMA) has approved four phosphodiesterase-5 inhibitors (PDE5is), primarily sildenafil, tadalafil, vardenafil, and avanafil in different dosage forms, as the treatment for men with ED. This study aimed to establish a comparative efficacy and safety profile of the four PDE5is for treating men with ED.
A comprehensive search was performed in PubMed-Medline, EMBASE, Scopus, Cochrane CENTRAL, and other databases to identify randomized, double-blinded, placebo-controlled trials on the management of ED with PDE5is. Studies reporting changes from baseline using International Index of Erectile Function (IIEF) domains, Global Assessment Question (GAQ)/Global Efficacy Questions (GEQ), and Sexual Encounter Profile (SEP) questionnaires were prioritized. Only on-demand dosages were selected for network meta-analysis (NMA), and the primary outcomes considered were 1) the proportion of participants achieving satisfactory erectile function (EF), defined as an IIEF–EF domain score > 26 and 2) the proportion of participants achieving no ED status, based on either the IIEF–EF domain or relevant questions from the GAQ, GEQ, or SEP 2 and 3.The risk of bias of the included studies was performed using the Cochrane Risk of Bias tool.
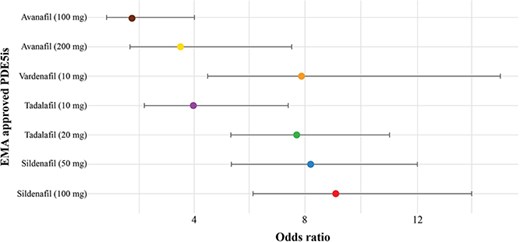
The NMA included 83 double-blinded, placebo-controlled, randomized trials reporting the efficacy and safety of four EMA-approved PDE5is. Sildenafil 100 mg significantly increased the odds of achieving normal EF by approximately nine times compared to placebo, indicating superior efficacy (Fig. 1). A dose–response relationship was evident for sildenafil and tadalafil. Treatment-related adverse events were also dose-dependent, with sildenafil and tadalafil presenting a higher rate compared to vardenafil and avanafil.
These findings suggest that sildenafil, particularly 100 mg, is the most effective PDE5i for achieving satisfactory erectile function and recovery from ED. These findings underscore the need for individualized treatment strategies to balance efficacy and safety.
AS, CW have no conflict of interest. EAJ have been a paid speaker and paid consultant for several pharmaceutical companies including Bayer, Ibsa, Menarini, Recordati, FQM, Otsuka, Kanna, Pfizer and Viatris.