-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea R Thurman, Isabella Johnson, Katherine A Cornell, Jessica Hatheway, Noel N Kim, Sharon J Parish, Clint Dart, David R Friend, Andrew Goldstein, Safety of topical sildenafil cream, 3.6% in a randomized, placebo-controlled trial for the treatment of female sexual arousal disorder, The Journal of Sexual Medicine, Volume 21, Issue 9, September 2024, Pages 793–799, https://doi.org/10.1093/jsxmed/qdae089
Close - Share Icon Share
Abstract
There are currently no Food and Drug Administration–approved treatments for female sexual arousal disorder (FSAD), which is physiologically analogous to male erectile dysfunction.
The study sought to test the systemic and local genital safety of topical sildenafil cream, 3.6% (sildenafil cream) among healthy premenopausal women with FSAD and their sexual partners over a 12-week treatment period.
This was a phase 2b, exploratory, randomized, placebo-controlled, double-blind study of sildenafil cream among healthy premenopausal women with FSAD. Safety was assessed by the frequency and incidence of treatment-emergent adverse events (TEAEs) among participants and their sexual partners. Participants recorded the incidence of TEAEs in a daily eDiary (electronic diary). Sexual partners were contacted within 72 hours of each sexual event in which investigational product was used. All participants used placebo cream for 1 month, during a single-blind run-in period, and then if eligible, were randomized 1:1 to sildenafil cream or placebo cream. Participants used their assigned investigational product over a 12-week double-blind dosing period. They attended monthly follow-up visits, in which their eDiary TEAE data were reviewed by the study staff and graded for severity and relationship to study product.
The frequency and incidence of TEAEs among participants and their sexual partners.
During the 12-week double-blind dosing period, there were 78 TEAEs reported by 29 of 99 sildenafil-assigned participants and 65 TEAEs reported by 28 of 94 placebo-assigned participants (P = .76). All TEAEs were mild or moderate in severity. The most common treatment-related TEAE among active and placebo-assigned participants was application site discomfort. There were no differences in the number of treatment-related TEAEs among sildenafil cream vs placebo cream users (P > .99). Four sildenafil cream participants and 3 placebo cream participants discontinued the study due to TEAEs involving application site discomfort (P > .99). There were 9 TEAEs reported by 7 of 91 sexual partners exposed to sildenafil cream vs 4 TEAEs reported by 4 of 84 sexual partners exposed to placebo cream (P = .54).
These data support further clinical development of topical sildenafil cream for the treatment of FSAD.
Safety was assessed among participants and their sexual partners after 1357 and 1160 sexual experiences in which sildenafil cream or placebo cream were used, respectively. The phase 2b study was powered for the primary objectives of efficacy, rather than safety.
These data demonstrate that topically applied sildenafil cream was safe and well tolerated by exposed users and their sexual partners.
Introduction
Female sexual arousal disorder (FSAD) is a persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate lubrication-swelling response of sexual excitement that causes marked distress or interpersonal difficulty.1,2 FSAD is estimated to negatively impact approximately 20% of women in the United States.3-5 Lack of arousal commonly leads to a lack of interest or desire because sexual activity is not enjoyable or reinforcing.6,7 Despite the high prevalence of FSAD and the potential impact it has on other aspects of sexual function in women, to date there are no U.S. Food and Drug Administration–approved pharmacological treatments for FSAD. Most commonly, treatment involves the administration of topical lubricants that help to mask the lack of vaginal lubrication associated with FSAD but are ineffective in enhancing genital/clitoral blood flow or alleviating the decrease in genital sensations that accompany FSAD.8
Paralleling the mechanism of penile erection in men, sexual stimulation in women leads to the release of nitric oxide (NO) from peripheral nonadrenergic, noncholinergic nerve terminals and endothelial cells.9 The diffusion of NO into the adjacent vascular smooth muscle cells stimulates the production of cyclic guanosine monophosphate (cGMP) by guanylyl cyclase, initiating a series of signaling cascades that result in smooth muscle relaxation allowing for increased genital engorgement.10 Sildenafil citrate acts by inhibiting cGMP-specific phosphodiesterase type 5, an enzyme that causes degradation of cGMP, a second messenger that regulates NO-mediated vasodilation in the corpus cavernosum of the penis.10 Immunohistochemistry data confirm that the NO-cGMP dependent phosphodiesterase type 5 isoenzyme is also expressed in the vascular smooth muscle cells of the corpus cavernous clitoris, the vagina, and the labia minora.11-16
Oral sildenafil citrate (Viagra) has been approved since 1998 for the treatment of erectile dysfunction (ED). Despite multiple attempts to demonstrate the efficacy of oral sildenafil citrate for the treatment of female sexual dysfunction (FSD) (including heterogeneous FSD populations consisting of women with hypoactive sexual desire disorder, female orgasmic disorder, and FSAD), statistically significant therapeutic effectiveness has only been shown in clearly defined subpopulations,17-19 and published placebo-controlled trials of oral sildenafil citrate administered to pre- and postmenopausal women afflicted with broad-spectrum FSD demonstrated marginal efficacy vs placebo, with a high incidence of systemic side effects.20-23
Therefore, topical sildenafil cream, 3.6% is being developed for the treatment of FSAD in women. The hypothesis is that by delivering sildenafil topically, specifically targeting genital anatomy central to the vascular arousal response, there will be less systemic exposure, followed by less systemic side effects, resulting in a more favorable safety profile. The safety and pharmacokinetics of topical sildenafil cream, 3.6% (sildenafil cream) were previously tested among normal healthy men and women (SST-6006-001-01 and SST-6006-008-01, data on file). The safety data for this Phase 2b study are reported in this article, while the primary efficacy data are reported separately.24
Methods
Clinical Study
The clinical trial was conducted at 49 sites in the United States and was approved by the Advarra Institutional Review Board (Pro00049161). Healthy premenopausal women 18 years of age or older and their sexual partners were screened. Volunteers and their sexual partners provided written informed consent prior to the performance of any study-related procedures. Women at risk of pregnancy used effective contraception during the study. Condom use was allowed for sexually transmitted infection prevention. Women or their partners were excluded if they had uncontrolled hypertension, a history of serious cardiac events, orthostatic hypotension, or other serious medical comorbidities. The full list of inclusion and exclusion criteria can be found at https://clinicaltrials.gov/study/NCT04948151. Table S1 outlines the schedule of evaluations, which was previously reported.24
In brief, volunteers and their sexual partners underwent informed consent and screening safety procedures at visit 1. After screening, a one-on-one clinical interview was conducted with each potential participant, using interviewers who were not employees of the study sponsor, in order to establish the diagnosis of FSAD and verify that FSAD was the woman’s main sexual dysfunction concern if other concomitant sexual dysfunction diagnoses or symptoms existed. Participants then entered a 28-day no drug run-in period, starting at visit 2, followed by a 28-day single-blind placebo run-in period, starting at visit 3. Participants were screen failed during these 2 months if they were not compliant with recording sexual experiences and AEs in the electronic diary (eDiary), or if they had a predetermined placebo response on the 15-question Female Sexual Distress Scale–Desire, Arousal, Orgasm.24 Eligible participants were randomized at visit 4 in a 1:1 manner to sildenafil cream vs placebo cream. The double-blind dosing period lasted 12 weeks, with monthly assessments at visits 5 (month 1), 6 (month 2) and 7 (month 3).
Randomization
Participants were randomized following the single-blind placebo run-in period using concealed interactive response technology. There were no allocation errors.
Study Product
The placebo cream contained the same ingredients as in the sildenafil cream but without the active ingredient, sildenafil citrate. The investigational product (IP) was stored at room temperature (20-25 °C) in a secure, temperature- and humidity-monitored area.
IP Dosing
Each month, the IP was dispensed in a 30-g tube, along with nine 2-g dosing cards. Participants were instructed to apply no more than one 2-g application of product per 24 hours and no more than 9 applications per month. The IP was applied approximately 10 to 20 minutes prior to the sexual event. One gram was applied externally to the anterior labial commissure, prepuce of the clitoris, glans, frenulum, vestibule, and labia minora. The other 1 g was applied to the anterior distal vagina to an approximate depth of 0 to 3 cm or about halfway between the distal and proximal interphalangeal joints, targeting the distal, lower one-third of the vagina. Use of over 9 applications per month was recorded as a protocol violation.
Safety Assessments
Participants were prompted daily to recorded TEAEs in the eDiary. The eDiary also prompted the participant every 24 hours to assess whether there had been a sexual event. If there was a sexual event, the eDiary provided the following prompts to the participant and/or her partner: (1) “Since your sexual event, do you have any complaints?” If the participant or partner answered “YES,” the eDiary would further prompt them with some of the more common side effects associated with oral sildenafil or genital creams, such as “Have you experienced any local irritation or discomfort?” Responses from participants who reported a TEAE following sexual activity were sent to the site electronically for evaluation and triage within 24 hours.
If the participant confirmed via the eDiary that a sexual event had taken place with her partner, study site staff contacted the partner within 72 hours of the event to confirm whether or not the partner had any complaints, and if so, the partner was further queried on common side effects associated with oral sildenafil.
The eDiary data was formally reviewed at each monthly follow-up visit. At these visits, the severity of adverse events and TEAEs and relatedness to study product or study procedures was determined by the investigator.
Orthostatic vital signs were obtained on the participant at each visit, with heart rate and blood pressure measured in the supine position, and 1 and 3 minutes after standing. The same orthostatic vital signs were measured among participating sexual partners, either at the clinic or via telemedicine at each visit.
Sample Size and Statistical Analysis
The sample size of this study was based on the co-primary product efficacy endpoints. The safety analysis population consisted of all women who applied IP at least once during the study, and therefore TEAEs were reported. The intention-to-treat (ITT) population consisted of women who were enrolled in the study at visit 4, were randomized, and received at least 1 dose of their assigned product during the double-blind dosing period. The frequency of TEAEs were described according to product group, relatedness to IP use or study procedures, and severity. TEAE frequency was compared by treatment group using the chi-square statistic or Fisher’s exact test, based on expected cell size. Vital sign data were normally distributed and therefore compared by visit and timing/position of the measurement (eg, supine) between the sildenafil cream and placebo cream groups using an independent samples t test. P values <.05 were considered significant.
Results
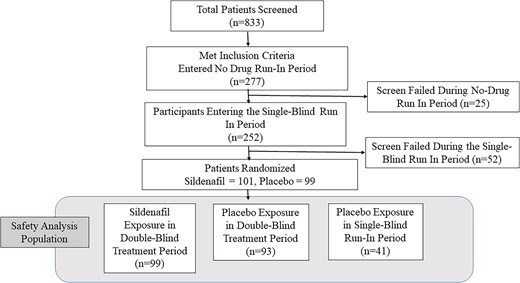
As shown in Figure 1, volunteers (n = 833) and their sexual partners (n = 605) underwent informed consent. Two hundred participants were randomized to either sildenafil cream (n = 101) or placebo cream (n = 99), and 99 sildenafil cream–assigned women and 94 placebo cream–assigned women received at least 1 dose of their assigned IP in the double-blind dosing period, representing the ITT population. An additional 41 women, who were exposed to at least 1 dose of placebo cream during the single-blind placebo run-in period but did not advance to becoming part of the ITT population for the double-blind dosing period, were also included in the safety analysis population, bringing the total to 134 placebo-exposed participants. Table 1 displays the demographics and baseline characteristics of subjects included in the safety analysis population.
Demographic and baseline characteristics of safety population participants and sexual partners.
| Participant . | |||||
|---|---|---|---|---|---|
| . | Sildenafil (n = 99) . | Placebo (n = 134) . | P value . | ||
| . | Mean . | SD . | Mean . | SD . | . |
| Age, y | 36.3 | 7.14 | 36.3 | 7.21 | .89 |
| Baseline BMI, kg/m2 | 27.1 | 4.7 | 27.2 | 4.6 | .95 |
| n | % Total product group | n | % Total product group | ||
| Cisgender female | 99 | 100 | 134 | 100 | NA |
| Ethnicity | |||||
| Hispanic or Latino | 14 | 14.1 | 24 | 17.9 | .44 |
| Not Hispanic or Latino | 85 | 85.9 | 110 | 82.1 | |
| Race | |||||
| American Indian or Alaskan Native | 0 | 0 | 2 | 1.5 | .37 |
| Asian | 5 | 5.1 | 7 | 5.2 | |
| Black or African American | 6 | 6.1 | 14 | 10.4 | |
| Native Hawaiian or Other Pacific Islander | 1 | 1.0 | 0 | 0 | |
| White | 84 | 84.8 | 110 | 82.1 | |
| Mixed race or other | 3 | 3.0 | 1 | 0.7 | |
| Employment status | |||||
| Employed | 84 | 84.8 | 108 | 80.6 | .40 |
| Not employed | 15 | 15.2 | 26 | 19.4 | |
| Highest level of education | |||||
| High school | 5 | 5.1 | 10 | 7.5 | .74 |
| College or postgraduate | 94 | 94.9 | 123 | 91.8 | |
| Missing or did not report | 0 | 0 | 1 | 1.0 | |
| Partner | |||||
| Sildenafil (n = 91) | Placebo (n = 112) | P value | |||
| Mean | SD | Mean | SD | ||
| Age, y | 38.5 | 8.60 | 37.6 | 8.48 | .64 |
| BMI, kg/m2 | 28.5 | 5.7 | 28.7 | 4.5 | .92 |
| n | % Total product group | N | % Total product group | ||
| Sex | |||||
| Female | 5 | 5.5 | 10 | 8.9 | .35 |
| Male | 86 | 94.5 | 102 | 91.1 | |
| Participant . | |||||
|---|---|---|---|---|---|
| . | Sildenafil (n = 99) . | Placebo (n = 134) . | P value . | ||
| . | Mean . | SD . | Mean . | SD . | . |
| Age, y | 36.3 | 7.14 | 36.3 | 7.21 | .89 |
| Baseline BMI, kg/m2 | 27.1 | 4.7 | 27.2 | 4.6 | .95 |
| n | % Total product group | n | % Total product group | ||
| Cisgender female | 99 | 100 | 134 | 100 | NA |
| Ethnicity | |||||
| Hispanic or Latino | 14 | 14.1 | 24 | 17.9 | .44 |
| Not Hispanic or Latino | 85 | 85.9 | 110 | 82.1 | |
| Race | |||||
| American Indian or Alaskan Native | 0 | 0 | 2 | 1.5 | .37 |
| Asian | 5 | 5.1 | 7 | 5.2 | |
| Black or African American | 6 | 6.1 | 14 | 10.4 | |
| Native Hawaiian or Other Pacific Islander | 1 | 1.0 | 0 | 0 | |
| White | 84 | 84.8 | 110 | 82.1 | |
| Mixed race or other | 3 | 3.0 | 1 | 0.7 | |
| Employment status | |||||
| Employed | 84 | 84.8 | 108 | 80.6 | .40 |
| Not employed | 15 | 15.2 | 26 | 19.4 | |
| Highest level of education | |||||
| High school | 5 | 5.1 | 10 | 7.5 | .74 |
| College or postgraduate | 94 | 94.9 | 123 | 91.8 | |
| Missing or did not report | 0 | 0 | 1 | 1.0 | |
| Partner | |||||
| Sildenafil (n = 91) | Placebo (n = 112) | P value | |||
| Mean | SD | Mean | SD | ||
| Age, y | 38.5 | 8.60 | 37.6 | 8.48 | .64 |
| BMI, kg/m2 | 28.5 | 5.7 | 28.7 | 4.5 | .92 |
| n | % Total product group | N | % Total product group | ||
| Sex | |||||
| Female | 5 | 5.5 | 10 | 8.9 | .35 |
| Male | 86 | 94.5 | 102 | 91.1 | |
Demographic and baseline characteristics of safety population participants and sexual partners.
| Participant . | |||||
|---|---|---|---|---|---|
| . | Sildenafil (n = 99) . | Placebo (n = 134) . | P value . | ||
| . | Mean . | SD . | Mean . | SD . | . |
| Age, y | 36.3 | 7.14 | 36.3 | 7.21 | .89 |
| Baseline BMI, kg/m2 | 27.1 | 4.7 | 27.2 | 4.6 | .95 |
| n | % Total product group | n | % Total product group | ||
| Cisgender female | 99 | 100 | 134 | 100 | NA |
| Ethnicity | |||||
| Hispanic or Latino | 14 | 14.1 | 24 | 17.9 | .44 |
| Not Hispanic or Latino | 85 | 85.9 | 110 | 82.1 | |
| Race | |||||
| American Indian or Alaskan Native | 0 | 0 | 2 | 1.5 | .37 |
| Asian | 5 | 5.1 | 7 | 5.2 | |
| Black or African American | 6 | 6.1 | 14 | 10.4 | |
| Native Hawaiian or Other Pacific Islander | 1 | 1.0 | 0 | 0 | |
| White | 84 | 84.8 | 110 | 82.1 | |
| Mixed race or other | 3 | 3.0 | 1 | 0.7 | |
| Employment status | |||||
| Employed | 84 | 84.8 | 108 | 80.6 | .40 |
| Not employed | 15 | 15.2 | 26 | 19.4 | |
| Highest level of education | |||||
| High school | 5 | 5.1 | 10 | 7.5 | .74 |
| College or postgraduate | 94 | 94.9 | 123 | 91.8 | |
| Missing or did not report | 0 | 0 | 1 | 1.0 | |
| Partner | |||||
| Sildenafil (n = 91) | Placebo (n = 112) | P value | |||
| Mean | SD | Mean | SD | ||
| Age, y | 38.5 | 8.60 | 37.6 | 8.48 | .64 |
| BMI, kg/m2 | 28.5 | 5.7 | 28.7 | 4.5 | .92 |
| n | % Total product group | N | % Total product group | ||
| Sex | |||||
| Female | 5 | 5.5 | 10 | 8.9 | .35 |
| Male | 86 | 94.5 | 102 | 91.1 | |
| Participant . | |||||
|---|---|---|---|---|---|
| . | Sildenafil (n = 99) . | Placebo (n = 134) . | P value . | ||
| . | Mean . | SD . | Mean . | SD . | . |
| Age, y | 36.3 | 7.14 | 36.3 | 7.21 | .89 |
| Baseline BMI, kg/m2 | 27.1 | 4.7 | 27.2 | 4.6 | .95 |
| n | % Total product group | n | % Total product group | ||
| Cisgender female | 99 | 100 | 134 | 100 | NA |
| Ethnicity | |||||
| Hispanic or Latino | 14 | 14.1 | 24 | 17.9 | .44 |
| Not Hispanic or Latino | 85 | 85.9 | 110 | 82.1 | |
| Race | |||||
| American Indian or Alaskan Native | 0 | 0 | 2 | 1.5 | .37 |
| Asian | 5 | 5.1 | 7 | 5.2 | |
| Black or African American | 6 | 6.1 | 14 | 10.4 | |
| Native Hawaiian or Other Pacific Islander | 1 | 1.0 | 0 | 0 | |
| White | 84 | 84.8 | 110 | 82.1 | |
| Mixed race or other | 3 | 3.0 | 1 | 0.7 | |
| Employment status | |||||
| Employed | 84 | 84.8 | 108 | 80.6 | .40 |
| Not employed | 15 | 15.2 | 26 | 19.4 | |
| Highest level of education | |||||
| High school | 5 | 5.1 | 10 | 7.5 | .74 |
| College or postgraduate | 94 | 94.9 | 123 | 91.8 | |
| Missing or did not report | 0 | 0 | 1 | 1.0 | |
| Partner | |||||
| Sildenafil (n = 91) | Placebo (n = 112) | P value | |||
| Mean | SD | Mean | SD | ||
| Age, y | 38.5 | 8.60 | 37.6 | 8.48 | .64 |
| BMI, kg/m2 | 28.5 | 5.7 | 28.7 | 4.5 | .92 |
| n | % Total product group | N | % Total product group | ||
| Sex | |||||
| Female | 5 | 5.5 | 10 | 8.9 | .35 |
| Male | 86 | 94.5 | 102 | 91.1 | |
During the double-blind dosing period, there were 1357 and 1160 sexual events recorded in which sildenafil cream or placebo cream was used among the ITT population, respectively. Table 2 shows the number of participants and sexual partners reporting at least 1 TEAE and the total number of TEAEs by severity and relatedness categories during the double-blind dosing period among the ITT population. There were no significant differences in the frequency of various TEAE reports by sildenafil cream vs placebo cream users during the double-blind dosing period. The mean time of onset of the first TEAE reported during double-blind dosing period was 21 ± 11 days and 20 ± 8 days for sildenafil cream– and placebo cream–assigned participants, respectively. During the double-blind dosing period, 2 sildenafil cream–assigned participants reported 3 TEAEs (burning at IP application site, erythema at IP application site, and dyspareunia with IP use), which were all deemed to be treatment related and led to discontinuation of IP. There were 2 TEAEs reported by placebo cream users in the double-blind dosing period that were not related to IP use (dyspareunia and vulvar fissure) but led to IP discontinuation nonetheless. Table 2 also demonstrates that during the double-blind dosing period, 7 partners exposed to sildenafil cream reported 9 TEAEs and 4 partners exposed to placebo cream reported 4 TEAEs.
TEAEs reported by participants and sexual partners during double-blind treatment period.
| Event category . | Sildenafil (n = 99 participants, n = 91 partners) . | Placebo (n = 94 participants, n = 84 partners) . | Fisher exact P value . | ||||
|---|---|---|---|---|---|---|---|
| . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | . |
| Participant TEAE data | |||||||
| Any TEAE | 29 | 29.3 | 78 | 28 | 29.8 | 65 | .76 |
| treatment-related TEAEa | 14 | 14.1 | 60 | 14 | 14.9 | 37 | >.99 |
| TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 2 | 2.1 | 2 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 1 | 1.1 | 1 | .50 |
| Any SAE | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Partner TEAE data | |||||||
| Any TEAE | 7 | 7.7 | 9 | 4 | 4.8 | 4 | .54 |
| Treatment-related TEAEa | 3 | 3.3 | 5 | 0 | 0 | 0 | .25 |
| TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
| Event category . | Sildenafil (n = 99 participants, n = 91 partners) . | Placebo (n = 94 participants, n = 84 partners) . | Fisher exact P value . | ||||
|---|---|---|---|---|---|---|---|
| . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | . |
| Participant TEAE data | |||||||
| Any TEAE | 29 | 29.3 | 78 | 28 | 29.8 | 65 | .76 |
| treatment-related TEAEa | 14 | 14.1 | 60 | 14 | 14.9 | 37 | >.99 |
| TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 2 | 2.1 | 2 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 1 | 1.1 | 1 | .50 |
| Any SAE | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Partner TEAE data | |||||||
| Any TEAE | 7 | 7.7 | 9 | 4 | 4.8 | 4 | .54 |
| Treatment-related TEAEa | 3 | 3.3 | 5 | 0 | 0 | 0 | .25 |
| TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
Abbreviation: IP, investigational product; NA, not applicable; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Definitely, probably, or possibly related.
TEAEs reported by participants and sexual partners during double-blind treatment period.
| Event category . | Sildenafil (n = 99 participants, n = 91 partners) . | Placebo (n = 94 participants, n = 84 partners) . | Fisher exact P value . | ||||
|---|---|---|---|---|---|---|---|
| . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | . |
| Participant TEAE data | |||||||
| Any TEAE | 29 | 29.3 | 78 | 28 | 29.8 | 65 | .76 |
| treatment-related TEAEa | 14 | 14.1 | 60 | 14 | 14.9 | 37 | >.99 |
| TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 2 | 2.1 | 2 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 1 | 1.1 | 1 | .50 |
| Any SAE | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Partner TEAE data | |||||||
| Any TEAE | 7 | 7.7 | 9 | 4 | 4.8 | 4 | .54 |
| Treatment-related TEAEa | 3 | 3.3 | 5 | 0 | 0 | 0 | .25 |
| TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
| Event category . | Sildenafil (n = 99 participants, n = 91 partners) . | Placebo (n = 94 participants, n = 84 partners) . | Fisher exact P value . | ||||
|---|---|---|---|---|---|---|---|
| . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | Participants reporting at least 1 TEAE . | % of Cohort reporting at least 1 TEAE . | TEAEs reported . | . |
| Participant TEAE data | |||||||
| Any TEAE | 29 | 29.3 | 78 | 28 | 29.8 | 65 | .76 |
| treatment-related TEAEa | 14 | 14.1 | 60 | 14 | 14.9 | 37 | >.99 |
| TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 2 | 2.1 | 2 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 2 | 2.0 | 3 | 1 | 1.1 | 1 | .50 |
| Any SAE | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Partner TEAE data | |||||||
| Any TEAE | 7 | 7.7 | 9 | 4 | 4.8 | 4 | .54 |
| Treatment-related TEAEa | 3 | 3.3 | 5 | 0 | 0 | 0 | .25 |
| TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
| Treatment-related TEAE leading to discontinuation of IP | 1 | 1.1 | 1 | 0 | 0 | 0 | >.99 |
Abbreviation: IP, investigational product; NA, not applicable; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Definitely, probably, or possibly related.
A post hoc analysis (Table S2) demonstrated that the number of sildenafil cream–assigned participants (randomized population, n = 101) reporting at least 1 type of TEAE was similar when subcategorized based on the quartile of total number of reported sexual events during the double-blind dosing period (all P values >.19). In fact, 2 sildenafil cream randomized participants contributed 53% (n = 41 of 78) of the total number of TEAEs observed in the sildenafil cream group. One of these participants reported 22 mild, related, and resolved TEAEs (mostly application site discomfort) and reported using IP 15 times during the double-blind dosing period. A second sildenafil cream randomized participant reported 19 mild, related, resolved TEAEs, which included genital irritation and/or itching and burning with product application. This participant had 23 sexual events during the double-blind dosing period. Additionally, the 2 participants who had genital irritation and burning with product application who elected to discontinue the study were both in the first quartile of reported IP exposed sexual events during the dosing period, reporting 2 and 4 sildenafil cream–exposed sexual events prior to discontinuing the study.
Table 3 displays the most common treatment-related (possibly, probably, or definitely related) TEAEs, by system organ class, reported by subjects or their sexual partners during the double-blind dosing period. As noted in Table 3, there were no significant differences in the system organ class of treatment-related TEAEs reported by sildenafil cream vs placebo cream users nor their sexual partners.
Most common treatment-related TEAEs by system organ class, reported by participants and sexual partners during double-blind dosing period.
| System organ class preferred term . | Sildenafil (n = 99 participants; n = 91 partners) . | Placebo (n = 94 participants; n = 84 partners) . | Fisher exact P value . | ||
|---|---|---|---|---|---|
| . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | . |
| Participant treatment-related TEAE data | |||||
| Any treatment-related adverse events | 14 | 14.1 | 14 | 14.9 | >.99 |
| Application site discomfort | 12 | 12.1 | 14 | 14.9 | .67 |
| Application site erythema | 3 | 3.0 | 3 | 3.2 | >.99 |
| Application site edema | 2 | 2.0 | 1 | 1.1 | >.99 |
| Vulvovaginal discomfort | 0 | 0 | 2 | 2.1 | .24 |
| Cervical friability, dysmenorrhea, or dyspareunia | 0 | 0 | 3 | 3.9 | .11 |
| Vulvovaginal erythema | 1 | 1.0 | 0 | 0 | >.99 |
| Partner treatment-related TEAE data | |||||
| Any treatment-related TEAE | 3 | 3.1 | 0 | 0 | .25 |
| Application site discomfort | 1 | 1.1 | 0 | 0 | >.99 |
| Headache | 1 | 1.1 | 0 | 0 | >.99 |
| genital hypothesia | 1 | 1.1 | 0 | 0 | >.99 |
| System organ class preferred term . | Sildenafil (n = 99 participants; n = 91 partners) . | Placebo (n = 94 participants; n = 84 partners) . | Fisher exact P value . | ||
|---|---|---|---|---|---|
| . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | . |
| Participant treatment-related TEAE data | |||||
| Any treatment-related adverse events | 14 | 14.1 | 14 | 14.9 | >.99 |
| Application site discomfort | 12 | 12.1 | 14 | 14.9 | .67 |
| Application site erythema | 3 | 3.0 | 3 | 3.2 | >.99 |
| Application site edema | 2 | 2.0 | 1 | 1.1 | >.99 |
| Vulvovaginal discomfort | 0 | 0 | 2 | 2.1 | .24 |
| Cervical friability, dysmenorrhea, or dyspareunia | 0 | 0 | 3 | 3.9 | .11 |
| Vulvovaginal erythema | 1 | 1.0 | 0 | 0 | >.99 |
| Partner treatment-related TEAE data | |||||
| Any treatment-related TEAE | 3 | 3.1 | 0 | 0 | .25 |
| Application site discomfort | 1 | 1.1 | 0 | 0 | >.99 |
| Headache | 1 | 1.1 | 0 | 0 | >.99 |
| genital hypothesia | 1 | 1.1 | 0 | 0 | >.99 |
Abbreviation: TEAE, treatment-emergent adverse event.
Most common treatment-related TEAEs by system organ class, reported by participants and sexual partners during double-blind dosing period.
| System organ class preferred term . | Sildenafil (n = 99 participants; n = 91 partners) . | Placebo (n = 94 participants; n = 84 partners) . | Fisher exact P value . | ||
|---|---|---|---|---|---|
| . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | . |
| Participant treatment-related TEAE data | |||||
| Any treatment-related adverse events | 14 | 14.1 | 14 | 14.9 | >.99 |
| Application site discomfort | 12 | 12.1 | 14 | 14.9 | .67 |
| Application site erythema | 3 | 3.0 | 3 | 3.2 | >.99 |
| Application site edema | 2 | 2.0 | 1 | 1.1 | >.99 |
| Vulvovaginal discomfort | 0 | 0 | 2 | 2.1 | .24 |
| Cervical friability, dysmenorrhea, or dyspareunia | 0 | 0 | 3 | 3.9 | .11 |
| Vulvovaginal erythema | 1 | 1.0 | 0 | 0 | >.99 |
| Partner treatment-related TEAE data | |||||
| Any treatment-related TEAE | 3 | 3.1 | 0 | 0 | .25 |
| Application site discomfort | 1 | 1.1 | 0 | 0 | >.99 |
| Headache | 1 | 1.1 | 0 | 0 | >.99 |
| genital hypothesia | 1 | 1.1 | 0 | 0 | >.99 |
| System organ class preferred term . | Sildenafil (n = 99 participants; n = 91 partners) . | Placebo (n = 94 participants; n = 84 partners) . | Fisher exact P value . | ||
|---|---|---|---|---|---|
| . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | Number of patients reporting at least 1 TEAEs . | % of Total group reporting at least 1 TEAE . | . |
| Participant treatment-related TEAE data | |||||
| Any treatment-related adverse events | 14 | 14.1 | 14 | 14.9 | >.99 |
| Application site discomfort | 12 | 12.1 | 14 | 14.9 | .67 |
| Application site erythema | 3 | 3.0 | 3 | 3.2 | >.99 |
| Application site edema | 2 | 2.0 | 1 | 1.1 | >.99 |
| Vulvovaginal discomfort | 0 | 0 | 2 | 2.1 | .24 |
| Cervical friability, dysmenorrhea, or dyspareunia | 0 | 0 | 3 | 3.9 | .11 |
| Vulvovaginal erythema | 1 | 1.0 | 0 | 0 | >.99 |
| Partner treatment-related TEAE data | |||||
| Any treatment-related TEAE | 3 | 3.1 | 0 | 0 | .25 |
| Application site discomfort | 1 | 1.1 | 0 | 0 | >.99 |
| Headache | 1 | 1.1 | 0 | 0 | >.99 |
| genital hypothesia | 1 | 1.1 | 0 | 0 | >.99 |
Abbreviation: TEAE, treatment-emergent adverse event.
Because oral sildenafil use can be associated with orthostatic hypotension, particularly in users taking nitrates for angina, we obtained orthostatic vital signs at baseline and then monthly during the double-blind dosing period among participants (Table S3) and their sexual partners. As noted in Table S3, there were no differences in vital signs taken at various positions between sildenafil- or placebo-assigned participants at any point in the study. There were no notable changes in blood pressure with standing. Finally, although baseline laboratories and electrocardiograms were not repeated at the end of the study, there were no TEAEs that necessitated repeating these baseline screening assessments.
Discussion
Topical sildenafil cream was safe and well tolerated by healthy premenopausal women and their sexual partners in 1357 documented sildenafil cream sexual exposures in the double-blind dosing period. These data, along with the co-primary preliminary efficacy data,24 will be used to support continued clinical development of this product for the treatment of FSAD. Many women’s health products use nonoral administration (eg, transdermal, transvaginal) to lower systemic exposure and potentially reduce systemic side effects.25-27 Previous clinical trials of oral sildenafil citrate in women had high rates of side effects using doses normally administered to men for ED (25-100 mg).20-23 The phase 2b safety data presented herein are consistent with past, smaller, placebo-controlled studies of topical sildenafil cream in women performed by Strategic Science and Technologies.28
Previously, single, topical, 1-g, 2-g, and 4-g doses of sildenafil cream and placebo cream were tested in a dose escalation study and in crossover, placebo-controlled studies among pre- (n = 15) and postmenopausal (n = 47) women to assess pharmacokinetics and safety by Strategic Science and Technologies in 2015 and 2018.28 All TEAEs were mild or moderate in severity, with no differences between active and placebo creams. In the dose escalation study in healthy postmenopausal women (SST-6006-008-01, data on file), the most common TEAE was genital burning sensation, reported by 12 subjects after exposure to the 4-g dose.28
We observed mild-to-moderate genital irritation and/or genital burning sensations with use of either the active sildenafil cream or placebo cream. Although the main constituent of both creams is purified water, both creams also contain salts, and are hyperosmolar to enhance absorption.
The most common side effects of oral sildenafil citrate use for ED are headache, flushing, dyspepsia, abnormal vision, nasal congestion, back pain, myalgia, nausea, dizziness, and rash.29 This side effect profile is reflective of the several hundred-fold higher systemic exposure measured after oral dosing compared with topical application.30 Data show that when oral sildenafil31 or oral tadalafil32 are given daily for pulmonary hypertension, the most common TEAEs are similar to when sildenafil is given orally on an as needed basis for ED (headache, flushing).33 As pulmonary hypertension is more common in women compared with men, the Food and Drug Administration safety data for daily oral phosphodiesterase type 5 inhibitor use are primarily (75%-78%) among women.31-33 Thus, there is no indication that women experience more severe adverse events when using substantially larger daily systemic doses of oral sildenafil (20 mg orally 3 or more times daily) for the treatment of pulmonary hypertension.31-33
Our safety data support that the side effects associated with topical sildenafil cream use were primarily related to local genital irritation. Our post hoc analysis found that the frequency of TEAEs was similar based on the quartile of total reported sexual exposures during the double-blind dosing period, supporting no cumulative toxicity from multiple exposures to sildenafil cream. Importantly, this study also described TEAEs among sexual partners, and TEAEs were reported directly by the sexual partner.
Strengths of this study included the detailed reporting of TEAEs by participants, via an eDiary with daily prompts, and direct TEAE reporting among sexual partners as noted previously. Limitations of these safety data are that the study was not powered to demonstrate statistically significant differences in the frequency and incidence of TEAEs. We used the Fisher exact test to compare the proportions of TEAEs between the IP groups, which is not dependent on sample size, to mitigate this risk. Consistent with good clinical practice, we instructed participants with unconsented sexual partners to not have a partnered sexual experience within 72 hours of IP application. We attempted to verify this safety guidance using the eDiary responses, but it is possible that unconsented sexual partners may have been exposed to IP despite these precautions. Given that the sexual partner TEAEs were infrequent and all of mild or moderate severity, the potential benefit of topical sildenafil outweighs the risks to users and their sexual partners. Similarly, we monitored postural vital signs in the clinic and/or via telemedicine for sexual partners but obviously could not measure postural vital signs immediately after IP use at home. Data from previous studies of topical sildenafil in women and men support that changes in heart rate with standing were likely a benign, normal, physiologic response to standing.28 We are reassured by the ample vital sign and TEAE data collected in this study that the systemic impacts of oral sildenafil citrate were not seen with topical application.
Conclusion
These data suggest that topical sildenafil was safe and well tolerated among users and exposed sexual partners, and support further clinical development of sildenafil cream, 3.6% as a potential first-in-category treatment for FSAD.
Author Contribution
C.D. provided independent data management and verification. N.N.K. and S.P. provided independent technical input into the study design, clinical interviews, and data interpretation and presentations.
CRediT Author Statement
A.R.T. (methodology, formal analysis, data curation, writing original draft, writing review and editing, visualization), I.J. (conceptualization, formal analysis, data curation, writing review and editing, visualization, project administration), K.A.C. (conceptualization, methodology, formal analysis, resources, data curation, writing review and editing, visualization, supervision, project administration), J.H. (methodology, formal analysis, resources, data curation, writing review and editing, visualization, supervision, project administration), N.N.K. (conceptualization, methodology, writing review and editing, visualization), S.J.P. (conceptualization, methodology, investigation, writing review and editing, visualization, supervision, project administration), C.D. (methodology, formal analysis, resources, data curation, writing review and editing), D.R.F. (conceptualization, methodology, resources, writing review and editing, visualization, supervision), A.G. (conceptualization, methodology, investigation, writing review and editing, visualization, supervision).
Funding
This work was funded by Daré Bioscience.
Conflicts of interest
A.R.T, .I.J., J.H., D.R.F., and A.G. are employees of Daré Bioscience. K.A.C. is an employee of Strategic Science & Technologies.



