-
PDF
- Split View
-
Views
-
Cite
Cite
Chris G. McMahon, Neil Shusterman, Brian Cohen, Pharmacokinetics, Clinical Efficacy, Safety Profile, and Patient-Reported Outcomes in Patients Receiving Subcutaneous Testosterone Pellets 900 mg for Treatment of Symptoms Associated with Androgen Deficiency, The Journal of Sexual Medicine, Volume 14, Issue 7, July 2017, Pages 883–890, https://doi.org/10.1016/j.jsxm.2017.04.734
Close - Share Icon Share
Abstract
Implantation of testosterone doses of at least 150 to 450 mg (ie, two to six pellets) is common clinical practice despite a lack of prospective data.
To evaluate pharmacokinetics, clinical efficacy, safety, and patient-reported outcomes in men with androgen deficiency who received implantation of testosterone pellets (900 mg) in an open-label study.
Men with androgen deficiency (serum testosterone < 300 ng/dL [10.4 nmol/L]) were screened and received 12 testosterone pellets (900 mg). Serum hormone measurements (total and free testosterone, dihydrotestosterone, and estradiol) were obtained on days 1, 5, 8, 15, 29, 57, 85, and 113. All hormones were assayed using validated liquid chromatography and tandem mass spectrometry.
Pharmacokinetics of selected hormones was determined. The patient-reported International Index of Erectile Function (IIEF), Center for Epidemiologic Studies Depression (CES-D), and Androgen Deficiency in the Aging Male (qADAM) questionnaires also were performed. Patients rated their satisfaction on a scale from 1 (very satisfied) to 5 (very dissatisfied). Adverse events were monitored throughout.
Fifteen patients were included (mean age = 54.5 years, SD = 8.6 years). Mean baseline total testosterone concentration was 241.6 ng/dL (SD = 88.8 ng/dL; mean = 8.4 nmol/L, SD = 3.1 nmol/L). Mean testosterone serum concentrations fluctuated during the first 2 weeks (range = 300–1,000 ng/dL, 10.4–34.7 nmol/L) but remained higher than or equal to 300 ng/dL (10.4 nmol/L) through day 113. Concentrations of free testosterone, dihydrotestosterone, and estradiol mirrored that of total testosterone. Male functioning (IIEF score), depression (CES-D total score), and androgen-deficiency symptoms (qADAM total score) improved from baseline. Most patients were “very satisfied” (40.0%) or “quite satisfied” (26.7%) with treatment. Testosterone pellets were well tolerated. Pellet extrusion and polycythemia occurred in one patient each.
Implantation of high doses (900 mg) of testosterone pellets are generally well tolerated and could provide clinical benefit for some patients.
This study provides standardized data for the implantation of 12 testosterone pellets. However, the open-label uncontrolled design of this study and its small and ethnically non-diverse patient population limit the interpretation of these data, particularly the patient-reported outcomes.
Implantation of 12 testosterone pellets (900 mg) was well tolerated and provided adequate and sustained serum testosterone concentrations. Additional randomized controlled trials are needed to confirm efficacy and safety findings.
Introduction
Androgen deficiency is characterized by inadequate gonadal function, including deficient spermatogenesis and decreased testosterone secretion.1,2 The prevalence of androgen deficiency depends on patient age and the concentration range used to define the condition. Between 2% and 6% of men in the general population have symptomatic testosterone deficiency,3 and up to 49% of men at least 45 years old have androgen deficiency, defined as a total testosterone concentration lower than 300 ng/dL (10.4 nmol/L).4–6 Current treatment guidelines recommend testosterone replacement therapy in men with classic androgen-deficiency symptoms, with the goal of achieving a serum testosterone concentration within the mid to normal range for healthy young men (ie, 280–800 ng/dL [9.7–27.8 nmol/L])1,3,7 and alleviating problematic symptoms (eg, improve well-being and sexual function and optimize bone density).1 Multiple formulations of testosterone replacement therapy, including intramuscular, subdermal, transdermal, oral, and buccal preparations, are available.3,7 Although most testosterone formulations are efficacious and well tolerated, each has potential limitations and safety issues.1,7 Testosterone injections (eg, testosterone enanthate and testosterone cypionate) provide inconsistent testosterone levels, with supraphysiologic testosterone concentrations at the time of injection waning to the androgen-deficient range by the end of the dosing interval.1,3 In addition, injection of testosterone has been associated with cases of pulmonary oil microembolism.1,8 Transdermal formulations, such as patches or gels, can be applied once daily and maintain normal testosterone levels but can cause skin irritation.1,8 Gel formulations also have a risk of skin-to-skin transference with other individuals such as children and women, have variable uptake depending on skin blood flow, and can alter dihydrotestosterone (DHT) levels.1,8 Oral formulations are convenient but provide variable serum testosterone levels and are not yet approved in the United States.1 Muco-adhesive (buccal) patches, which effectively maintain testosterone levels within a normal range, must be applied twice a day, often have an unpleasant taste, and can adversely affect gum health.1,8
Subcutaneously implanted testosterone pellets (Testopel; Endo Pharmaceuticals, Malvern, PA, USA) were approved for treatment of androgen deficiency by the US Food and Drug Administration (FDA) in 19729 and were designed to allow consistent delivery of serum testosterone concentrations for several months.1,10,11 FDA approval was based on observations and data extrapolations from use of testosterone propionate injections, which are no longer available9; therefore, pharmacokinetic and dosing studies using the currently available formulation of testosterone pellets are greatly needed. Testosterone pellets are 3- × 8-mm pellets that are surgically implanted into the subcutaneous space. Each pellet contains crystalline testosterone 75 mg and provides gradual release of testosterone in the subdermal space as the pellet dissolves.9 The current recommended dosage of testosterone pellets is 150 to 450 mg (ie, two to six pellets) every 3 to 6 months12; however, the medical literature indicates that insertion of at least 10 pellets (≥750 mg) might be common in clinical practice.13–15
Several retrospective and open-label studies have demonstrated sustained testosterone concentrations of at least 300 ng/dL (10.4 nmol/L) for up to 4 months in men with androgen deficiency who received testosterone pellets.13,15,16 Higher short-term testosterone concentrations were observed in patients who received higher doses (ie, had implantation of a larger number of pellets),13 although supraphysiologic testosterone levels were uncommon.13 Because implantation of high doses of testosterone pellets have not been thoroughly evaluated, this open-label study evaluated the pharmacokinetics, patient-reported outcomes associated with symptoms of androgen deficiency, and tolerability profile of implantation of 12 75-mg testosterone pellets (900 mg) in men with androgen deficiency.
Methods
Study Design
This was an open-label three-center study that evaluated the pharmacokinetics, patient-reported outcomes associated with symptoms of androgen deficiency, and safety profile of implantation of a larger number of testosterone pellets (12 pellets; 900 mg) than currently recommended in prescribing information (2–6 pellets; 150–450 mg). No patients received compensation for trial participation. All centers were institutions with a specific interest in sexual medicine or andrology and typically managed men with erectile dysfunction, ejaculatory dysfunction, testosterone deficiency, or infertility. Patients were recruited from practice databases of existing or previous patients or from online clinical trial recruitment advertisements. Eligible patients were men 18 to 70 years old who had a serum testosterone concentration lower than 300 ng/dL (10.4 nmol/L) in samples collected at 8:00 am plus or minus 90 minutes at the screening visit (determined by chemiluminescence immunoassay). Included patients had to have a body mass index of 20 to 40 kg/m2 and be considered by investigators to be in good health.
Patients were excluded if they had used pelleted or long-acting depot testosterone products within 6 months or short-acting injectable testosterone ester products within 6 weeks of screening or topical or oral testosterone products within 14 days before the study. Use of medications that could interfere with androgen metabolism (eg, spironolactone, cimetidine, or 5α-reductase inhibitors) was not permitted within 4 weeks of screening or during the study. Patients with a history of liver disease, cardiovascular disease (myocardial infarction, unstable angina, or heart failure), active hepatitis, hepatic neoplasm, or aspartate aminotransferase or alanine aminotransferase values at least 2.0 times the upper limit of normal also were excluded.
The study protocol for each site was approved by Bellberry Limited, (Eastwood, SA, Australia). The study was conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice and the ethical principles according to the Declaration of Helsinki. All patients provided written informed consent. Eligible patients received implantation of 12 75-mg crystalline testosterone pellets (Testopel) subcutaneously into the upper lateral aspect of the thigh at 8:00 am (after pharmacokinetic sampling on day 1) using an aseptic technique under local anesthesia.
Assessments
Blood samples for pharmacokinetic evaluations were obtained by venipuncture on days 1, 5, 8, 15, 29, 57, 85, and 113 at 8:00 am plus or minus 90 minutes. Assessments of serum testosterone, DHT, estradiol, and free testosterone at all post-screening time points were performed using validated liquid chromatography assays with tandem mass spectrometry. All samples were assayed alongside surrogate and endogenous quality control samples. The total testosterone assay had a lower limit of detection of 5 ng/dL and an upper limit of detection of 1,000 ng/dL. The upper and lower limits of detection for other assays were 5 and 1,000 ng/dL for DHT, 1 and 200 pg/mL for free testosterone, and 1 and 100 pg/mL for estradiol. Intra- and interassay coefficients of variation were lower than 6% for all assays. Area under the concentration-time curve (AUC) from zero to time of last measurable concentration (AUC0-tlast), maximum observed concentration (Cmax), time to maximum observed concentration, and average concentration (Cavg) were calculated using observed serum concentrations. The percentage of patients who achieved a Cavg of serum total testosterone within normal limits and the percentage of patients who had a Cmax higher than 1,000 ng/dL (34.7 nmol/L) were calculated.
Patients completed the International Index of Erectile Function (IIEF; Australian version), Center for Epidemiologic Studies Depression (CES-D), and Androgen Deficiency in the Aging Male (qADAM) questionnaires at screening and during study visits on days 15, 29, 85, and 113. The validated IIEF questionnaire consists of 15 questions organized into five domains of sexual function: erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction.17 Each question was rated by the patient on a scale of 1 (least severe) to 5 (most severe), with an optional response of 0 (no sexual activity) on questions related to sexual intercourse (ie, questions 1–10). The CES-D also is a validated patient-report questionnaire. It is composed of 20 questions pertaining to how patients felt or behaved during the previous week and uses a Likert-type scale that ranges from 0 (rarely) to 3 (most or all of the time), with higher scores indicative of greater depression.18 The qADAM questionnaire consists of 10 questions that evaluate various aspects of daily living (eg, energy level, libido, work performance, etc) on a Likert-type scale from 1 (worst) to 5 (best), with lower scores indicative of more androgen-deficiency symptoms.19 One question of the qADAM (item 10 regarding height loss) was not included in post-baseline case report forms; therefore, post-baseline qADAM scores included only nine questions. Patient satisfaction was assessed on day 113 by asking the patient to rate his satisfaction with treatment on a scale from 1 (very satisfied) to 5 (very dissatisfied). Adverse events (AEs) and vital signs were monitored throughout the study. The Medical Dictionary for Regulatory Activities 17.0 was used to classify all AEs. Hematology and serum chemistry were assessed at screening and at the last visit (day 113).
Statistical Analyses
Pharmacokinetic parameters were summarized using descriptive statistics. Calculation of geometric means and percentage of coefficient of variation for AUC0-tlast and Cmax were performed after logarithmic transformation of concentration values. Descriptive statistics were summarized at each assessment time point for the IIEF domains, CES-D, and qADAM. For the IIEF, the domain score was considered missing if more than 50% of the questions related to that domain were unanswered. CES-D score was considered missing if more than four responses were unavailable. Change from baseline for each score was estimated using a mixed-model with repeated measurements. Positive changes from baseline in the IIEF and qADAM indicated improvement of function and satisfaction and androgen-deficiency symptoms, respectively; negative changes from baseline in CES-D score indicated improvement of depression. Satisfaction with study medication was summarized on day 113 using a frequency distribution. Statistical significance was achieved if the 95% CI of the parameter measurement did not include zero. Because this was a pharmacokinetic study and no specific hypotheses were tested, a formal sample size calculation was not performed. However, enrollment of approximately 24 patients was considered sufficient to provide the information required to adequately assess the study objectives.
Results
Patient Population
Of 30 patients screened for participation, 15 patients were enrolled. The study was stopped after 15 of the 24 planned patients were enrolled because of slow patient recruitment. All enrolled patients (N = 15) were included in the efficacy and safety analyses. One patient was excluded from the pharmacokinetic evaluation because of pellet extrusion on day 12. All 15 patients were white (100%), with a mean age of 54.5 years (SD = 8.6 years) and a mean body mass index of 31.2 kg/m2 (SD = 4.3 kg/m2). Patients were generally in good health and had few comorbid conditions (Table 1 ). Mean prostate-specific antigen concentration at baseline was 0.6 μg/L (SD = 0.9 μg/L), with values ranging from 0.06 to 3.5 μg/L, indicating that patients were not at an increased risk for prostate cancer (Table 1). Mean baseline total serum testosterone concentration was 241.6 ng/dL (SD = 88.8 ng/dL; mean = 8.4 nmol/L, SD = 8.4 nmol/L), which was below the lower limit of the normal range (ie, <300 ng/dL [10.4 nmol/L]; Table 1).
| Parameter . | Testosterone pellets 900 mg (N = 15) . |
|---|---|
| Age (y), mean (range) | 54.5 (38–70) |
| Age group, n (%) | |
| <45 y | 2 (13.3) |
| 45–64 y | 11 (73.3) |
| ≥65 y | 2 (13.3) |
| White race, n (%) | 15 (100) |
| BMI (kg/m2), mean (SD) | 31.2 (4.3) |
| Prostate-specific antigen, mean (SD) | 0.6 (0.9) |
| Comorbid conditions, n (%) | |
| Hypertension | 4 (26.7) |
| Type 1 diabetes mellitus | 1 (6.7) |
| Type 2 diabetes mellitus | 1 (6.7) |
| Colon cancer | 1 (6.7) |
| Depression | 2 (13.3) |
| Benign prostatic hyperplasia | 1 (6.7) |
| Erectile dysfunction | 1 (6.7) |
| Baseline hormone concentrations, mean (SD) | |
| Total testosterone (ng/dL) | 241.6 (88.8) |
| Free testosterone (pg/mL) | 58.6 (22.3) |
| DHT (ng/dL) | 17.5 (12.6) |
| Estradiol (pg/mL) | 15.9 (3.8) |
| Parameter . | Testosterone pellets 900 mg (N = 15) . |
|---|---|
| Age (y), mean (range) | 54.5 (38–70) |
| Age group, n (%) | |
| <45 y | 2 (13.3) |
| 45–64 y | 11 (73.3) |
| ≥65 y | 2 (13.3) |
| White race, n (%) | 15 (100) |
| BMI (kg/m2), mean (SD) | 31.2 (4.3) |
| Prostate-specific antigen, mean (SD) | 0.6 (0.9) |
| Comorbid conditions, n (%) | |
| Hypertension | 4 (26.7) |
| Type 1 diabetes mellitus | 1 (6.7) |
| Type 2 diabetes mellitus | 1 (6.7) |
| Colon cancer | 1 (6.7) |
| Depression | 2 (13.3) |
| Benign prostatic hyperplasia | 1 (6.7) |
| Erectile dysfunction | 1 (6.7) |
| Baseline hormone concentrations, mean (SD) | |
| Total testosterone (ng/dL) | 241.6 (88.8) |
| Free testosterone (pg/mL) | 58.6 (22.3) |
| DHT (ng/dL) | 17.5 (12.6) |
| Estradiol (pg/mL) | 15.9 (3.8) |
BMI = body mass index; DHT = dihydrotestosterone.
| Parameter . | Testosterone pellets 900 mg (N = 15) . |
|---|---|
| Age (y), mean (range) | 54.5 (38–70) |
| Age group, n (%) | |
| <45 y | 2 (13.3) |
| 45–64 y | 11 (73.3) |
| ≥65 y | 2 (13.3) |
| White race, n (%) | 15 (100) |
| BMI (kg/m2), mean (SD) | 31.2 (4.3) |
| Prostate-specific antigen, mean (SD) | 0.6 (0.9) |
| Comorbid conditions, n (%) | |
| Hypertension | 4 (26.7) |
| Type 1 diabetes mellitus | 1 (6.7) |
| Type 2 diabetes mellitus | 1 (6.7) |
| Colon cancer | 1 (6.7) |
| Depression | 2 (13.3) |
| Benign prostatic hyperplasia | 1 (6.7) |
| Erectile dysfunction | 1 (6.7) |
| Baseline hormone concentrations, mean (SD) | |
| Total testosterone (ng/dL) | 241.6 (88.8) |
| Free testosterone (pg/mL) | 58.6 (22.3) |
| DHT (ng/dL) | 17.5 (12.6) |
| Estradiol (pg/mL) | 15.9 (3.8) |
| Parameter . | Testosterone pellets 900 mg (N = 15) . |
|---|---|
| Age (y), mean (range) | 54.5 (38–70) |
| Age group, n (%) | |
| <45 y | 2 (13.3) |
| 45–64 y | 11 (73.3) |
| ≥65 y | 2 (13.3) |
| White race, n (%) | 15 (100) |
| BMI (kg/m2), mean (SD) | 31.2 (4.3) |
| Prostate-specific antigen, mean (SD) | 0.6 (0.9) |
| Comorbid conditions, n (%) | |
| Hypertension | 4 (26.7) |
| Type 1 diabetes mellitus | 1 (6.7) |
| Type 2 diabetes mellitus | 1 (6.7) |
| Colon cancer | 1 (6.7) |
| Depression | 2 (13.3) |
| Benign prostatic hyperplasia | 1 (6.7) |
| Erectile dysfunction | 1 (6.7) |
| Baseline hormone concentrations, mean (SD) | |
| Total testosterone (ng/dL) | 241.6 (88.8) |
| Free testosterone (pg/mL) | 58.6 (22.3) |
| DHT (ng/dL) | 17.5 (12.6) |
| Estradiol (pg/mL) | 15.9 (3.8) |
BMI = body mass index; DHT = dihydrotestosterone.
Pharmacokinetic Parameters
After pellet implantation, maximum serum total testosterone concentrations occurred within 2 weeks (median = 13.5 days). Mean serum total testosterone increased to 817.9 ng/dL (SD = 270.0 ng/dL; mean = 28.4 nmol/L, SD = 9.4 nmol/L) by day 5 but was followed by a temporary decrease at day 8 and another increase in concentration at day 15 (Figure 1 A). During this time, total testosterone concentrations did not decrease below normal serum testosterone levels and remained higher than 300 ng/dL (10.4 nmol/L) throughout the study. The average serum total testosterone (Cavg) ranged from 300 to 1,000 ng/dL (10.4–34.7 nmol/L) for all (100%) patients (Table 2 ). Most patients (93%; 13 of 14) had a concentration maximum no higher than 1,500 ng/dL (52.0 nmol/L); however, 7% of patients had maximum serum total testosterone levels from 1,501 ng/dL (52.1 nmol/L) to 1,799 ng/dL (62.4 nmol/L).
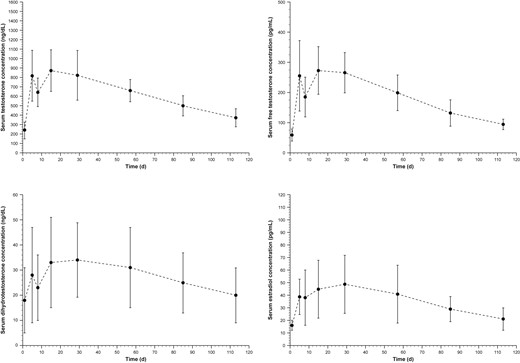
Panels A to D show concentration time curves for serum testosterone, free testosterone, DHT, and estradiol, respectively. Men deficient in androgens (serum testosterone concentration < 300 ng/dL) received implantation of 12 testosterone pellets (900 mg). Blood samples for pharmacokinetic evaluations were obtained before pellet implantation on day 1 and throughout the study at 8:00 am plus or minus 90 minutes. DHT = dihydrotestosterone.
| Hormone . | Tmax (d), median (range) . | Cmax (ng/dL), mean (%CV) . | Cavg (ng/dL), mean (SD) . | AUC0-tlast (ng/dL/d), mean (%CV) . | ||
|---|---|---|---|---|---|---|
| Days 1–113 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | |
| Serum total testosterone | 13.5 (2–32) | 1,021.6 (24.9) | 703.8 (140.9) | 638.3 (124.2) | 59,927.6 (20.9) | 71,720.5 (19.9) |
| DHT | 26 (2–84) | 38.1 (18.0) | 29.9 (14.4) | 28.2 (13.7) | 2,566.5 (51.2) | 3,167.5 (49.6) |
| Free testosterone | 14 (2–53) | 327.6 (79.4) | 212.6 (47.5) | 190.3 (40.8) | 18,128.0 (25.6) | 20,979.4 (24.2) |
| Estradiol | 27 (2–84) | 57.0 (20.1) | 40.3 (17.5) | 36.6 (15.2) | 3,426.8 (36.7) | 4,110.4 (35.9) |
| Hormone . | Tmax (d), median (range) . | Cmax (ng/dL), mean (%CV) . | Cavg (ng/dL), mean (SD) . | AUC0-tlast (ng/dL/d), mean (%CV) . | ||
|---|---|---|---|---|---|---|
| Days 1–113 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | |
| Serum total testosterone | 13.5 (2–32) | 1,021.6 (24.9) | 703.8 (140.9) | 638.3 (124.2) | 59,927.6 (20.9) | 71,720.5 (19.9) |
| DHT | 26 (2–84) | 38.1 (18.0) | 29.9 (14.4) | 28.2 (13.7) | 2,566.5 (51.2) | 3,167.5 (49.6) |
| Free testosterone | 14 (2–53) | 327.6 (79.4) | 212.6 (47.5) | 190.3 (40.8) | 18,128.0 (25.6) | 20,979.4 (24.2) |
| Estradiol | 27 (2–84) | 57.0 (20.1) | 40.3 (17.5) | 36.6 (15.2) | 3,426.8 (36.7) | 4,110.4 (35.9) |
AUC0-tlast = area under the plasma concentration time curve from time 0 to last measurable concentration; %CV = percentage of coefficient of variation; Cavg = average plasma concentration; Cmax = maximum plasma concentration; DHT = dihydrotestosterone; Tmax = time to maximum plasma concentration.
| Hormone . | Tmax (d), median (range) . | Cmax (ng/dL), mean (%CV) . | Cavg (ng/dL), mean (SD) . | AUC0-tlast (ng/dL/d), mean (%CV) . | ||
|---|---|---|---|---|---|---|
| Days 1–113 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | |
| Serum total testosterone | 13.5 (2–32) | 1,021.6 (24.9) | 703.8 (140.9) | 638.3 (124.2) | 59,927.6 (20.9) | 71,720.5 (19.9) |
| DHT | 26 (2–84) | 38.1 (18.0) | 29.9 (14.4) | 28.2 (13.7) | 2,566.5 (51.2) | 3,167.5 (49.6) |
| Free testosterone | 14 (2–53) | 327.6 (79.4) | 212.6 (47.5) | 190.3 (40.8) | 18,128.0 (25.6) | 20,979.4 (24.2) |
| Estradiol | 27 (2–84) | 57.0 (20.1) | 40.3 (17.5) | 36.6 (15.2) | 3,426.8 (36.7) | 4,110.4 (35.9) |
| Hormone . | Tmax (d), median (range) . | Cmax (ng/dL), mean (%CV) . | Cavg (ng/dL), mean (SD) . | AUC0-tlast (ng/dL/d), mean (%CV) . | ||
|---|---|---|---|---|---|---|
| Days 1–113 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | Days 1–85 . | Days 1–113 . | |
| Serum total testosterone | 13.5 (2–32) | 1,021.6 (24.9) | 703.8 (140.9) | 638.3 (124.2) | 59,927.6 (20.9) | 71,720.5 (19.9) |
| DHT | 26 (2–84) | 38.1 (18.0) | 29.9 (14.4) | 28.2 (13.7) | 2,566.5 (51.2) | 3,167.5 (49.6) |
| Free testosterone | 14 (2–53) | 327.6 (79.4) | 212.6 (47.5) | 190.3 (40.8) | 18,128.0 (25.6) | 20,979.4 (24.2) |
| Estradiol | 27 (2–84) | 57.0 (20.1) | 40.3 (17.5) | 36.6 (15.2) | 3,426.8 (36.7) | 4,110.4 (35.9) |
AUC0-tlast = area under the plasma concentration time curve from time 0 to last measurable concentration; %CV = percentage of coefficient of variation; Cavg = average plasma concentration; Cmax = maximum plasma concentration; DHT = dihydrotestosterone; Tmax = time to maximum plasma concentration.
Similar to serum testosterone, mean free testosterone concentrations (Figure 1B) increased at day 5, decreased at day 8, and then increased again on day 15; however, concentrations remained within 1% to 4% of the serum total testosterone concentration. DHT concentrations also increased initially, followed by a decrease at day 8 and a subsequent increase on day 15 (Figure 1C); mean concentrations remained within the normal range (25–75 ng/dL [0.9–2.6 nmol/L]) at most time points. Mean estradiol concentrations increased until day 29 and then gradually decreased (Figure 1D). Pharmacokinetic parameters of free testosterone, DHT, and estradiol were consistent with anticipated changes in men with androgen deficiency receiving testosterone replacement (Table 2).
Clinical Efficacy and Patient Satisfaction
Mean changes from baseline in all IIEF domain scores were positive, indicating improvement in sexual functionality with testosterone pellet implantation (Table 3 ). Statistically significant improvement was observed in erectile function, sexual desire, intercourse satisfaction, and overall satisfaction scores at the end of the study. Depressive symptoms, measured by CES-D total score (range = 0–60, with higher scores indicating increased depression), improved throughout the study from a baseline mean score of 17.9 (SD = 6.9) to 16.8 (SD = 7.49; mean change = −1.1) on day 15; 17.7 (SD = 9.20, mean change = −0.3) on day 29; 15.8 (SD = 5.4; mean change = −2.1) on day 85; and 16.7 (SD = 6.7, mean change = −1.2) on day 113; however, these changes did not reach statistical significance. Mean qADAM total score (range = 9–45, with lower scores indicating greater deficiency in male functioning) was 26.7 (SD = 5.0) at baseline and increased to 28.5 (SD = 5.7) on day 15; 28.7 (SD = 5.0) on day 29; 29.2 (SD = 4.4) on day 85; and 29.0 (SD = 5.8) on day 113. Mean changes in qADAM total score were positive at all postimplantation time points (1.9 for day 15; 2.1 for day 29; 2.5 for day 85; and 2.3 for day 113), indicating improvement in androgen-deficiency symptoms; however, changes only reached statistical significance at day 85 (mean change from baseline score = 2.5, 95% CI = 0.3–4.8). At the end of the study, most patients were “very satisfied” (40.0%) or “quite satisfied” (26.7%) with testosterone pellet implantation.
| IIEF domain (score range) . | Baseline, mean (SD) . | Mean change from baseline (95% CI) . | |||
|---|---|---|---|---|---|
| Day 15 . | Day 29 . | Day 85 . | Day 113 . | ||
| Erectile function† (1–30) | 8.5 (7.48) | 4.9 (0.9–9.0)∗∗ | 7.8 (3.5–12.1)∗∗ | 7.7 (3.2–12.3)∗∗ | 6.3 (1.2–11.5)∗∗ |
| Orgasmic function‡ (0–10) | 5.1 (3.74) | 1.2 (−0.9 to 3.4) | 1.1 (−0.1 to 2.3)§ | 1.7 (0.1–3.3)∗∗ | 0.9 (−0.9 to 2.7) |
| Sexual desire‖ (2–10) | 4.5 (2.03) | 1.8 (0.6–3.0)∗∗ | 1.8 (0.9–2.7)∗∗ | 2.1 (1.4–2.8)∗∗ | 1.7 (0.7–2.6)∗∗ |
| Intercourse satisfaction¶ (0–15) | 2.1 (3.45) | 2.5 (0.6–4.3)∗∗ | 3.9 (1.3–6.5)∗∗ | 4.1 (1.7–6.5)∗∗ | 3.6 (1.2–6.0)∗∗ |
| Overall satisfaction# (2–10) | 3.2 (1.82) | 1.6 (0.3–2.9)∗∗ | 2.2 (0.9–3.5)∗∗ | 2.0 (0.4–3.6)∗∗ | 2.0 (0.2–3.8)∗∗ |
| IIEF domain (score range) . | Baseline, mean (SD) . | Mean change from baseline (95% CI) . | |||
|---|---|---|---|---|---|
| Day 15 . | Day 29 . | Day 85 . | Day 113 . | ||
| Erectile function† (1–30) | 8.5 (7.48) | 4.9 (0.9–9.0)∗∗ | 7.8 (3.5–12.1)∗∗ | 7.7 (3.2–12.3)∗∗ | 6.3 (1.2–11.5)∗∗ |
| Orgasmic function‡ (0–10) | 5.1 (3.74) | 1.2 (−0.9 to 3.4) | 1.1 (−0.1 to 2.3)§ | 1.7 (0.1–3.3)∗∗ | 0.9 (−0.9 to 2.7) |
| Sexual desire‖ (2–10) | 4.5 (2.03) | 1.8 (0.6–3.0)∗∗ | 1.8 (0.9–2.7)∗∗ | 2.1 (1.4–2.8)∗∗ | 1.7 (0.7–2.6)∗∗ |
| Intercourse satisfaction¶ (0–15) | 2.1 (3.45) | 2.5 (0.6–4.3)∗∗ | 3.9 (1.3–6.5)∗∗ | 4.1 (1.7–6.5)∗∗ | 3.6 (1.2–6.0)∗∗ |
| Overall satisfaction# (2–10) | 3.2 (1.82) | 1.6 (0.3–2.9)∗∗ | 2.2 (0.9–3.5)∗∗ | 2.0 (0.4–3.6)∗∗ | 2.0 (0.2–3.8)∗∗ |
IIEF = International Index of Erectile Function.
Higher values indicate a more positive rating on the scale. Change is defined as visit score minus baseline score, with a positive change score reflecting improvement and a negative change score reflecting worsening. Mean change estimate and its 95% CI are computed from a mixed model with a factor of visits and repeat measurements within a patient by using all available data from baseline and days 15, 29, 85, and 113. For all domains and time points, n = 15 unless otherwise noted.
IIEF questions 1 to 5 and 15.
IIEF questions 9 and 10.
n = 14.
IIEF questions 11 and 12.
IIEF questions 6 to 8.
IIEF questions 13 and 14.
Statistically significant improvement vs baseline (ie, 95% CI does not contain zero).
| IIEF domain (score range) . | Baseline, mean (SD) . | Mean change from baseline (95% CI) . | |||
|---|---|---|---|---|---|
| Day 15 . | Day 29 . | Day 85 . | Day 113 . | ||
| Erectile function† (1–30) | 8.5 (7.48) | 4.9 (0.9–9.0)∗∗ | 7.8 (3.5–12.1)∗∗ | 7.7 (3.2–12.3)∗∗ | 6.3 (1.2–11.5)∗∗ |
| Orgasmic function‡ (0–10) | 5.1 (3.74) | 1.2 (−0.9 to 3.4) | 1.1 (−0.1 to 2.3)§ | 1.7 (0.1–3.3)∗∗ | 0.9 (−0.9 to 2.7) |
| Sexual desire‖ (2–10) | 4.5 (2.03) | 1.8 (0.6–3.0)∗∗ | 1.8 (0.9–2.7)∗∗ | 2.1 (1.4–2.8)∗∗ | 1.7 (0.7–2.6)∗∗ |
| Intercourse satisfaction¶ (0–15) | 2.1 (3.45) | 2.5 (0.6–4.3)∗∗ | 3.9 (1.3–6.5)∗∗ | 4.1 (1.7–6.5)∗∗ | 3.6 (1.2–6.0)∗∗ |
| Overall satisfaction# (2–10) | 3.2 (1.82) | 1.6 (0.3–2.9)∗∗ | 2.2 (0.9–3.5)∗∗ | 2.0 (0.4–3.6)∗∗ | 2.0 (0.2–3.8)∗∗ |
| IIEF domain (score range) . | Baseline, mean (SD) . | Mean change from baseline (95% CI) . | |||
|---|---|---|---|---|---|
| Day 15 . | Day 29 . | Day 85 . | Day 113 . | ||
| Erectile function† (1–30) | 8.5 (7.48) | 4.9 (0.9–9.0)∗∗ | 7.8 (3.5–12.1)∗∗ | 7.7 (3.2–12.3)∗∗ | 6.3 (1.2–11.5)∗∗ |
| Orgasmic function‡ (0–10) | 5.1 (3.74) | 1.2 (−0.9 to 3.4) | 1.1 (−0.1 to 2.3)§ | 1.7 (0.1–3.3)∗∗ | 0.9 (−0.9 to 2.7) |
| Sexual desire‖ (2–10) | 4.5 (2.03) | 1.8 (0.6–3.0)∗∗ | 1.8 (0.9–2.7)∗∗ | 2.1 (1.4–2.8)∗∗ | 1.7 (0.7–2.6)∗∗ |
| Intercourse satisfaction¶ (0–15) | 2.1 (3.45) | 2.5 (0.6–4.3)∗∗ | 3.9 (1.3–6.5)∗∗ | 4.1 (1.7–6.5)∗∗ | 3.6 (1.2–6.0)∗∗ |
| Overall satisfaction# (2–10) | 3.2 (1.82) | 1.6 (0.3–2.9)∗∗ | 2.2 (0.9–3.5)∗∗ | 2.0 (0.4–3.6)∗∗ | 2.0 (0.2–3.8)∗∗ |
IIEF = International Index of Erectile Function.
Higher values indicate a more positive rating on the scale. Change is defined as visit score minus baseline score, with a positive change score reflecting improvement and a negative change score reflecting worsening. Mean change estimate and its 95% CI are computed from a mixed model with a factor of visits and repeat measurements within a patient by using all available data from baseline and days 15, 29, 85, and 113. For all domains and time points, n = 15 unless otherwise noted.
IIEF questions 1 to 5 and 15.
IIEF questions 9 and 10.
n = 14.
IIEF questions 11 and 12.
IIEF questions 6 to 8.
IIEF questions 13 and 14.
Statistically significant improvement vs baseline (ie, 95% CI does not contain zero).
Safety
Twelve AEs were reported by 6 of the 15 patients. The most common AE reported by patients was implant site pain, with five events reported by five patients (Table 4 ). All AEs were mild to moderate in intensity and, except for one AE, were considered by investigators to be related to study treatment. Three patients reported mild implant site pain and two patients experienced implant site pain of moderate intensity. One patient experienced implant site infection that was moderate. This occurred on day 2 with extrusion of five testosterone pellets on day 12, after which implant site pain and infection resolved. Polycythemia was reported by one patient on day 29; the AE resolved on day 190 and was not associated with any other AEs. No clinically meaningful changes in hematology, serum chemistry values, or vital signs were observed. No severe AEs, AE-related discontinuations, or serious AEs were reported.
| Adverse event . | Patients (N = 15), n (%) . |
|---|---|
| ≥1 adverse event | 6 (40.0) |
| Implant site pain | 5 (33.3) |
| Device expulsion | 1 (6.7) |
| Headache | 1 (6.7) |
| Implant site infection | 1 (6.7) |
| Implant site inflammation | 1 (6.7) |
| Implant site pruritus | 1 (6.7) |
| Implant site swelling | 1 (6.7) |
| Polycythemia | 1 (6.7) |
| Adverse event . | Patients (N = 15), n (%) . |
|---|---|
| ≥1 adverse event | 6 (40.0) |
| Implant site pain | 5 (33.3) |
| Device expulsion | 1 (6.7) |
| Headache | 1 (6.7) |
| Implant site infection | 1 (6.7) |
| Implant site inflammation | 1 (6.7) |
| Implant site pruritus | 1 (6.7) |
| Implant site swelling | 1 (6.7) |
| Polycythemia | 1 (6.7) |
Treatment-emergent adverse events were not necessarily considered related to the study drug by the investigator.
| Adverse event . | Patients (N = 15), n (%) . |
|---|---|
| ≥1 adverse event | 6 (40.0) |
| Implant site pain | 5 (33.3) |
| Device expulsion | 1 (6.7) |
| Headache | 1 (6.7) |
| Implant site infection | 1 (6.7) |
| Implant site inflammation | 1 (6.7) |
| Implant site pruritus | 1 (6.7) |
| Implant site swelling | 1 (6.7) |
| Polycythemia | 1 (6.7) |
| Adverse event . | Patients (N = 15), n (%) . |
|---|---|
| ≥1 adverse event | 6 (40.0) |
| Implant site pain | 5 (33.3) |
| Device expulsion | 1 (6.7) |
| Headache | 1 (6.7) |
| Implant site infection | 1 (6.7) |
| Implant site inflammation | 1 (6.7) |
| Implant site pruritus | 1 (6.7) |
| Implant site swelling | 1 (6.7) |
| Polycythemia | 1 (6.7) |
Treatment-emergent adverse events were not necessarily considered related to the study drug by the investigator.
Discussion
The FDA-approved testosterone pellets for use in 1972 and recommended implantation of two to six pellets (150–450 mg) subcutaneously. However, the medical literature suggests that higher doses (>10 pellets; ≥750 mg) might be common in clinical practice.13–15 The pharmacokinetics, clinical efficacy, and tolerability of a high-dose regimen were unclear. In the present study, implantation of testosterone pellets (900 mg) in men showing signs of androgen deficiency provided sustained serum testosterone concentrations for approximately 4 months and improved sexual function, with minimal tolerability issues.
Similar to the pharmacokinetics of other biodegradable implants,20,21 testosterone pellets provided an initial increase in serum total testosterone concentrations followed by a slower and more constant release rate. Serum total testosterone levels peaked at approximately 2 weeks after implantation, which contrasts sharply with the rapid supra-therapeutic concentrations of testosterone caused by short-acting testosterone injections.1 A slight decrease in total testosterone concentrations was observed at day 8 but recovered by day 15. However, even during the slight decrease, testosterone concentrations remained above levels of androgen deficiency (ie, ≥300 ng/dL [10.4 nmol/L]).
All patients demonstrated testosterone concentrations higher than 300 ng/dL (10.4 nmol/L) through day 85 and most patients (71%) had testosterone concentrations higher than 300 ng/dL (10.4 nmol/L) for at least 3 months, indicating that most patients would require new implantation after 3 months and many could wait until 4 months. This is consistent with retrospective data that showed maintenance of testosterone concentrations of at least 300 ng/dL (10.4 nmol/L) for 4 months after implantation of 8 to 9 or 10 to 12 pellets,13 and an open-label study in which 86% (24 of 28) of patients who received 6 to 12 testosterone pellets (450–900 mg) maintained testosterone concentrations higher than 315 ng/dL (10.9 nmol/L) at 4 months.16
Changes from baseline in other sex hormones (DHT, free testosterone, and estradiol) were as expected and mirrored serum total testosterone concentrations. However, these results contrast with data from a large (N = 273) retrospective study that demonstrated significantly increased estradiol concentrations in androgen-deficient men who received implantation of 10 to 12 testosterone pellets.14 The reason for this discrepancy is currently unknown.
Testosterone pellets have been shown to significantly improve sexual dysfunction.16 In the present study, IIEF scores improved from baseline throughout the study. Depression and androgen-deficiency symptoms also showed improvement, although changes in the latter reached statistical significance only on day 85. These findings support data from an open-label study that showed mean IIEF scores were significantly higher (indicating improvement) at 1 month (P = .003) and 3 months (P = .001) after implantation of 6 to 12 testosterone pellets (450–900 mg) in men with androgen deficiency.16 Taken together, improvement in depression and other androgen-deficiency symptoms might beneficially affect patient satisfaction and adherence to the treatment regimen.10
Implantation of 12 testosterone pellets was generally well tolerated. Pellet extrusion and infection have been cited as a concern for other testosterone pellet products22; however, pellet extrusion and infection were observed in only one patient (6.7%) in the present study. Other studies using Testopel pellets have reported low extrusion rates (4 of 380 patients [1%]13 and 1 of 28 patients [4%]16).
This study was limited by its small and ethnically non-diverse patient population. In addition, its open-label uncontrolled design might have influenced patient response to questionnaires and reporting of AEs; therefore, caution should be used in interpreting the data from the patient-reported outcomes. Despite these limitations, the consistency of the positive results regarding serum testosterone levels and sexual function with high-dose (900-mg) testosterone pellet implantation in men with androgen deficiency is encouraging.
Conclusion
Implantation of 12 75-mg testosterone pellets provided adequate and sustained serum testosterone concentrations and was well tolerated. Subjective ratings of sexual dysfunction and androgen deficiency for up to 4 months in men with androgen deficiency improved over time; however, additional randomized placebo-controlled studies are needed to confirm these findings. This study and previous studies have demonstrated the clinical efficacy and safety of implantation of at least six testosterone pellets13,15,16 and suggest that patients could benefit from implantation of high doses (900 mg) of testosterone pellets.
Statement of authorship
Category 1
- (a)
Conception and Design
Brian Cohen
- (b)
Acquisition of Data
Chris G. McMahon; Brian Cohen
- (c)
Analysis and Interpretation of Data
Chris G. McMahon; Neil Shusterman; Brian Cohen
Category 2
- (a)
Drafting the Article
Chris G. McMahon; Neil Shusterman; Brian Cohen
- (b)
Revising It for Intellectual Content
Chris G. McMahon; Neil Shusterman; Brian Cohen
Category 3
- (a)
Final Approval of the Completed Article
Chris G. McMahon; Neil Shusterman; Brian Cohen
Acknowledgments
The authors thank Synchrony Medical Communications, LLC (West Chester, PA, USA) for providing writing and editorial support under the direction of the authors; this support was funded by Endo Pharmaceuticals Inc.
Funding
This study was funded by Auxilium Pharmaceuticals, Inc.
References
Author notes
Former employee of Endo Pharmaceuticals.
Conflicts of Interest: Dr McMahon is a consultant and investigator for Absorption Pharmaceuticals, Ixchelsis, Neurohealing Pharmaceuticals, Eli Lilly and Company, Johnson & Johnson, Janssen Cilag Asia Pacific, and Menarini. Dr Shusterman and Mr Cohen are former employees of Endo Pharmaceuticals Inc.



