-
PDF
- Split View
-
Views
-
Cite
Cite
Geoff Hackett, Michael Kirby, David Edwards, Thomas Hugh Jones, Kevan Wylie, Nick Ossei-Gerning, Janine David, Asif Muneer, British Society for Sexual Medicine Guidelines on Adult Testosterone Deficiency, with Statements for UK Practice, The Journal of Sexual Medicine, Volume 14, Issue 12, December 2017, Pages 1504–1523, https://doi.org/10.1016/j.jsxm.2017.10.067
Close - Share Icon Share
Abstract
Testosterone deficiency (TD) is an increasingly common problem with significant health implications, but its diagnosis and management can be challenging.
To review the available literature on TD and provide evidence-based statements for UK clinical practice.
Evidence was derived from Medline, EMBASE, and Cochrane searches on hypogonadism, testosterone (T) therapy, and cardiovascular safety from May 2005 to May 2015. Further searches continued until May 2017.
To provide a guideline on diagnosing and managing TD, with levels of evidence and grades of recommendation, based on a critical review of the literature and consensus of the British Society of Sexual Medicine panel.
25 statements are provided, relating to 5 key areas: screening, diagnosis, initiating T therapy, benefits and risks of T therapy, and follow-up. 7 statements are supported by level 1, 8 by level 2, 5 by level 3, and 5 by level 4 evidence.
To help guide UK practitioners on effectively diagnosing and managing primary and age-related TD.
A large amount of literature was carefully sourced and reviewed, presenting the best evidence available at the time. However, some statements provided are based on poor-quality evidence. This is a rapidly evolving area of research and recommendations are subject to change. Guidelines can never replace clinical expertise when making treatment decisions for individual patients, but rather help to focus decisions and take personal values and preferences and individual circumstances into account. Many issues remain controversial, but in the meantime, clinicians need to manage patient needs and clinical expectations armed with the best clinical evidence and the multidisciplinary expert opinion available.
Improving the diagnosis and management of TD in adult men should provide somatic, sexual, and psychological benefits and subsequent improvements in quality of life.
Panel composition
The British Society of Sexual Medicine (BSSM) panel consists of a group of experts in urology, andrology, cardiology, psychiatry, and sexual medicine.
Sources of information
The BSSM UK policy statements on testosterone deficiency (TD), published in 2016,1 were based on evidence derived from Medline, EMBASE, and Cochrane searches on hypogonadism, testosterone (T) therapy, and cardiovascular (CV) safety from May 2005 to May 2015, which yielded 1,714 articles, including 52 clinical trials and 32 placebo-controlled randomized controlled trials (RCTs). Further searches continued until May 2017. Levels of evidence (LoEs) and grades of recommendation were based on the Oxford Criteria for Evidence-Based Medicine.2
Introduction
T is the principal androgen in men. It is essential for the development and maintenance of secondary male characteristics.3 When T levels decrease, patients can experience physical and psychological effects, which can compromise their general well-being, sexuality, and fertility.4,5
These statements have been developed for UK practice and aim to address the widespread media and scientific concerns over the appropriate treatment of TD with T therapy.
They apply to adult men only. Women are not included, because there is currently no consensus for using T in women and the T products available are not licensed for use in women.
Definition
TD is a well-established and significant medical condition.1 It is defined as a clinical and biochemical syndrome associated with advancing age and comorbidities (LoE = 2, Grade = B)6 and is characterized by a deficiency in serum androgen levels (with or without decreased genomic sensitivity to androgens1) and relevant signs and symptoms.6,7
TD can adversely affect multiple organ systems and result in significant decreases in quality of life, including changes in sexual function4,6,7 (LoE = 2, Grade = B).6
Epidemiology
Estimates regarding the prevalence of TD vary widely. The European Male Aging Study (EMAS) evaluated more than 3,000 men 40 to 79 years old according to biochemistry and symptoms. Results showed an overall prevalence of 2.1% in men 40 to 79 years old and rates of 0.1% in men 40 to 49 years old, 0.6% in men 50 to 59 years old, 3.2% in men 60 to 69 years old, and 5.1% in men 70 to 79 years old (in which the syndrome of TD included ≥3 sexual symptoms associated with a total T [TT] level < 11 nmol/L and a free T [FT] level < 220 pmol/L [<0.22 nmol/L]).8 However, 75% of men maintained normal T levels into old age, suggesting that TD is not merely a function of aging. The prevalence of secondary TD was 11.8%, with 2% having primary TD and 9.5% having compensated (subclinical) TD, worthy of observation but not treatment with T.9
TD is more common in older men and those with obesity, comorbidities, and poor health status.4
Basic physiology
In eugonadal men, the regulation of T production is controlled by the hypothalamic-pituitary-gonadal axis.3 In the brain, the hypothalamus secretes gonadotropin-releasing hormone, which stimulates the anterior pituitary gland to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In the testes, LH stimulates the Leydig cells to produce T, and FSH stimulates the seminiferous tubules for sperm maturation. Through a negative feedback mechanism, T inhibits gonadotropin-releasing hormone and LH secretion, and the hormone inhibin B, secreted by Sertoli cells, inhibits the release of FSH.3,10
Androgen receptors (ARs) are present in many body tissues. They allow the body to respond appropriately to T by attaching (binding) to it. The resulting AR complex binds to DNA, regulating the activity of androgen-responsive genes. The AR gene contains a DNA segment known as CAG, which is repeated multiple times. In most people, the number of CAG repeats ranges from less than 10 to approximately 36.11 The length of these repeats in the AR gene can influence androgen sensitivity,12 androgen action,13 and androgenic phenotypical effects, even in the presence of normal T levels.14
T has multiple effects on the body. In the brain, it stimulates libido and aggression and aids cognition, memory, and feelings. In the kidneys, it promotes erythropoiesis. In the skin, it supports collagen production and stimulates hair growth and sebum production. In the heart, it affects cardiac output and coronary and peripheral blood flow, decreases the corrected QT interval, and decreases reperfusion injury. T also affects muscle mass and strength, bone growth, density and erythropoiesis, growth of the sex organs, spermatogenesis, and erectile function.1,15–18
Etiology
TD occurs when the body cannot produce sufficient T to function normally. This can result from disruption of at least 1 level of the hypothalamic-pituitary-gonadal axis (LoE = 1, Grade = A)6:
- •
Testes (primary TD, Figure 1 )
- •
Hypothalamus and pituitary gland (secondary TD)
- •
Hypothalamus,19,20 pituitary, and testes (combined primary and secondary TD; adapted from Grossman and Matsumoto19)

Primary hypogonadism. FSH = follicle-stimulating hormone; LH = luteinizing hormone. Figure 1 is available in color at www.jsm.jsexmed.org.
Secondary TD is the most common form.9,21 The term “functional” (late-onset, age-related, or adult-onset) TD has recently been introduced to describe androgen-deficiency–like features and low T levels in men older than 50 years associated with conditions such as obesity and metabolic syndrome in the absence of intrinsic, structural hypothalamic-pituitary-testicular axis pathology and specific pathologic conditions suppressing the hypothalamic-pituitary-testicular axis (eg, microprolactinoma or endogenous Cushing syndrome19; Figure 2 presents other causes of secondary TD). However, the BSSM is concerned that that this definition might lead to men with potentially important comorbid conditions going untreated.
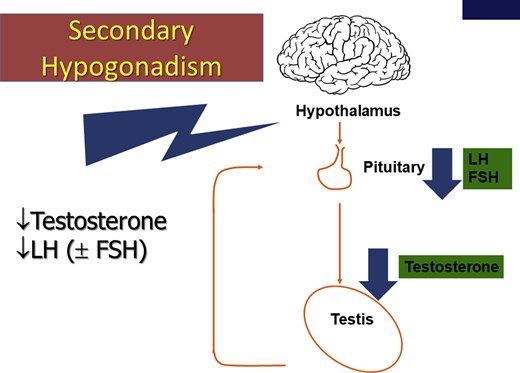
Secondary hypogonadism. FSH = follicle-stimulating hormone; LH = luteinizing hormone. Figure 2 is available in color at www.jsm.jsexmed.org.
Combined primary and secondary TD, sometimes known as mixed TD, is associated with low T levels, impaired spermatogenesis, and variable FSH and LH levels, depending on whether primary or secondary testicular failure predominates.22 Combined primary and secondary TD occurs with certain conditions such as sickle cell disease, hemochromatosis, thalassemia, and mutations in the DAX-1 gene and with glucocorticoid treatment and alcoholism.22–24
Compensated TD occurs when men have normal levels of T but higher gonadotropin levels. Characterized by increased LH levels and older age, compensated TD has been proposed as a genuine clinical subgroup of late-onset hypogonadism, from which some men could eventually progress to overt primary TD.9 Further studies are required to investigate the long-term impact of compensated TD.
TD also can result if the action of T is impaired because of decreased bioavailability of the hormone (resulting from variations in sex hormone binding globulin [SHBG])6 or because of AR changes that can affect androgen activity6,25 (LoE = 2, Grade = A).6
Certain medications can suppress T levels, including oral glucocorticoids, opioids, and antipsychotics26–29 (Table 1 ).
| Sexual | Physical |
| Delayed puberty | Decreased body hair |
| Small testes | Gynecomastia |
| Infertility | Decreased muscle mass and strength |
| Decreased sexual desire and activity | Hot flushes or sweats |
| Decreased frequency of sexual thoughts | Sleep disturbances |
| Erectile dysfunction | Fatigue |
| Delayed ejaculation | Osteoporosis, height loss, low trauma fractures |
| Decreased volume of ejaculate | |
| Decreased or absent morning or night-time erections | |
| Cardiometabolic | Psychological |
| Increased BMI or obesity | Changes in mood (eg, anger, irritability, sadness, depression) |
| Visceral obesity | Decreased well-being or poor self-rated health |
| Metabolic syndrome | Decreased cognitive function (including impaired concentration, verbal memory, and spatial performance) |
| Insulin resistance and T2DM |
| Sexual | Physical |
| Delayed puberty | Decreased body hair |
| Small testes | Gynecomastia |
| Infertility | Decreased muscle mass and strength |
| Decreased sexual desire and activity | Hot flushes or sweats |
| Decreased frequency of sexual thoughts | Sleep disturbances |
| Erectile dysfunction | Fatigue |
| Delayed ejaculation | Osteoporosis, height loss, low trauma fractures |
| Decreased volume of ejaculate | |
| Decreased or absent morning or night-time erections | |
| Cardiometabolic | Psychological |
| Increased BMI or obesity | Changes in mood (eg, anger, irritability, sadness, depression) |
| Visceral obesity | Decreased well-being or poor self-rated health |
| Metabolic syndrome | Decreased cognitive function (including impaired concentration, verbal memory, and spatial performance) |
| Insulin resistance and T2DM |
BMI = body mass index; T2DM = type 2 diabetes mellitus.
TD is often associated with increased waist circumference, obesity, metabolic syndrome, and impaired health status.1 In a primary care population of men at least 45 years, the reported odds ratios for TD with comorbid conditions were 2.38 for obesity, 2.09 for T2DM, 1.84 for hypertension, 1.47 for dyslipidemia, 1.40 for chronic obstructive pulmonary disease, and 1.20 for lower urinary tract symptoms related to benign prostatic hyperplasia.32 Significant TD is associated with an increased risk of chronic anemia and osteoporosis.1 Table 2 presents a list of the conditions associated with an increased prevalence of TD.
| Sexual | Physical |
| Delayed puberty | Decreased body hair |
| Small testes | Gynecomastia |
| Infertility | Decreased muscle mass and strength |
| Decreased sexual desire and activity | Hot flushes or sweats |
| Decreased frequency of sexual thoughts | Sleep disturbances |
| Erectile dysfunction | Fatigue |
| Delayed ejaculation | Osteoporosis, height loss, low trauma fractures |
| Decreased volume of ejaculate | |
| Decreased or absent morning or night-time erections | |
| Cardiometabolic | Psychological |
| Increased BMI or obesity | Changes in mood (eg, anger, irritability, sadness, depression) |
| Visceral obesity | Decreased well-being or poor self-rated health |
| Metabolic syndrome | Decreased cognitive function (including impaired concentration, verbal memory, and spatial performance) |
| Insulin resistance and T2DM |
| Sexual | Physical |
| Delayed puberty | Decreased body hair |
| Small testes | Gynecomastia |
| Infertility | Decreased muscle mass and strength |
| Decreased sexual desire and activity | Hot flushes or sweats |
| Decreased frequency of sexual thoughts | Sleep disturbances |
| Erectile dysfunction | Fatigue |
| Delayed ejaculation | Osteoporosis, height loss, low trauma fractures |
| Decreased volume of ejaculate | |
| Decreased or absent morning or night-time erections | |
| Cardiometabolic | Psychological |
| Increased BMI or obesity | Changes in mood (eg, anger, irritability, sadness, depression) |
| Visceral obesity | Decreased well-being or poor self-rated health |
| Metabolic syndrome | Decreased cognitive function (including impaired concentration, verbal memory, and spatial performance) |
| Insulin resistance and T2DM |
BMI = body mass index; T2DM = type 2 diabetes mellitus.
TD is often associated with increased waist circumference, obesity, metabolic syndrome, and impaired health status.1 In a primary care population of men at least 45 years, the reported odds ratios for TD with comorbid conditions were 2.38 for obesity, 2.09 for T2DM, 1.84 for hypertension, 1.47 for dyslipidemia, 1.40 for chronic obstructive pulmonary disease, and 1.20 for lower urinary tract symptoms related to benign prostatic hyperplasia.32 Significant TD is associated with an increased risk of chronic anemia and osteoporosis.1 Table 2 presents a list of the conditions associated with an increased prevalence of TD.
The clinical manifestations of TD result from decreased serum androgen concentrations or activity, with or without an identified underlying cause (LoE = 1, Grade = A).6
Clinical diagnosis of TD
The diagnosis of symptomatic TD requires the presence of characteristic signs and symptoms (Level = 2, Grade = A) plus decreased serum concentrations of TT or FT (LoE = 2, Grade = A).26
The assessment of gonadotropins is required to determine the origin of the TD (see section on Laboratory Diagnosis).
Signs, symptoms, and comorbidities
TD has well established symptoms (Table 2 ).1 The 4th International Consultation for Sexual Medicine (ICSM)6 made the following recommendations for the clinical diagnosis of TD based on the following signs and symptoms:
- •
Sexual dysfunction, especially low sexual desire, decreased morning and night-time erections, and erectile dysfunction (ED) are prominent, commonly presenting symptoms particularly suggestive of TD when associated with each other (LoE = 1, Grade = A).6
- •
Less specific symptoms such as fatigue, sleep disturbance, loss of physical strength, decreased energy and motivation, and depressed mood are often present (LoE = 1, Grade = B).6
- •
Visceral obesity and decreases in muscle mass and bone mineral density are commonly observed (LoE = 1, Grade = A).6
- •
Hot flushes and changes in cognition and memory can be associated with TD (LoE = 3, Grade = C).6
- •
On physical examination, features suggestive of TD include decreased body hair, decreased testicular size, and gynecomastia, but these are not always present (LoE = 1, Grade = B).6 Fine wrinkling of the skin, especially around the mouth, also can be apparent.
| Andrologic and endocrinologic . | Metabolic diseases associated with insulin resistance . | Cardiovascular diseases . | Other chronic diseases . | Pharmacologic . |
|---|---|---|---|---|
| Delayed puberty | Obesity | Hypertension | Chronic obstructive pulmonary disease | Oral glucocorticoid treatment |
| Cryptorchidism | Metabolic syndrome | Coronary artery disease | Obstructive sleep apnea | Regular opioid use |
| Pituitary disease | Type 2 diabetes | Cerebrovascular disease | End-stage renal disease | Antipsychotic medications |
| Infertility | Chronic heart failure | Cirrhosis | Androgen deprivation therapy | |
| Varicocele | Atrial fibrillation | Osteoporosis | Methadone maintenance therapy | |
| Rheumatoid arthritis | Antiretroviral therapy | |||
| HIV | Chemotherapy + radiation | |||
| cancer | Anticonvulsant therapy |
| Andrologic and endocrinologic . | Metabolic diseases associated with insulin resistance . | Cardiovascular diseases . | Other chronic diseases . | Pharmacologic . |
|---|---|---|---|---|
| Delayed puberty | Obesity | Hypertension | Chronic obstructive pulmonary disease | Oral glucocorticoid treatment |
| Cryptorchidism | Metabolic syndrome | Coronary artery disease | Obstructive sleep apnea | Regular opioid use |
| Pituitary disease | Type 2 diabetes | Cerebrovascular disease | End-stage renal disease | Antipsychotic medications |
| Infertility | Chronic heart failure | Cirrhosis | Androgen deprivation therapy | |
| Varicocele | Atrial fibrillation | Osteoporosis | Methadone maintenance therapy | |
| Rheumatoid arthritis | Antiretroviral therapy | |||
| HIV | Chemotherapy + radiation | |||
| cancer | Anticonvulsant therapy |
| Andrologic and endocrinologic . | Metabolic diseases associated with insulin resistance . | Cardiovascular diseases . | Other chronic diseases . | Pharmacologic . |
|---|---|---|---|---|
| Delayed puberty | Obesity | Hypertension | Chronic obstructive pulmonary disease | Oral glucocorticoid treatment |
| Cryptorchidism | Metabolic syndrome | Coronary artery disease | Obstructive sleep apnea | Regular opioid use |
| Pituitary disease | Type 2 diabetes | Cerebrovascular disease | End-stage renal disease | Antipsychotic medications |
| Infertility | Chronic heart failure | Cirrhosis | Androgen deprivation therapy | |
| Varicocele | Atrial fibrillation | Osteoporosis | Methadone maintenance therapy | |
| Rheumatoid arthritis | Antiretroviral therapy | |||
| HIV | Chemotherapy + radiation | |||
| cancer | Anticonvulsant therapy |
| Andrologic and endocrinologic . | Metabolic diseases associated with insulin resistance . | Cardiovascular diseases . | Other chronic diseases . | Pharmacologic . |
|---|---|---|---|---|
| Delayed puberty | Obesity | Hypertension | Chronic obstructive pulmonary disease | Oral glucocorticoid treatment |
| Cryptorchidism | Metabolic syndrome | Coronary artery disease | Obstructive sleep apnea | Regular opioid use |
| Pituitary disease | Type 2 diabetes | Cerebrovascular disease | End-stage renal disease | Antipsychotic medications |
| Infertility | Chronic heart failure | Cirrhosis | Androgen deprivation therapy | |
| Varicocele | Atrial fibrillation | Osteoporosis | Methadone maintenance therapy | |
| Rheumatoid arthritis | Antiretroviral therapy | |||
| HIV | Chemotherapy + radiation | |||
| cancer | Anticonvulsant therapy |
Many signs and symptoms of TD are multifactorial in origin and associated with various lifestyle and psychological factors and with normal aging, so they can be found in men with normal T levels.4,34
Although it is commonly stated that T decreases with age,35 more than 80% of men maintain normal T levels into old age.9 The age-related increase in SHBG does cause a decrease in FT and bioavailable T, but the increased rates of TD seen in older men are primarily related to an increased prevalence of obesity, type 2 diabetes mellitus (T2DM), and chronic illness.36
The signs and symptoms of TD vary depending on age at onset, duration, and severity.1,4 Although the prevalence of TD increases with advancing age, it can occur in adult men of any age (LoE = 1, Grade = B).6 The more signs and symptoms a man has, the greater the probability that he has genuine TD.26,37
The prevalence of hypogonadal symptoms increases when TT levels decrease below 12.1 nmol/L26,37 (LoE = 2b, Grade = A).26 However, they also are seen at higher levels. A cross-sectional cohort study investigating the structure of complaints in 434 hypogonadal men 50 to 86 years old reported loss of erections at a TT level of approximately 8 nmol/L, diabetes and depression at 10 nmol/L, obesity at 12 nmol/L, and decreased vigor at 15 nmol/L. The investigators found no evidence for a uniform structure of T concentrations and complaints and concluded that in aging men psychosomatic complaints and metabolic risk relate to T in a symptom-specific manner.37
Rationale for T therapy
Because many adult men with TD are sick and/or obese, weight loss if overweight, lifestyle modification, and optimal management of comorbidities are important first steps.4,38,39
However, a 4.3-year follow-up from the EMAS40 found that biochemical reversal of secondary hypogonadism was not associated with an improvement in symptoms. In addition, an RCT of obese hypogonadal men treated with severe weight loss and long-acting T undecanoate (TU) injections or placebo for 12 months found that although decreased weight and body mass index (BMI) were achieved in the 2 groups, only the TU group preserved lean muscle and achieved symptomatic benefit.41,42 Therefore, we believe that current evidence does not support the recommendation for lifestyle intervention alone as first-line therapy. Patients with low T present with distressing symptoms and therapy should be relevant to the effective relief of those symptoms. Therefore, we endorse lifestyle modification in conjunction with T therapy in men with symptomatic TD (LoE = 2, Grade = A).
T therapy is indicated for men with symptoms of TD supported by low biochemical levels. Because the 3 most common symptoms of deficiency are ED, loss of early morning erections, and low sexual desire, most candidates for T therapy present with sexual dysfunction and a desire for treatment. The increasing realization that ED is a strong marker for coronary heart disease risk and recent recommendations on obesity and diabetes by the American Association of Clinical Endocrinologists and the American College of Endocrinology43,44 are likely to increase the number of men detected by routine screening. Against this recognition of the importance of T in cardiometabolic disease, it is inevitable that the past 20 years has seen a large increase in men being treated.45,46
In men, multiple symptoms such as loss of vitality, tiredness, decreased strength, and tiredness also might demand attention. In men with no other comorbidities, T therapy alone might reverse the symptoms, but where other comorbidities exist, ED-specific therapy (usually a phosphodiesterase type 5 inhibitor [PDE5i]) will often be required. Studies have suggested that the best responses in sexual symptoms are seen with baseline levels below 8 nmol/L, that sexual desire improves by 6 weeks, but that ED improvement could take longer. Benefits in loss of fat mass and increase of lean muscle mass can take at least 12 months. Longer-term studies have suggested that symptoms improve progressively over many years. Because most men will only receive 1 trial of T therapy in their lifetime, we recommend a minimum of 6 months, assuming effective levels are achieved. At that point, continuation should be discussed once the patient has received sufficient accurate information for an informed decision.
Men whose T therapy has failed despite multiple doses (>8) of a PDE5i can frequently be salvaged by correcting a TT level lower than 12 nmol/L, although 1 trial showed significance only at levels below 10.4 nmol/L.47 T has been shown to upregulate PDE5 and most trials of oral therapy have excluded men with untreated TD. Failure of oral therapy in these men also could be related to previously undetected low desire or ejaculatory or orgasmic dysfunction or dislike of on-demand therapy by the couple. T therapy could address these issues.48 With the availability of generic PDE5is, avoiding the progression to expensive second- and third-line therapies will become increasingly cost effective.
Evidence for T therapy in men with TD
In a retrospective study of men with TD, T therapy was associated with an average decrease in American Urological Association symptom index and International Prostate Symptom Score of −7.42 in those with severe lower urinary tract symptoms (LUTS) at baseline.49 T therapy also has been associated with an improvement in depression scores in RCTs.50
In the TIMES218 and BLAST51 studies, which recruited patients with T2DM and/or metabolic syndrome, T therapy was associated with small benefits in lipid metabolism, but blood pressure was unaffected. T therapy appeared to attenuate insulin resistance most markedly in patients with poorly controlled T2DM. These benefits were amplified when combined with lifestyle modification.51
A long-term registry study lasting longer than 5 years showed progressive weight loss with T therapy, decreases in waist circumference and BMI, total and low-density lipoprotein cholesterol, triglycerides, systolic and diastolic blood pressure, blood glucose, and C-reactive protein, and an increase in high-density lipoprotein cholesterol.52,53
A more recent RCT from Australia evaluated severely obese men with hypogonadism in a double-blinded RCT involving long-acting TU or placebo plus intense dieting in the first 10 weeks. At 12 months, there was no significant difference in weight loss, BMI, and waist circumference, but the men on TU preserved all their lean muscle, whereas men on the diet alone lost an additional 3.5 kg of lean muscle compared with the TU group. The active group also showed significant loss of visceral and appendicular fat plus increased grip strength.42
In a meta-analysis of 59 RCTs involving 5,078 subjects, T therapy was not associated with any significant changes in body weight, BMI, or waist circumference, but it consistently improved lean muscle and decreased fat mass.54
The Testosterone Trials were a set of 7 coordinated, double-blinded, placebo-controlled trials conducted at 12 sites55 involving 790 men at least 65 years old with serum T concentrations lower than 275 ng/dL. Participants received T or placebo gel for 1 year. Dosing was adjusted to maintain T concentrations within the normal range for young men (19–40 years of age). Efficacy was assessed at baseline and then at 3, 6, 9, and 12 months.56
In men who participated in at least 1 of 3 the Testosterone Trials (Sexual Function Trial, Physical Function Trial, and Vitality Trial), T therapy was associated with significant improvements in sexual desire, sexual activity, and erectile function and modest improvements in 6-minute walking test, mood, and depression.56
The Bone Trial55 found that T therapy significantly increased volumetric bone mineral density and estimated bone strength. These changes were greater in trabecular than in peripheral bone and in the spine than in the hip. Larger and longer trials are required to determine whether this treatment also lowers fracture risk.57 Earlier evidence had shown that T therapy consistently improved bone mineral density in the lumbar spine but was not associated with significant improvements in hip scores.58
The Anaemia Trial55 found that T therapy significantly increased hemoglobin levels in men with anemia from unexplained or known causes,59 whereas the Cognitive Function Trial55 found no effect on memory or other cognitive function.60
Several meta-analyses of RCTs, notably by Corona et al61 in 2017, concluded that T therapy in men with TD significantly improves sexual desire and erectile function (particularly in men with T levels < 8 nmol/L) and increases sexual activity, satisfaction, and orgasm.1
A limitation of some of the aforementioned RCTs is that they were of relatively short duration, usually 3 to 12 months. The evidence strongly suggests that trials of T therapy should last at least 6 months.1,51
TD is commonly associated with decreased fertility.4 In younger men, T therapy lowers LH and FSH levels and frequently causes infertility after 6 to 12 months; but this is reversible in 60% to 70% of men within 9 to 12 months.62
TD and increased CV and all-cause mortality
Numerous long-term studies and various reviews and meta-analyses have provided evidence to support the association between TD and increased CV and all-cause mortality,1,63–68 although evidence for a pathogenic link is lacking.1,69
A systematic review and meta-analysis evaluating the association between endogenous T and mortality concluded that low levels of endogenous T were associated with an increased risk of all-cause and CV death in community-based studies of men, with a decrease of 2.1 SD in TT being associated with a 25% increase in mortality. However, most of these studies had issues with cohort selection and choice.63
2 systematic reviews and meta-analyses evaluating the association between endogenous T and all-cause63 and CV disease (CVD)63,64 mortality reported a protective effect of increased TT. Research examining the data from 1,954 subjects using several statistical models found that even after strict adjustment for comorbidities, there was a consistent link between T level and mortality risk throughout, without proving causation.65
In a prospective study involving 581 men with T2DM, patients were followed for a mean of 5.81 years. Low T was defined as a TT level lower than 10.4 nmol/L. 51 men received T therapy for at least 2 years. Mortality rates were 20% in the low T group vs 9.1% in the normal T group, independent of comorbidities and therapies, and 9.4% in those with TD in the treated group.66
In a 10-year Australian study involving 3,690 older men, TT and FT levels in the normal range were associated with lower all-cause and CV mortality. This was the first evidence to suggest that low and high levels are associated with all-cause mortality and that higher levels of dihydrotestosterone lower CV risk.67
A Swedish study involving 1,109 subjects at least 40 years old, with a mean follow up of 14.1 years, suggested a strong association between low baseline T and incident myocardial infarction (MI).68
Although these studies demonstrated a consistent association between low T and CVD incidence and mortality, this did not prove a pathogenic link.1,69 However, the conclusions from a review on T and mortality were that low T could be a “marker” of illness.70
6 studies, mostly involving small samples, found that low TT and FT were associated with coronary artery disease (CAD), but 4 found no association. 4 studies found inverse associations between low TT and FT and CAD severity.69,71 However, such studies do not determine whether low TT or FT is a cause or a consequence of CAD.69
Possible increased CV risk with T therapy
Although it has been proposed that serum dihydrotestosterone can increase the risk of CVD through mechanisms involving inflammation, coagulation, and vasoreactivity,72,73 the results of studies investigating this association have been conflicting.
Concerns regarding T therapy in elderly men stem initially from the premature discontinuation of the Testosterone in Older Men (TOM) trial,74 which involved 209 frail, elderly men with a mean age of 74 years. This study was not powered to detect differences in CV events, but it was stopped early because of 23 CV-related events (2 deaths) in the 106 men in the T group vs 5 in the placebo group, despite positive results in study end points. The primary limitation of interpreting CV risk from this trial is that most adverse CV events were of questionable significance (eg, palpitations and non-specific electrocardiographic changes) and were not systematically queried. It also involved treatment initiation with topical T gel 100 mg (twice the recommended starting dose) and then rapid escalation up to 150 mg/day (above the manufacturer’s recommended dose), and many of the events were reported with inadequate validation and mainly occurred in those subjects receiving the higher doses.1,75 Similar dosing regimens used several years later in 790 men in the Testosterone Trials failed to reproduce these findings, and in the 12-month study, there were 3 deaths reported on T and 7 on placebo.56
A retrospective American study76 involved 8,709 men with a baseline TT level no higher than 10.4 nmol/L who were undergoing angiography. During a mean follow-up of 840 days, 681 of the 7,486 patients not receiving T therapy died, 420 had MIs, and 486 had strokes. Of the 1,223 patients receiving T therapy, 67 died, 23 had MIs, and 33 had strokes. Complex statistical analysis using more than 50 covariates concluded there was a greater risk in the T therapy group. However, there were concerns regarding the exclusion of 1,132 patients who experienced events because they were prescribed T therapy after the event, when they should have been included in the untreated group, increasing the events by 70%. When challenged, the investigators revised the number to 132, but admitted that 104 women had been mistakenly included in the results.1 There also were no data confirming a correct diagnosis of TD syndrome before T therapy and none on compliance with therapy. Treated patients had baseline T levels 1.2 nmol/L below that of the untreated group and therefore would have been at increased risk, especially if undertreated, a possibility confirmed by the investigators.
A study analyzing prescribing data in men treated with T therapy, without records of blood results or symptoms, defined non-fatal coronary events as the major end point, assessed in the 12 months before and 3 months after therapy.77 However, the benefits of T therapy take longer than this to appear and other studies have excluded the first 3 months treatment from analysis because of the likelihood of events relating to the pre-existing condition. Crucially, data on fatal CV events and all-cause mortality were not collected, despite T therapy in other studies reporting a major impact on mortality rather than event numbers. 12-month post-treatment data were collected but not presented. Before treatment, the event rates within the groups were strangely identical. There was a small increase in non-fatal cardiac events in men taking T therapy, which was more marked in those with increased risk. Overall, events were fewer than predicted from comparable research. The lack of mortality data demonstrates a failing to realize that a treatment that decreased mortality was likely to increase non-fatal events. In addition, the study design was not prospective, which casts doubts on the validity of retrospective assessment for the 12-month pretreatment period. Although this study has been widely quoted in public media, it is discredited by several design flaws and statistical analyses.1
In the Cardiovascular Trial set of the Testosterone Trials, 170 men were assessed by cardiac computed tomographic angiography for baseline and 1-year plaque burden. Compared with placebo, T therapy was associated with a significantly greater increase in non-calcified plaque volume over 12 months. Unfortunately, there was a significantly greater baseline plaque level in the placebo group that resulted in multiple interventions including stents and bypass grafting.78 However, the lack of change in coronary calcium in this study raises questions as to the reliability of the result of non-calcified plaque. The investigators reported no coronary events or significant drug changes in either cohort throughout the study.
A recent systematic review and meta-analysis evaluated whether T therapy was associated with an increased risk of serious CV events compared with other treatments or placebo. It included 39 RCTs and 10 observational studies. The meta-analysis used data from 30 RCTs. Compared with placebo, T therapy was not associated with any significant risk in MI, stroke, or mortality. However, the strength of the evidence for the 3 outcomes was classed as low, because of the risk of bias in the included RCTs and imprecision. These results reflect those of several other systematic reviews and meta-analyses that have yielded inconclusive findings, representing the many limitations of the individual studies. Patient-level data could not be used, many studies did not specify the reasons for study withdrawal, and whether the adverse events were identified using prespecified criteria for safety end points, potentially biasing the results, and the impact of the pharmaceutical industry funding was not explored. The results from the observational studies varied greatly and thus were not pooled statistically. The strengths of this study included rigorous and comprehensive screening of several databases, the inclusion of trials and observational studies, focusing on specific disaggregated CV end points of clinical interests, the use of meta-analytic approaches suitable for sparse data, evaluation of the stability of the results in several sensitivity analyses, and, in contrast to previous studies, the provision of strength-of-evidence ratings for the evidence. Until the results of prospective trials become available, rigorously designed observational evidence will continue to expand the growing evidence base on the risks and benefits of T therapy and its optimal clinical application.79
Studies suggesting decreased CV risk with T therapy
In a retrospective study involving 1,031 hypogonadal men, 372 of whom took T therapy, the cumulative mortality was 21% in the untreated group vs 10% in the treated group. The greatest effect was observed in younger men and those with T2DM.80
In a prospective study involving 581 men with T2DM, patients were followed for a mean of 5.81 years. Low T was defined as a TT level lower than 10.4 nmol/L. 51 men received T therapy for at least 2 years. Mortality rates were 20% in the low T group vs 9.1% in the normal T group, independent of comorbidities and therapies, and 9.4% in those with TD in the treated group.66
The 2 aforementioned studies were criticized for possible selection bias, but their strengths were reliable pretreatment diagnosis and accurate reporting of medications.1,69,81
A real-life observational registry study82 assessed the long-term effectiveness and safety of parenteral TU used for up to 10 years in 656 men with a mean age of 60.7 years. It concluded that long-term treatment was well tolerated, with excellent adherence. Furthermore, mortality related to CVD was significantly decreased in the group taking T vs the untreated group.
A retrospective study followed 857 men with T2DM for 4 years after baseline T measurement. Patients were randomized to long-acting TU or placebo. Results showed that low baseline TT and FT levels were associated with increased all-cause mortality. T therapy and the use of PDE5is were independently associated with lower all-cause mortality, with the greatest benefit from the 2 treatments being seen in older men.83,84
Sharma et al85 retrospectively evaluated 83,010 male veterans with recorded low TT levels. Subjects were categorized into 3 groups: T therapy with resulting normalization of TT levels (group 1), T therapy without normalization of TT levels (group 2), and no T therapy (group 3). All-cause mortality (hazard ratio = 0.53, 95% CI = 0.50–0.55), risk of MI (hazard ratio = 0.82, 95% CI = 0.71–0.95), and stroke (hazard ratio = 0.70, 95% CI = 0.51–0.96) were significantly lower in group 1 vs 2 (n = 25,701, median age = 66 years, mean follow-up = 4.6 years).
A study comparing acute MI rates in 6,355 men receiving at least 1 T injection compared with a matched placebo group over 8 years found no overall increase in events. In those at greatest risk, there was a significant decrease in events and mortality. There was no increased risk from venous thromboembolism.86
In a virtual controlled study, researchers examined electronic medical records from 1996 to 2011 to identify 5,695 men with a low initial TT level, a subsequent T level, and up to 3 years of follow-up. T levels were correlated with the use of T supplementation. The primary outcomes were a composite of death, non-fatal MI, and stroke (major adverse cardiac events) and death alone. T supplementation in men with low T levels was associated with a lower incidence of major adverse cardiac events and death over 3 years compared with no or ineffective supplementation. The results suggest that the positive impact of T therapy was mainly on mortality as opposed to the number of events, and the benefits were associated with the achievement of therapeutic levels of T. There was no suggestion of increased risk with sustained higher serum levels.87
More recently, the same group reported a significant decrease in CV events in a cohort of hypogonadal men with angiographically diagnosed CAD.88
In a study of 10,311 T-treated men compared with 28,029 controls, Wallis et al89 found a decrease in all-cause and CV mortality with T therapy achieved and maintained in the normal range, with an increase in mortality in the first 6 months compared with normal, most likely because of the impact of underlying undertreated TD. This study also reported a 40% decrease in new diagnoses of prostate cancer (pCa) in the treated group compared with the control group.
Cheetham et al90 retrospectively reported on 8,808 T-treated and 35,527 untreated men with low T and found a 33% decrease in cardiac events associated with T therapy.
These studies present the most compelling evidence to date for the safety of T therapy in patients, with a decrease in mortality in clearly defined TD treated to the therapeutic range, suggesting that studies with negative outcomes usually included inadequate diagnosis and little evidence of effective therapeutic levels or adequate follow-up and failed to exclude the possibility that increased risk is related to TD and not its treatment.69
Registry studies have published data collected over 6 years of follow-up, with no suggestion of increased mortality.91
Findings from meta-analyses
A meta-analysis of 27 placebo-controlled trials of T therapy lasting longer than 12 weeks concluded that it could increase the risk of CV-related events. However, most studies involved small cohorts with a small number of events.92 The investigators were criticized for their selection and inclusion of studies,1,93 and their findings were heavily skewed1 by over-reliance on the TOM study74 and the studies by Vigen et al76 and Finkle et al.77 Another meta-analysis, published the same year, concluded that T therapy was not associated with increased risk, and in certain cohorts there was evidence of fewer events, especially in men with cardiometabolic disease.93
In response to these publications and US media publicity, the Food and Drug Administration issued a request for more information on the safety of T from future studies,94 whereas a bulletin from the European Medicines Agency expressed no concerns.95
T therapy and risk of pCa
Concerns about T therapy and pCa date back to 1941, when Huggins and Hodges96 reported that marked decreases in T by castration or estrogen treatment caused metastatic cancer to regress, but that administration of exogenous T caused pCa to grow.
The paradox of why lowering T causes pCa to regress, but raising T fails to cause pCa to grow, can be explained by the saturation model, which posits a finite ability of androgens to stimulate pCa growth.97 The AR becomes saturated in human prostate tissue at approximately 8 nmol/L in vivo,98,99 and there appears to be no further appreciable growth with increasing serum T concentrations beyond a saturation point of approximately 8 to 8.7 nmol/L.97 This explains why men with T below the saturation point of approximately 8.7 nmol/L at baseline are likely to see an increase in prostate-specific antigen (PSA) at treatment initiation, whereas PSA is unlikely to increase in men with T above this level.100
A meta-analysis of 19 randomized, placebo-controlled T therapy studies, published in 2005, which included 651 men at least 45 years old with low or low to normal T level, found no statistically significant differences in pCa, PSA levels higher than 4.0 ng/mL, or urinary symptom scores between those receiving T therapy and those receiving placebo.101
A collaborative analysis of 18 prospective studies, comparing 3,886 men with incident pCa with 6,438 age-matched controls, showed no association between T levels and pCa. There was no difference in the relative risk of pCa between men with the highest 20% of T levels and those with the lowest 20%.102
More recently, the placebo arm of the REDUCE trial, which investigated dutasteride for preventing pCa and included results from 3,255 men who had prostate biopsy examinations at 2 and 4 years, showed no association between serum T or dihydrotestosterone and pCa risk. The risk of pCa was no greater in men with high T levels than those with low T levels.103
Guidelines from the European Association of Urology (EAU),4 the BSSM,5 the ICSM,6 the International Society of Sexual Medicine (ISSM),7 the Endocrine Society (ES),22 and the International Study of the Aging Male (ISSAM)26 conclude that there is no compelling evidence that T therapy is associated with an increased risk of pCa. The ISSAM26 stated that there is no evidence that T therapy converts subclinical prostatic lesions into clinically detectable pCa (LoE = 2, Grade = B), and the ICSM6 stated that there is no compelling evidence that T therapy increases the risk of pCa or is associated with pCa progression (LoE = 1, Grade = C).
Recent research has suggested that lower T levels are associated with a risk of poorly differentiated cancers and greater risk of positive biopsy results,104 and there is strong evidence linking low T concentrations to aggressive high-grade pCa, higher rates of positive biopsy results, biochemical recurrence, and disease progression in men under active surveillance.105
However, estimates suggest that to detect a 30% difference in pCa rates between T- and placebo-treated men, approximately 6,000 men 65 to 80 years old with low T levels would need to be randomized to T or placebo treatment for an average of 5 years. Because such an RCT would probably include relatively healthy older men with unequivocally low T levels but without pCa or PSA levels higher than 4 ng/mL, it would require screening of a significantly larger number of older men to enroll the required number of eligible participants. Because it seems unlikely that a study of this size will be funded, it will remain uncertain whether long-term T therapy affects the incidence of clinically overt pCa.106,107
Careful assessment of the prostate before starting T therapy and regular monitoring for prostate disease once the patient is on T therapy remain essential. Early increases in PSA after initiation of T therapy could unmask an occult prostate carcinoma that was undetected at baseline.
Routine measurement of T
The EAU108 and BSSM5 guidelines on male sexual dysfunction recommend that all men with ED should have an initial measurement of T as the minimal standard and that rechecking is indicated in the event of poor response to PDE5is.
National Institute for Health and Care Excellence guidance109 recommends that men with T2DM should be asked about ED annually and assessed, educated, and supported as necessary.
In 2016, the American Association of Clinical Endocrinologists and American College of Endocrinology guidelines on the management of obesity110 recommended that all men with a waist circumference larger than 102 cm or BMI higher than 30 kg/m2 be assessed for TD by history, clinical examination, and measurement of T. They recommended measurement of T in all men with diabetes. They pointed out that weight loss and lifestyle change should always form part of the management, but that a significant increase in T is not usually seen unless more than 5% to 10% of weight loss is achieved. They also recommended T measurement in men with chronic disease such as HIV and chronic renal disease.
Screening for low T is not recommended in the absence of TD symptoms in all other populations (including those with CVD, chronic pulmonary diseases, cirrhosis, end-stage renal diseases, rheumatoid arthritis, and cancer), because although such conditions are potentially associated with an increased prevalence of low T, there is a lack of evidence for benefit of T therapy in asymptomatic individuals (LoE = 3, Grade = B).6
| Recommendations—screening . | LoE . | Grade . |
|---|---|---|
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with T2DM, BMI > 30 kg/m2 or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
| Recommendations—screening . | LoE . | Grade . |
|---|---|---|
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with T2DM, BMI > 30 kg/m2 or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
| Recommendations—screening . | LoE . | Grade . |
|---|---|---|
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with T2DM, BMI > 30 kg/m2 or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
| Recommendations—screening . | LoE . | Grade . |
|---|---|---|
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with T2DM, BMI > 30 kg/m2 or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
History taking and questionnaires
History taking should include signs and symptoms suggestive of TD (Table 1), pharmacologic treatment with corticosteroids or opiates, abuse of drugs such as marijuana, alcohol, and anabolic steroids, and previous treatment or use of T. It is important to assess and exclude systemic illness, ongoing acute disease, malabsorption, and malnutrition.4
Clinical assessment can be supported by the use of validated questionnaires such as the Aging Male Symptoms Scale (available at: http://www.issam.ch/AMS_English.pdf and http://www.issam.ch/AMS_English_Evaluation.pdf) and the ANDROTEST111 to provide a quantitative baseline assessment of baseline symptoms and evaluate the clinical response to treatment. We endorse the use of validated questionnaires to demonstrate changes in symptoms with T therapy.
Because the signs and symptoms can be non-specific,4 patients at risk of or suspected of having TD should receive a thorough physical and biochemical workup5,26 (LoE = 2, Grade = A).26
Physical examination
Physical examination in men with suspected TD should include measurement of height, weight, BMI, and waist circumference,22,26,112 assessment of the degree and distribution of body hair (including facial and pubic), and examination for the presence of acanthosis nigricans associated with insulin resistance,26,113–116 presence and degree of breast enlargement, appearance of the penis, presence of subcutaneous plaque, testicular size and consistency, and scrotum size and abnormalities.4,26 The prostate also should be examined by digital rectal examination (DRE).4
Laboratory diagnosis
Because the diurnal rhythm of serum T means levels are highest in the early morning, T measurement before 11 am is particularly important for men younger than 40 years.6 Although diurnal variation is substantially blunted in older men,26 it might still be evident, even in the elderly, supporting the recommendation of restricting T measurement to the morning hours for all age groups.117
Serum T should be measured from 7 to 11 am (LoE = 2a, Grade = A) on at least 2 occasions with a reliable method (LoE = 1, Grade = A),26 preferably 4 weeks apart and, if possible, not during an acute illness. Because T levels are influenced by insulin, glucose 75 g was shown to lower T by 25%.118
Although there is a lack of evidence supporting the necessity of evaluating fasting T, the EAU recommended that T be measured in the fasting state,4 which fits conveniently into the routine blood testing for dyslipidemia, hypertension, and diabetes. Until further evidence is accumulated, we also believe that fasting testing is appropriate for a first test. Fasting levels were found to be up to 30% higher than non-fasting levels in volunteer and normal population studies involving a 75-g glucose load.118,119 We believe it is doubtful whether these findings would be applicable to a hypogonadal population in which diurnal variation in T is typically absent. We recognize that most published RCTs involved fasting levels. We recommend that clinicians should be aware that patients do not routinely fast and therefore might have daily levels below those detected by fasting measurement.
Assays of TT, performed by most laboratories in the everyday clinical setting, are readily available and relatively inexepensive.6 Although widely recommended as the standard for research, equilibrium dialysis is most widely used clinically, most notably in the EMAS studies, in which the results of equilibrium dialysis diagnosis were found to correlate well with isotope dilution gas chromatographic mass spectrometry.120
Assays of FT based on analogue displacement immunoassays produce unpredictable results and are not recommended. In obese men, the free androgen index, calculated as TT/SHBG × 100, is used by some laboratories but we recommend the calculated FT derived from SHBG and albumin through the equation of Vermeulen et al.121 An online FT calculator and downloadable app, sponsored by the Primary Care Testosterone Advisory Group, can be found at http://www.pctag.uk/testosterone-calculator/. The ISSAM also provides an online FT calculator (available at: http://www.issam.ch/freetesto.htm). The free androgen index is not as reliable as FT or bioavailable T.122
It is important that clinicians familiarize themselves with their local laboratories. TD is a diagnosis made from clinical symptoms and abnormal biochemistry. Clinicians need to appreciate that “reference ranges” quoted by laboratories represent the normal population and that the” action levels” recommended by the BSSM refer to men with clinical symptoms of TD, just as reference ranges on normal lipids are inappropriate for men with T2DM and for secondary prevention in coronary heart disease. Wide variations in laboratory practice for T testing in the United Kingdom can lead to variations in patient care and potentially result in some patients with TD not being identified. Improvements in the standardization of T assays and the consistency of reporting between laboratories are required.
When TT levels are close to the lower normal range (8–12 nmol/L), the FT level also should be checked4,5 (LoE = 1, Grade = A).4 When TT levels are lower than 12 nmol/L, serum LH and FSH levels also should be measured. When TT level is borderline or low to normal, particularly in obese and older men, SHBG levels also should be checked (LoE = 2, Grade = C).6 For known or suspected abnormal SHBG levels, FT also should be measured (LoE = 1, Grade = A). LH levels should be assessed to differentiate between primary and secondary TD (LoE = 2, Grade = A).4 Serum prolactin levels should be measured when LH and FSH levels are low. A very low TT level (<5.2 nmol/L) and low LH and FSH levels are more likely to be associated with hyperprolactinemia or when secondary TD from a pituitary tumor or other pituitary disease is suspected.
For other investigations, in men with T levels lower than 5.2 nmol/L and increased prolactin levels or decreased LH and FSH levels, pituitary magnetic resonance imaging should be performed to exclude a pituitary adenoma.6,22
The EAU4 recommends screening for concomitant osteoporosis in men older than 50 years with established TD (LoE = 2, Grade = B).
| Recommendations—diagnosis . | LoE . | Grade . |
|---|---|---|
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure FT in men with levels close to the lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG levels | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
| Recommendations—diagnosis . | LoE . | Grade . |
|---|---|---|
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure FT in men with levels close to the lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG levels | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
| Recommendations—diagnosis . | LoE . | Grade . |
|---|---|---|
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure FT in men with levels close to the lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG levels | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
| Recommendations—diagnosis . | LoE . | Grade . |
|---|---|---|
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure FT in men with levels close to the lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG levels | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
Thresholds for T therapy
Regarding the thresholds for treatment intervention in symptomatic men, BSSM1,5 and ISSM123 guidelines recommend the following:
- •
TT level lower than 8 nmol/L or FT level lower than 180 pmol/L (<0.180 nmol/L; based on 2 separate levels from 8 to 11 am) usually requires T therapy.1
- •
TT level higher than 12 nmol/L or FT level higher than 225 pmol/L (>0.225 nmol/L) does not require T therapy.1
- •
Levels from 8 to 12 nmol/L might require a trial of T therapy for a minimum of 6 months based on symptoms (LoE = 3, Grade = C).123
BSSM guidelines also state:
- •
A FT level lower than 225 pmol/L (0.225 nmol/L) provides supportive evidence for T therapy in the presence of appropriate symptoms.124
Additional recommendations include:
- •
Increased LH levels and T levels below normal or in the lower quartile range indicate testicular failure, so T therapy should be considered.26,125
- •
Increased LH levels in men with normal T levels but symptoms of TD should be considered as having TD.26
- •
Recent data from the EMAS124 found that clinical symptoms were more closely related to calculated FT (LoE = 2, Grade = B).
| Recommendations—initiating T therapy . | LoE . | Grade . |
|---|---|---|
| Perform CV, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess CV risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
| Recommendations—initiating T therapy . | LoE . | Grade . |
|---|---|---|
| Perform CV, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess CV risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
| Recommendations—initiating T therapy . | LoE . | Grade . |
|---|---|---|
| Perform CV, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess CV risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
| Recommendations—initiating T therapy . | LoE . | Grade . |
|---|---|---|
| Perform CV, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess CV risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
T therapy
There are many different T preparations available (Table 3 ), which differ in their formulations, routes of administration, dosage intervals, and safety profiles. Aromatizable T is recommended for replacement therapy.26
| Formulation . | Route of administration . | Frequency of administration . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Testosterone 1% and 2% gel available | Transdermal gel | Applied daily, requires dose titration | Fast onset; provide uniform and normal serum levels for 24 hours | Skin irritation at application site Potential for interpersonal transfer Non-compliance long term |
| Testosterone undecanoate | Oral capsules | Once or twice daily | Lymphatic absorption decrease liver involvement | Levels fluctuate Daily or twice-daily commitment Must be taken with food |
| Testosterone undecanoate | Intramuscular injection | Every 10–14 wk adjusted to maintain trough testosterone level > 12 nmol/L | Steady-state levels Lower frequency of administration improves compliance | Long duration of action prevents drug withdrawal in the event of adverse side effects |
| Testosterone enanthate | Intramuscular injection | Every 2–3 wk | Short duration of action allows drug withdrawal if there are adverse side effects | Levels fluctuate |
| Testosterone propionate | Intramuscular injection | Currently available as 1 of 4 testosterone esters used in sustanon 250, which is usually administered every 3 wk; sustanon’s duration of action prevents drug withdrawal in the event of adverse side effects127 | ||
| Formulation . | Route of administration . | Frequency of administration . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Testosterone 1% and 2% gel available | Transdermal gel | Applied daily, requires dose titration | Fast onset; provide uniform and normal serum levels for 24 hours | Skin irritation at application site Potential for interpersonal transfer Non-compliance long term |
| Testosterone undecanoate | Oral capsules | Once or twice daily | Lymphatic absorption decrease liver involvement | Levels fluctuate Daily or twice-daily commitment Must be taken with food |
| Testosterone undecanoate | Intramuscular injection | Every 10–14 wk adjusted to maintain trough testosterone level > 12 nmol/L | Steady-state levels Lower frequency of administration improves compliance | Long duration of action prevents drug withdrawal in the event of adverse side effects |
| Testosterone enanthate | Intramuscular injection | Every 2–3 wk | Short duration of action allows drug withdrawal if there are adverse side effects | Levels fluctuate |
| Testosterone propionate | Intramuscular injection | Currently available as 1 of 4 testosterone esters used in sustanon 250, which is usually administered every 3 wk; sustanon’s duration of action prevents drug withdrawal in the event of adverse side effects127 | ||
| Formulation . | Route of administration . | Frequency of administration . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Testosterone 1% and 2% gel available | Transdermal gel | Applied daily, requires dose titration | Fast onset; provide uniform and normal serum levels for 24 hours | Skin irritation at application site Potential for interpersonal transfer Non-compliance long term |
| Testosterone undecanoate | Oral capsules | Once or twice daily | Lymphatic absorption decrease liver involvement | Levels fluctuate Daily or twice-daily commitment Must be taken with food |
| Testosterone undecanoate | Intramuscular injection | Every 10–14 wk adjusted to maintain trough testosterone level > 12 nmol/L | Steady-state levels Lower frequency of administration improves compliance | Long duration of action prevents drug withdrawal in the event of adverse side effects |
| Testosterone enanthate | Intramuscular injection | Every 2–3 wk | Short duration of action allows drug withdrawal if there are adverse side effects | Levels fluctuate |
| Testosterone propionate | Intramuscular injection | Currently available as 1 of 4 testosterone esters used in sustanon 250, which is usually administered every 3 wk; sustanon’s duration of action prevents drug withdrawal in the event of adverse side effects127 | ||
| Formulation . | Route of administration . | Frequency of administration . | Advantages . | Disadvantages . |
|---|---|---|---|---|
| Testosterone 1% and 2% gel available | Transdermal gel | Applied daily, requires dose titration | Fast onset; provide uniform and normal serum levels for 24 hours | Skin irritation at application site Potential for interpersonal transfer Non-compliance long term |
| Testosterone undecanoate | Oral capsules | Once or twice daily | Lymphatic absorption decrease liver involvement | Levels fluctuate Daily or twice-daily commitment Must be taken with food |
| Testosterone undecanoate | Intramuscular injection | Every 10–14 wk adjusted to maintain trough testosterone level > 12 nmol/L | Steady-state levels Lower frequency of administration improves compliance | Long duration of action prevents drug withdrawal in the event of adverse side effects |
| Testosterone enanthate | Intramuscular injection | Every 2–3 wk | Short duration of action allows drug withdrawal if there are adverse side effects | Levels fluctuate |
| Testosterone propionate | Intramuscular injection | Currently available as 1 of 4 testosterone esters used in sustanon 250, which is usually administered every 3 wk; sustanon’s duration of action prevents drug withdrawal in the event of adverse side effects127 | ||
Oral therapies are rarely used in the United Kingdom because of the need for multiple dosing regimens and fears of hepatotoxicity. The choice usually lies between the transdermal route and long-acting TU. The features of each method should be discussed with the patient. Based on current evidence, we can see no justification for selecting one system over another except for patient choice.
The BSSM recommends initiating T therapy only in men with bothersome symptoms (Table 4), in conjunction with weight-loss advice, lifestyle modification, and treatment of comorbidities (LoE = 2, Grade = A). Weight loss and lifestyle modification alone have failed to demonstrate effective improvement in clinical symptoms, even after more than 4 years, and patients need to be informed of this.41,42,128
British Society for Sexual Medicine recommendations for UK practice with levels of evidence and grades of recommendation
| . | LoE . | Grade . |
|---|---|---|
| Recommendations—screening | ||
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with type 2 diabetes, BMI > 30 kg/m2, or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
| Recommendations—diagnosis | ||
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T level | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure free T in men with levels close to lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG level | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
| Recommendations—initiating T therapy | ||
| Perform cardiovascular, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess cardiovascular risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
| Recommendations—benefits and risks of T therapy | ||
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome. | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when TRT is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
| Recommendations—follow-up | ||
| Assess response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at a lower dose | 4 | C |
| Assess prostate health by PSA and DRE before commencing TRT followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess cardiovascular risk before TRT is initiated and monitor cardiovascular risk factors throughout therapy | 1b | A |
| . | LoE . | Grade . |
|---|---|---|
| Recommendations—screening | ||
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with type 2 diabetes, BMI > 30 kg/m2, or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
| Recommendations—diagnosis | ||
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T level | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure free T in men with levels close to lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG level | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
| Recommendations—initiating T therapy | ||
| Perform cardiovascular, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess cardiovascular risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
| Recommendations—benefits and risks of T therapy | ||
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome. | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when TRT is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
| Recommendations—follow-up | ||
| Assess response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at a lower dose | 4 | C |
| Assess prostate health by PSA and DRE before commencing TRT followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess cardiovascular risk before TRT is initiated and monitor cardiovascular risk factors throughout therapy | 1b | A |
BMI = body mass index; DRE = digital rectal examination; ED = erectile dysfunction; LH = luteinizing hormone; LoE = level of evidence; PSA = prostate-specific antigen; T = testosterone; TD = testosterone deficiency; TRT = testosterone replacement therapy; TT = total testosterone.
British Society for Sexual Medicine recommendations for UK practice with levels of evidence and grades of recommendation
| . | LoE . | Grade . |
|---|---|---|
| Recommendations—screening | ||
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with type 2 diabetes, BMI > 30 kg/m2, or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
| Recommendations—diagnosis | ||
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T level | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure free T in men with levels close to lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG level | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
| Recommendations—initiating T therapy | ||
| Perform cardiovascular, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess cardiovascular risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
| Recommendations—benefits and risks of T therapy | ||
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome. | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when TRT is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
| Recommendations—follow-up | ||
| Assess response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at a lower dose | 4 | C |
| Assess prostate health by PSA and DRE before commencing TRT followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess cardiovascular risk before TRT is initiated and monitor cardiovascular risk factors throughout therapy | 1b | A |
| . | LoE . | Grade . |
|---|---|---|
| Recommendations—screening | ||
| Screen for TD in adult men with consistent and multiple signs of TD | 3 | C |
| Screen all men presenting with ED, loss of spontaneous erections, or low sexual desire | 1 | A |
| Screen for TD in all men with type 2 diabetes, BMI > 30 kg/m2, or waist circumference > 102 cm | 2 | A |
| Screen for TD in all men on long-term opiate, antipsychotic, or anticonvulsant medication | 2 | B |
| Recommendations—diagnosis | ||
| Restrict diagnosis of TD to men with persistent symptoms suggesting TD and confirmed low T level | 3 | C |
| Measure fasting T levels in the morning before 11 am, acknowledging that, in normal life, non-fasting levels could be up to 30% lower | 2 | A |
| Repeat TT assessment on ≥2 occasions by a reliable method; in addition, measure free T in men with levels close to lower normal range (8–12 nmol/L) or those with suspected or known abnormal SHBG level | 1 | A |
| Measure LH serum levels to differentiate primary from secondary TD | 2 | A |
| Base decisions on therapy on published action levels rather than laboratory reference ranges | 4 | B |
| Recommendations—initiating T therapy | ||
| Perform cardiovascular, prostate, breast, and hematologic assessments before start of treatment | 1a | A |
| Offer T therapy to symptomatic men with TD syndrome for treated localized low-risk prostate cancer (Gleason score < 8, stages 1–2, preoperative PSA level < 10 ng/mL, and not starting before 1 y of follow-up) and without evidence of active disease (based on measurable PSA level, DRE result, and evidence of metastatic disease) | 3 | B |
| Assess cardiovascular risk factors before commencing T therapy and optimize secondary prevention in men with established disease | 1a | A |
| Recommendations—benefits and risks of T therapy | ||
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome. | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when TRT is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
| Recommendations—follow-up | ||
| Assess response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at a lower dose | 4 | C |
| Assess prostate health by PSA and DRE before commencing TRT followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess cardiovascular risk before TRT is initiated and monitor cardiovascular risk factors throughout therapy | 1b | A |
BMI = body mass index; DRE = digital rectal examination; ED = erectile dysfunction; LH = luteinizing hormone; LoE = level of evidence; PSA = prostate-specific antigen; T = testosterone; TD = testosterone deficiency; TRT = testosterone replacement therapy; TT = total testosterone.
For sexual dysfunction, a patient’s desire to achieve a satisfactory sexual response is a major driver for T therapy in isolation or with erectogenic therapy.5 Men with documented TD and poor morning erections, ED, and/or decreased libido are candidates for T therapy.26
T therapy is appropriate for treating ED, particularly at TT levels lower than 8 nmol/L5,7 or calculated FT levels lower than 225 pmol/L and for salvaging ED treatment failures with oral medication, particularly at TT levels lower than 10.4 nmol/L.47 Furthermore, appropriate intervention with T therapy lessens the need for more invasive second- and third-line treatments in these patients.129 Although these indications for T therapy are often considered vitally important to the patient, they could be considered of low importance by the physician not specializing in sexual dysfunction. Because sexual dysfunction is often multifaceted in nature, involving physical, behavioral, psychological, interpersonal, and contextual aspects,130 psychosexual therapy also might be indicated.1
A combination of T and PDE5i treatment should be considered in patients with TD and ED who do not respond to either treatment in isolation, especially in those with multiple risk factors for ED in addition to TD (LoE = 2, Grade = A).
In men with T2DM, improvements in symptoms appear to parallel improvements in T levels.51
Although TD is often associated with decreased fertility, treatment with exogenous T decreases endogenous T production through negative feedback in the hypothalamic-pituitary-gonadal axis,4 resulting in decreased LH and FSH levels and decreased spermatogenesis. If a man wishes to father children in the near future, then the risk of T therapy on fertility needs to be discussed, because long-term use is associated with azoospermia. A wish for paternity is one of the main contraindications to T therapy.4 For secondary TD, possible alternatives include human chorionic gonadotropin, selective estrogen receptor modulators (eg, clomiphene), and aromatase inhibitors. Selective estrogen receptor modulators and aromatase inhibitors are particularly useful in men with metabolic disturbances. However, these drugs should not be used when pituitary function is compromised.
There is no scientific basis for withholding T therapy from men with TD based on age.1
The clinical and physiologic responses to T therapy, particularly the benefits of increased muscle mass and strength,54 might be more clinically and economically significant in older men, because decreased muscle mass and lower limb strength are strongly related to frailty and an increased rate of falls.131
Recent research has suggested a greater decrease in all-cause mortality in men older than 75 years treated with T.84 The traditional view that younger men experience greater benefit from improvement in sexual symptoms associated with T therapy has not been supported by recent studies.132
When T therapy is used in men with benign prostatic hyperplasia, symptomatic improvement of LUTS has been reported.6
The clinical response to T therapy appears unrelated to the underlying etiology,133 because recent studies have shown benefits in men without “classic TD” and because such “textbook” conditions are relatively uncommon in the general population. It is really a matter of clinical judgement and patient expectation as to whether the underlying conditions should be addressed first,1 but because many men with TD are sick and/or obese, evidence suggests that better outcomes can be achieved if lifestyle modifications, including weight loss, and T therapy are combined.4,38
It seems that responsiveness to T therapy is dependent not only on serum T concentration but also on the length of CAG repeats on the AR. Longer CAG repeats are associated with more severe symptoms and decreased response to standard-dose therapy. The wide variations in CAG repeats within different ethnic groups is particularly important in the ethnically diverse UK population.25,134
For treatment duration, in 2 RCTs involving 447 men 45 to 90 years old, cessation of T therapy resulted in relapse and reversal of benefits within 6 months, so it is likely to be required life long.47,135
Adverse effects of T therapy
Serious adverse events related to T therapy are relatively rare. They are more significant in elderly patients and often dependent on the method of delivery. Some adverse events are related to supraphysiologic levels and can be lowered or stopped altogether by adjusting the dose or switching to a different formulation.6
Intramuscular injections are associated with pain at the injection site, changes in mood, energy, and sexual desire, and, rarely, coughing straight after the injection.1
Transdermal gels carry a risk of interpersonal transfer, skin irritation, and fluctuations in absorption.1
The 17α-alkylated formulations are associated with a risk of hepatotoxicity, cholestasis, peliosis hepatis, hepatic tumors, and a marked decrease in high-density lipoprotein cholesterol.1 These treatments should no longer be prescribed.5
Other side effects seen with T therapy include polycythemia (which is more common in older men treated with injectable T preparations),5 with increases in hematocrit and hemoglobin, gynecomastia, edema, acne, and other skin reactions.1
Obese men are more likely to develop adverse effects from T therapy than men of normal weight.5
Contraindications to T therapy
The main contraindications to T therapy are4:
- •
Locally advanced or metastatic pCa
- •
Male breast cancer
- •
An active desire to have children
- •
Hematocrit higher than 54%
- •
Severe chronic heart failure (New York Heart Association class IV)
Additional contraindications include unevaluated prostate nodule or induration, PSA level higher than 4 ng/mL6,22 (or >3 ng/mL in men at high risk of pCa, such as African Americans and those with first-degree relatives who have pCa),22 severe, untreated sleep apnea,26 and severe LUTS associated with benign prostatic hyperplasia as indicated by the American Urological Association symptom index and International Prostate Symptom Score higher than 19.6,22
A critical update of the ES guidelines published in 2015,136 which examined the body of level 1 evidence literature published since 2010, stated that patients with increased PSA levels should be referred for urologic consultation before initiating T replacement therapy, and untreated sleep apnea and severe LUTS might not be absolute contraindications to T therapy.
| Recommendations—benefits and risks of T therapy . | LoE . | Grade . |
|---|---|---|
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when T replacement therapy is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
| Recommendations—benefits and risks of T therapy . | LoE . | Grade . |
|---|---|---|
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when T replacement therapy is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
| Recommendations—benefits and risks of T therapy . | LoE . | Grade . |
|---|---|---|
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when T replacement therapy is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
| Recommendations—benefits and risks of T therapy . | LoE . | Grade . |
|---|---|---|
| Beyond 6 mo there is evidence of benefit for T therapy in body composition, bone mineralization, and features of metabolic syndrome | 3 | A |
| T therapy improves sexual desire, erectile function, and sexual satisfaction | 1 | A |
| Decreases in BMI and waist size and improved glycemic control and lipid profile are observed in hypogonadal men receiving T therapy | 2 | A |
| Trials of T therapy should be ≥6 mo and maximal benefit is often seen beyond 12 mo | 2 | A |
| Fully inform the patient about expected benefits and side effects of therapy and facilitate a joint decision by an informed patient and physician | 3 | A |
| Fully discuss the adverse effect of T therapy and its future reversibility on future fertility for each patient and his partner and offer alternative treatment as necessary | 1b | B |
| In patients with adult-onset TD, when T replacement therapy is prescribed, offer weight-loss and lifestyle advice as standard management | 2 | A |
| In severely symptomatic patients with TT levels < 8 nmol/L, lifestyle and dietary advice alone is unlikely to produce meaningful clinical improvement within a relevant clinical period | 2 | B |
Follow-up and monitoring
Before initiating T therapy, hematologic assessment should be performed, prostate health should be assessed by DRE and PSA, the man’s breasts should be checked, CV risk factors should be assessed, and men with CVD should receive evaluation of CV symptoms and optimization of secondary prevention.4
After initiation of T therapy, we recommend evaluating patients at 3, 6 and 12 months and then every 12 months thereafter to assess serum T levels, confirm symptomatic improvement, and check for any changes in hematocrit, PSA level, and DRE results. Follow-up every 3 months might be necessary in some patients, including those with a suboptimal treatment response or safety issues, such as a high or increasing hematocrit or PSA levels.6
Monitoring levels of TT, SHBG, and albumin (to calculate bioavailable or FT) is required to ensure normal T levels are being achieved. Currently, there are insufficient data to define optimal serum T levels during treatment. The aim of therapy is to restore serum T levels to the mid-normal range for specific age groups.4 Impairment of sexual function and nocturnal erections are usually associated with TT levels lower than 7 nmol/L, with the maximal benefit of T appearing to result with levels of at least 12 to 16 nmol/L.6,137
For monitoring purposes, we recommend a therapeutic target in the mid to upper range (15–30 nmol/L), making reference ranges of little value. For patients on transdermal preparations, blood samples should be taken 2 to 4 hours after application of gel or lotion, being careful to avoid any skin contamination. For injections, the timing for blood testing is irrelevant, because the trough levels, which assess the duration of effect of the formulation, are all that are required.
Hematocrit levels should remain below 54%4,6,26 (LoE = 2, Grade = B).6 Dose adjustments and/or periodic venesection might be required to achieve this.5
PSA increases greater than 1.4 ng/mL during any 1-year period after initiation of T therapy or a PSA velocity greater than 0.4 ng/mL per year during sequential PSA measurements over more than 2 years warrant a urologic evaluation and more intensive surveillance for pCa thereafter.5
Men with CVD should be monitored carefully, with clinical assessment and measurement of T and hematocrit performed regularly.4
Bone mineral density should be monitored only in men with abnormal bone mineral density before initiating T therapy,4,6 and repeat dual energy x-ray absorptiometry is recommended after 1 to 2 years of T therapy.6
Although monitoring of serum lipids and glycemia is not required for safety during T therapy, it could be necessary for monitoring the efficacy of other aspects of treatment (LoE = 2, Grade = A).6
Assessment of treatment outcome and decisions regarding continuation of T therapy should be based on improvement in signs and symptoms of TD. Failure to benefit within a reasonable timeframe (defined as 6 months for libido, sexual function, muscle function, and improved body fat) should prompt discontinuation of treatment. Further investigation for other causes of the symptoms is essential.5
| Recommendations—follow-up . | LoE . | Grade . |
|---|---|---|
| Assess the response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at lower dose | 4 | C |
| Assess prostate health by PSA assessment and DRE before commencing T replacement therapy followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess CV risk before T replacement therapy is initiated and monitor CV risk factors throughout therapy | 1b | A |
| Recommendations—follow-up . | LoE . | Grade . |
|---|---|---|
| Assess the response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at lower dose | 4 | C |
| Assess prostate health by PSA assessment and DRE before commencing T replacement therapy followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess CV risk before T replacement therapy is initiated and monitor CV risk factors throughout therapy | 1b | A |
| Recommendations—follow-up . | LoE . | Grade . |
|---|---|---|
| Assess the response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at lower dose | 4 | C |
| Assess prostate health by PSA assessment and DRE before commencing T replacement therapy followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess CV risk before T replacement therapy is initiated and monitor CV risk factors throughout therapy | 1b | A |
| Recommendations—follow-up . | LoE . | Grade . |
|---|---|---|
| Assess the response to therapy at 3, 6, and 12 mo and every 12 mo thereafter | 4 | C |
| Aim for a target TT level of 15–30 nmol/L to achieve optimal response | 4 | C |
| Monitor hematocrit before treatment, at 3–6 mo, 12 mo, and every 12 mo thereafter; decrease dosage, or switch preparation, if hematocrit is >0.54; if hematocrit remains high, consider stopping and reintroduce at lower dose | 4 | C |
| Assess prostate health by PSA assessment and DRE before commencing T replacement therapy followed by PSA at 3–6 mo, 12 mo, and every 12 mo thereafter | 4 | C |
| Assess CV risk before T replacement therapy is initiated and monitor CV risk factors throughout therapy | 1b | A |
Conclusion
TD is a well-established and significant medical condition, encompassing somatic, sexual, and psychological effects. It also is associated with increased CV and all-cause mortality. Clearly defined clinical symptoms should prompt swift evaluation of these and the underlying risk factors, and further investigations should be performed when appropriate.
To identify individuals who should be screened for TD, consideration should be given to routinely asking men if they have any sexual concerns, particularly those at high risk. These include men with diabetes, osteoporosis or fragility fractures, CVD, ED, and depression and those on long-term opiate or oral glucocorticoid therapy.
T therapy is based on evidence, effective, and safe, and treatment-related sustained normalization of serum T levels is probably associated with lower mortality.
However, although the multiple benefits of T therapy are highly relevant to the patient, they are often underestimated by specialist physicians focused on specific outcomes. Because sexual dysfunction is often multifaceted in nature, the treatment of men with sexual desire, arousal, and ejaculation problems should be individualized and can include T therapy, erectogenic agents, and psychosexual therapy.
Until a definitive well-powered long-term study is published, treatment of TD with T therapy should be guided by the best available evidence.
Statement of authorship
Category 1
- (a)
Conception and Design
Geoff Hackett; Michael Kirby; Asif Muneer
- (b)
Acquisition of Data
Geoff Hackett; Michael Kirby; Thomas Hugh Jones; Nick Ossei-Gerning
- (c)
Analysis and Interpretation of Data
Geoff Hackett; Michael Kirby; Asif Muneer
Category 2
- (a)
Drafting the Article
Geoff Hackett; Michael Kirby
- (b)
Revising It for Intellectual Content
Geoff Hackett; Michael Kirby; Thomas Hugh Jones; Nick Ossei-Gerning; Janine David; Asif Muneer
Category 3
- (a)
Final Approval of the Completed Article
Geoff Hackett; Michael Kirby; Thomas Hugh Jones; Nick Ossei-Gerning; Janine David; Asif Muneer
Funding
None.
References
Author notes
Past Chairman of the British Society for Sexual Medicine.
Chairman of British Society for Sexual Medicine.
Conflicts of Interest: The authors report no conflicts of interest.



