-
PDF
- Split View
-
Views
-
Cite
Cite
Mario Maggi, Darell Heiselman, Jack Knorr, Smriti Iyengar, Darius A. Paduch, Craig F. Donatucci, Impact of Testosterone Solution 2% on Ejaculatory Dysfunction in Hypogonadal Men, The Journal of Sexual Medicine, Volume 13, Issue 8, August 2016, Pages 1220–1226, https://doi.org/10.1016/j.jsxm.2016.05.012
Close - Share Icon Share
ABSTRACT
Hypogonadism is defined as decreased testosterone levels in men. Hypogonadism can be accompanied by erectile, orgasmic, and ejaculatory dysfunction.
To evaluate whether treatment with testosterone solution 2% (testosterone) could improve ejaculatory function in a cohort of hypogonadal men.
Sexually active, hypogonadal men at least 18 years old (total testosterone < 300 ng/dL) were randomized to receive testosterone or placebo for 12 weeks.
Effects of testosterone on primary outcomes were evaluated using the International Index of Erectile Function (IIEF) and the Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form (MSHQ-EjD-SF) questionnaires. Treatment differences were calculated using analysis of covariance.
In total, 715 men (mean age = 55 years) were randomized to placebo (n = 357) or testosterone (n = 358). Most sexually active men who reported IIEF scores had some degree of erectile dysfunction (IIEF erectile function score < 26). Although ejaculatory function score (MSHQ-EjD-SF) improved in the testosterone group compared with placebo (P < .001), improvement on the “bother” item did not reach statistical significance. Treatment-related adverse events in the testosterone group affecting at least 1% of patients were increased hematocrit, upper respiratory tract infection, arthralgia, burning sensation, fatigue, increased prostate-specific antigen, erythema, and cough. Few patients in either treatment group developed at least one adverse event leading to discontinuation (testosterone = 1.98% vs placebo = 3.09%; P = .475).
Hypogonadal men receiving testosterone solution 2% therapy experience significantly greater improvement in ejaculatory function, compared with placebo, as assessed by the MSHQ-EjD-SF. However, improvement in “bother” was not statistically different between the two groups. Testosterone therapy was generally well tolerated.
Introduction
Hypogonadism, biochemically defined as a decreased concentration of serum testosterone, can result in decreased libido, erectile dysfunction, decreased bone density, decreased lean body mass, increased body fat, and fatigue.1,2 Erectile dysfunction is one of the main reasons men with hypogonadism seek treatment.3 However, disorders of the endocrine system are rarely the cause of erectile dysfunction,4 and only 12% of men with erectile dysfunction also have hypogonadism.5
Sexual dysfunctions in men also include ejaculatory dysfunction, disorders of arousal, and orgasmic dysfunction. Blanker et al6 found a prevalence of ejaculatory dysfunction of 13% in older men. In an international study, the prevalence of the inability to reach orgasm was 14.4%.7 In addition, low testosterone may increase the risk for some forms of ejaculatory dysfunction.8
Testosterone solution 2% (hereafter also referred to as testosterone, testosterone treatment, or testosterone solution) is approved by the U.S. Food and Drug Administration for testosterone replacement therapy in adult men for conditions associated with a deficiency or absence of endogenous testosterone. These conditions include primary hypogonadism (congenital or acquired) and hypogonadotropic hypogonadism (congenital or acquired) when testosterone deficiency has been confirmed by clinical features and biochemical tests.9 Previous studies have shown that testosterone solution can increase serum testosterone to normal levels10 and improve sexual drive.11
Aims
Because hypogonadism is commonly associated with ejaculatory dysfunction, the present analysis of data from a large study of testosterone replacement therapy was undertaken to determine whether testosterone replacement therapy could improve this function (as measured by the Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form [MSHQ-EjD-SF]) in sexually active hypogonadal men. Another report from this study, examining different end points, has been published.11
Methods
This study was conducted in accordance with consensus ethics principles derived from international ethics guidelines, including the Declaration of Helsinki, the Council for International Organizations of Medical Sciences International Ethical Guidelines, the International Conference on Harmonization Good Clinical Practices Guideline, and applicable laws and regulations.
Subjects
Men were eligible to be included in the study if they were least 18 years of age with a total testosterone level lower than 300 ng/dL (10.4 nmol/L) at both visits 1 and 2 (at least 1 week apart; Figure 1 ) and if they had at least one symptom of testosterone deficiency (decreased energy or decreased sexual drive). Ejaculatory dysfunction was not one of the inclusion criteria. Other inclusion criteria were a prostate-specific antigen level lower than 4 ng/mL, no use of oral or topical testosterone therapy within 14 days before visit 1, and, if prescribed, stable doses of lipid-lowering medications, insulin, antidepressants, anxiolytics, or therapy for benign prostatic hyperplasia for at least 3 months before visit 1.
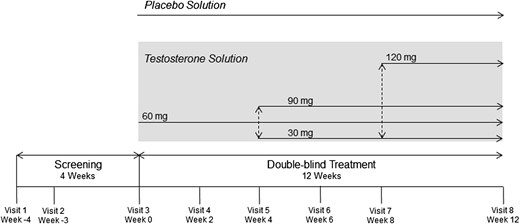
Exclusion criteria included use of long-acting intramuscular testosterone undecanoate or testosterone pellets 6 months before screening, body mass index higher than 37 kg/m2, severe lower urinary tract symptoms and significant prostate enlargement as determined by the investigator, prolactin levels higher than 30 ng/mL, glycosylated hemoglobin levels higher that 11%, hematocrit levels of at least 50% (>54% for sites located at geographic elevations ≥ 4,500 feet), or dermatologic conditions in the underarm area that would interfere with testosterone absorption or be exacerbated by topical testosterone replacement therapy. Other exclusion criteria included current treatment with chemotherapy or antiandrogens, long-term use of systemic glucocorticoids, use of anabolic steroids or estrogenizing agents within 12 months before visit 1, history of frequent opioid use, luteinizing hormone-releasing hormone antagonist or agonist treatment, clomiphene or other antiestrogen treatment, and use of finasteride, dutasteride, warfarin, phenprocoumon, or dopamine receptor agonists. Patients also were excluded if they had a history of central nervous system injuries or disease, malignant hypertension, unstable angina or coronary artery disease, liver disease, renal disease, or human immunodeficiency virus infection, or clinical suspicion or history of prostate cancer.
Study Design
This was a multicenter, randomized, double-blinded, placebo-controlled, parallel-group, 16-week (4-week screening, 12-week treatment) study in patients with testosterone deficiency. To verify testosterone deficiency, morning testosterone levels were drawn during the first screening visit (visit 1) and then at least 1 week later (visit 2). Patients were randomized at visit 3 to testosterone solution vs placebo solution (1:1 ratio) for 12 weeks (Figure 1). Treatment group assignment was determined by a computer-generated random sequence that used an interactive voice response system. To maintain blinding, participants were required to apply a single dose of study drug from each of four bottles to the axillae each day. Each of these four bottles could contain 0 or 30 mg of testosterone per one pump accentuation, thereby permitting a dosage range of 0 (placebo) to 120 mg. Patients randomized to testosterone solution began the double-blinded phase on 60 mg once daily. Dosage adjustment, if required, was implemented at visit 5 (week 4) and visit 7 (week 8), based on total testosterone levels collected at the previous visit (central laboratory analyses were conducted for all sites by Covance Central Laboratory Services, Inc, Indianapolis, IN, USA).
Main Outcome Measures
The International Index of Erectile Function (IIEF) is a multidimensional questionnaire that was self-administered by each patient who was sexually active (or wished to be).12 The IIEF consists of 15 questions in five domains. Erectile dysfunction was assessed using the IIEF erectile function domain (questions 1–5 and 15). The IIEF erectile function domain score recorded at visit 3 was used to classify erectile dysfunction severity for each patient. The IIEF sexual desire domain (questions 11 and 12) was used to provide an assessment of levels of sexual desire. The other IIEF domains evaluated included intercourse satisfaction (questions 6–8), orgasmic function (questions 9 and 10), and overall satisfaction (questions 13 and 14).
Ejaculatory function was assessed using the MSHQ-EjD-SF.13 The MSHQ-EjD-SF instrument contains four items and evaluates the frequency of ejaculation with sexual activity, the perceived strength and volume of ejaculate, and the degree of bother perceived by the patients. The four items are: (i) in the past month how often was the patient able to ejaculate when having sexual activity (rated 1 = none of the time/could not ejaculate, up to 5 = all the time); (ii) how would he rate the strength or force of his ejaculation (0 = could not ejaculate, up to 5 = as strong as it always was); (iii) how would he rate the amount or volume of semen or fluid when he ejaculates (0 = could not ejaculate, up to 5 = as much as it always was); and (iv) if he had any ejaculation difficulties or was unable to ejaculate, was he bothered by this (0 = no problem with ejaculation, up to 5 = extremely bothered). The MSHQ-EjD-SF was completed at randomization and at the end of the double-blinded treatment period (visit 8, week 12). The MSHQ-EjD-SF ejaculatory function score was calculated as the sum of responses to questions 1 through 3. The MSHQ-EjD-SF bother score was the numerical response to question 4.
Statistical Analysis
Summary statistics for continuous measurements included sample size, mean, SD, median, minimum, and maximum for actual measurements and change from baseline measurements. For categorical measurements, counts and percentages were tabulated for each category.
Analysis of covariance (ANCOVA) was used as the primary analysis method to evaluate change from baseline to week 12 (or at early discontinuation, last observation carried forward) in all efficacy variables. The ANCOVA model included terms for treatment group and the baseline value of the efficacy variable. Type III sum of squares for least-squares means was used for the statistical comparison using ANCOVA. Last post-baseline observation carried forward was used to impute missing end points.
Change from baseline to end point in IIEF and MSHQ-EjD-SF scores was analyzed in the intent-to-treat population using ANCOVA. In addition, Pearson correlation coefficients were calculated to evaluate the correlation between change from baseline to end point in free (and total) testosterone levels vs change from baseline to end point in the IIEF and MSHQ-EjD-SF domains. Pearson correlation coefficients between end-point free (and total) testosterone levels vs end points in the IIEF and MSHQ-EjD-SF domains also were calculated.
The baseline (visit 3) total testosterone level was the average of the two screening samples at visit 1 and visit 2. Treatment effects were evaluated based on a two-sided significance level of 0.05 and 95% confidence intervals were presented for the difference in least-squares means between treatment groups. Differences in safety parameters between treatment arms were assessed using the Fisher exact test. No adjustments for multiplicity were applied to these efficacy analyses, comparisons of baseline characteristics, or safety parameters.
Results
In total, 715 men (mean age = 55 years) were randomized to placebo (n = 357) or testosterone (n = 358). Demographics and patient characteristics were similar between groups (Table 1 ). Most patients were younger than 65 years (80%), white (79%), and had symptoms of decreased sexual drive and low energy (76%). Approximately 80% of sexually active patients (n = 600) who reported IIEF scores had mild to severe erectile dysfunction (IIEF erectile function score < 26). Baseline measurements of sexually active patients are presented in Table 2 .
Summary of demographics and patient characteristics (intent-to-treat population)
| Parameter . | Placebo (n = 357) . | Testosterone solution (n = 358) . | Total (N = 715) . |
|---|---|---|---|
| Age (y), mean (SD) | 55.9 (11.35) | 54.7 (10.58) | 55.3 (10.98) |
| Age category, n (%) | |||
| <65 y | 283 (79.27) | 289 (80.73) | 572 (80.00) |
| ≥65 y | 74 (20.73) | 69 (19.27) | 143 (20.00) |
| Race, n (%) | |||
| American Indian or Alaska Native | 3 (0.84) | 1 (0.28) | 4 (0.56) |
| Asian | 38 (10.64) | 45 (12.57) | 83 (11.61) |
| Black or African American | 26 (7.28) | 24 (6.70) | 50 (6.99) |
| Native Hawaiian or other islander | 2 (0.56) | 0 | 2 (0.28) |
| White | 282 (78.99) | 281 (78.49) | 563 (78.74) |
| Multiple | 6 (1.68) | 7 (1.96) | 13 (1.82) |
| Body mass index (kg/m2), mean (SD) | 30.9 (4.21) | 30.3 (4.06) | 30.6 (4.14) |
| Symptom of hypogonadism, n (%) | |||
| Only low energy | 49 (13.73) | 47 (13.13) | 96 (13.43) |
| Only decreased sexual drive | 39 (10.92) | 40 (11.17) | 79 (11.05) |
| Low energy and decreased sexual drive | 269 (75.35) | 271 (75.70) | 540 (75.52) |
| Primary cause of hypogonadism, n (%)∗ | |||
| Unknown | 145 (40.6) | 136 (38.0) | 281 (39.3) |
| Aging | 71 (19.9) | 64 (17.9) | 135 (18.9) |
| Idiopathic, acquired | 53 (14.8) | 60 (16.8) | 113 (15.8) |
| Diabetes mellitus† | 24 (6.7) | 28 (7.8) | 52 (7.3) |
| Parameter . | Placebo (n = 357) . | Testosterone solution (n = 358) . | Total (N = 715) . |
|---|---|---|---|
| Age (y), mean (SD) | 55.9 (11.35) | 54.7 (10.58) | 55.3 (10.98) |
| Age category, n (%) | |||
| <65 y | 283 (79.27) | 289 (80.73) | 572 (80.00) |
| ≥65 y | 74 (20.73) | 69 (19.27) | 143 (20.00) |
| Race, n (%) | |||
| American Indian or Alaska Native | 3 (0.84) | 1 (0.28) | 4 (0.56) |
| Asian | 38 (10.64) | 45 (12.57) | 83 (11.61) |
| Black or African American | 26 (7.28) | 24 (6.70) | 50 (6.99) |
| Native Hawaiian or other islander | 2 (0.56) | 0 | 2 (0.28) |
| White | 282 (78.99) | 281 (78.49) | 563 (78.74) |
| Multiple | 6 (1.68) | 7 (1.96) | 13 (1.82) |
| Body mass index (kg/m2), mean (SD) | 30.9 (4.21) | 30.3 (4.06) | 30.6 (4.14) |
| Symptom of hypogonadism, n (%) | |||
| Only low energy | 49 (13.73) | 47 (13.13) | 96 (13.43) |
| Only decreased sexual drive | 39 (10.92) | 40 (11.17) | 79 (11.05) |
| Low energy and decreased sexual drive | 269 (75.35) | 271 (75.70) | 540 (75.52) |
| Primary cause of hypogonadism, n (%)∗ | |||
| Unknown | 145 (40.6) | 136 (38.0) | 281 (39.3) |
| Aging | 71 (19.9) | 64 (17.9) | 135 (18.9) |
| Idiopathic, acquired | 53 (14.8) | 60 (16.8) | 113 (15.8) |
| Diabetes mellitus† | 24 (6.7) | 28 (7.8) | 52 (7.3) |
In the opinion of the principal investigator. Only causes accounting for at least 5% of patients; listed in descending order.
Glycosylated hemoglobin level higher than 11% was an exclusion criterion for this study.
Summary of demographics and patient characteristics (intent-to-treat population)
| Parameter . | Placebo (n = 357) . | Testosterone solution (n = 358) . | Total (N = 715) . |
|---|---|---|---|
| Age (y), mean (SD) | 55.9 (11.35) | 54.7 (10.58) | 55.3 (10.98) |
| Age category, n (%) | |||
| <65 y | 283 (79.27) | 289 (80.73) | 572 (80.00) |
| ≥65 y | 74 (20.73) | 69 (19.27) | 143 (20.00) |
| Race, n (%) | |||
| American Indian or Alaska Native | 3 (0.84) | 1 (0.28) | 4 (0.56) |
| Asian | 38 (10.64) | 45 (12.57) | 83 (11.61) |
| Black or African American | 26 (7.28) | 24 (6.70) | 50 (6.99) |
| Native Hawaiian or other islander | 2 (0.56) | 0 | 2 (0.28) |
| White | 282 (78.99) | 281 (78.49) | 563 (78.74) |
| Multiple | 6 (1.68) | 7 (1.96) | 13 (1.82) |
| Body mass index (kg/m2), mean (SD) | 30.9 (4.21) | 30.3 (4.06) | 30.6 (4.14) |
| Symptom of hypogonadism, n (%) | |||
| Only low energy | 49 (13.73) | 47 (13.13) | 96 (13.43) |
| Only decreased sexual drive | 39 (10.92) | 40 (11.17) | 79 (11.05) |
| Low energy and decreased sexual drive | 269 (75.35) | 271 (75.70) | 540 (75.52) |
| Primary cause of hypogonadism, n (%)∗ | |||
| Unknown | 145 (40.6) | 136 (38.0) | 281 (39.3) |
| Aging | 71 (19.9) | 64 (17.9) | 135 (18.9) |
| Idiopathic, acquired | 53 (14.8) | 60 (16.8) | 113 (15.8) |
| Diabetes mellitus† | 24 (6.7) | 28 (7.8) | 52 (7.3) |
| Parameter . | Placebo (n = 357) . | Testosterone solution (n = 358) . | Total (N = 715) . |
|---|---|---|---|
| Age (y), mean (SD) | 55.9 (11.35) | 54.7 (10.58) | 55.3 (10.98) |
| Age category, n (%) | |||
| <65 y | 283 (79.27) | 289 (80.73) | 572 (80.00) |
| ≥65 y | 74 (20.73) | 69 (19.27) | 143 (20.00) |
| Race, n (%) | |||
| American Indian or Alaska Native | 3 (0.84) | 1 (0.28) | 4 (0.56) |
| Asian | 38 (10.64) | 45 (12.57) | 83 (11.61) |
| Black or African American | 26 (7.28) | 24 (6.70) | 50 (6.99) |
| Native Hawaiian or other islander | 2 (0.56) | 0 | 2 (0.28) |
| White | 282 (78.99) | 281 (78.49) | 563 (78.74) |
| Multiple | 6 (1.68) | 7 (1.96) | 13 (1.82) |
| Body mass index (kg/m2), mean (SD) | 30.9 (4.21) | 30.3 (4.06) | 30.6 (4.14) |
| Symptom of hypogonadism, n (%) | |||
| Only low energy | 49 (13.73) | 47 (13.13) | 96 (13.43) |
| Only decreased sexual drive | 39 (10.92) | 40 (11.17) | 79 (11.05) |
| Low energy and decreased sexual drive | 269 (75.35) | 271 (75.70) | 540 (75.52) |
| Primary cause of hypogonadism, n (%)∗ | |||
| Unknown | 145 (40.6) | 136 (38.0) | 281 (39.3) |
| Aging | 71 (19.9) | 64 (17.9) | 135 (18.9) |
| Idiopathic, acquired | 53 (14.8) | 60 (16.8) | 113 (15.8) |
| Diabetes mellitus† | 24 (6.7) | 28 (7.8) | 52 (7.3) |
In the opinion of the principal investigator. Only causes accounting for at least 5% of patients; listed in descending order.
Glycosylated hemoglobin level higher than 11% was an exclusion criterion for this study.
Baseline measurements (intent-to-treat population, sexually active patients)
| . | Placebo . | Testosterone solution . | ||
|---|---|---|---|---|
| n . | Mean (SD) . | n . | Mean (SD) . | |
| IIEF | ||||
| Orgasmic function | 266 | 5.5 (3.82) | 273 | 6.1 (3.50) |
| Erectile function | 265 | 14.2 (9.30) | 273 | 16.4 (9.60) |
| Sexual desire | 265 | 5.0 (1.96) | 273 | 5.1 (2.04) |
| Intercourse satisfaction | 267 | 5.2 (4.46) | 274 | 6.2 (4.57) |
| Overall satisfaction | 262 | 4.4 (2.29) | 273 | 4.5 (2.26) |
| MSHQ-EjD-SF | ||||
| Ejaculatory function | 317 | 8.6 (4.45) | 318 | 8.8 (4.07) |
| Bother | 317 | 1.9 (1.71) | 314 | 2.1 (1.77) |
| . | Placebo . | Testosterone solution . | ||
|---|---|---|---|---|
| n . | Mean (SD) . | n . | Mean (SD) . | |
| IIEF | ||||
| Orgasmic function | 266 | 5.5 (3.82) | 273 | 6.1 (3.50) |
| Erectile function | 265 | 14.2 (9.30) | 273 | 16.4 (9.60) |
| Sexual desire | 265 | 5.0 (1.96) | 273 | 5.1 (2.04) |
| Intercourse satisfaction | 267 | 5.2 (4.46) | 274 | 6.2 (4.57) |
| Overall satisfaction | 262 | 4.4 (2.29) | 273 | 4.5 (2.26) |
| MSHQ-EjD-SF | ||||
| Ejaculatory function | 317 | 8.6 (4.45) | 318 | 8.8 (4.07) |
| Bother | 317 | 1.9 (1.71) | 314 | 2.1 (1.77) |
IIEF = International Index of Erectile Function; MSHQ-EjD-SF = Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form.
Baseline measurements (intent-to-treat population, sexually active patients)
| . | Placebo . | Testosterone solution . | ||
|---|---|---|---|---|
| n . | Mean (SD) . | n . | Mean (SD) . | |
| IIEF | ||||
| Orgasmic function | 266 | 5.5 (3.82) | 273 | 6.1 (3.50) |
| Erectile function | 265 | 14.2 (9.30) | 273 | 16.4 (9.60) |
| Sexual desire | 265 | 5.0 (1.96) | 273 | 5.1 (2.04) |
| Intercourse satisfaction | 267 | 5.2 (4.46) | 274 | 6.2 (4.57) |
| Overall satisfaction | 262 | 4.4 (2.29) | 273 | 4.5 (2.26) |
| MSHQ-EjD-SF | ||||
| Ejaculatory function | 317 | 8.6 (4.45) | 318 | 8.8 (4.07) |
| Bother | 317 | 1.9 (1.71) | 314 | 2.1 (1.77) |
| . | Placebo . | Testosterone solution . | ||
|---|---|---|---|---|
| n . | Mean (SD) . | n . | Mean (SD) . | |
| IIEF | ||||
| Orgasmic function | 266 | 5.5 (3.82) | 273 | 6.1 (3.50) |
| Erectile function | 265 | 14.2 (9.30) | 273 | 16.4 (9.60) |
| Sexual desire | 265 | 5.0 (1.96) | 273 | 5.1 (2.04) |
| Intercourse satisfaction | 267 | 5.2 (4.46) | 274 | 6.2 (4.57) |
| Overall satisfaction | 262 | 4.4 (2.29) | 273 | 4.5 (2.26) |
| MSHQ-EjD-SF | ||||
| Ejaculatory function | 317 | 8.6 (4.45) | 318 | 8.8 (4.07) |
| Bother | 317 | 1.9 (1.71) | 314 | 2.1 (1.77) |
IIEF = International Index of Erectile Function; MSHQ-EjD-SF = Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form.
Effect of Treatment With Testosterone on Ejaculatory Function
Treatment with testosterone had positive effects on study end points. Figures 2 to 4 present frequency histograms of the responses of placebo- and testosterone-treated patients to questions 1 to 3 of the MSHQ-EjD-SF. For each question (How often have you been able to ejaculate when having sexual activity? How would you rate the strength or force of your ejaculation? How would you rate the amount or volume of semen or fluid when you ejaculate?), ratings from testosterone-treated patients were significantly more positive compared with placebo-treated patients (P < .001, all three questions). Further, a significant difference in the change from baseline to end point was noted for MSHQ-EjD-SF ejaculatory function score (total score from the first three questions) between the testosterone and placebo groups (P < .001). However, the difference between treatment groups for the MSHQ-EjD-SF bother item was not statistically significant (Figure 5 ).

Percentages of responses to the question, How often have you been able to ejaculate when having sexual activity?, from the Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form. Figure 2 is available in color online at www.jsm.jsexmed.org.
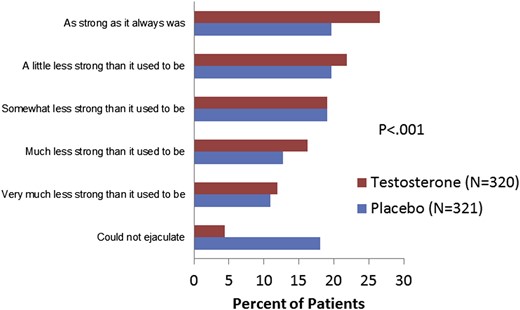
Percentages of responses to the question, How would you rate the strength or force of your ejaculation?, from the Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form. Figure 3 is available in color online at www.jsm.jsexmed.org.

Percentages of responses to the question, How would you rate the amount or volume of semen or fluid when you ejaculate?, from the Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form. Figure 4 is available in color online at www.jsm.jsexmed.org.
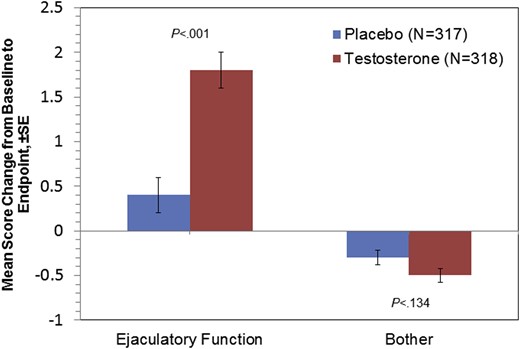
Summary of change from baseline to end point (week 12, last observation carried forward, intent-to-treat population) on the Men's Sexual Health Questionnaire, Ejaculatory Dysfunction, Short Form. SE = standard error. Figure 5 is available in color online at www.jsm.jsexmed.org.
When all patients (placebo and testosterone groups combined) were considered together, all end-point IIEF erectile function domain scores were correlated with end-point total testosterone (r = 0.13–0.20, all statistically significant).11 However, when considering just the testosterone group, only one correlation (between end-point testosterone and end-point overall satisfaction) was statistically significant (r = 0.15, 95% confidence interval = 0.03–0.27). For the MSHQ-EjD-SF, end-point ejaculatory function was significantly correlated with end-point total testosterone when all patients (placebo and testosterone groups combined) were considered together (r = 0.15, 95% confidence interval = 0.06–0.22). For the testosterone group, there were no statistically significant correlations between end-point scores on the MSHQ-EjD-SF and end-point total testosterone. Using a measurement of free testosterone or using change from baseline to end point for all measurements did not improve these correlations.
Safety
Overall, approximately 41% of patients experienced a treatment-emergent, however minor, adverse event. Treatment-emergent adverse events occurring in at least 1% of the testosterone group (n = 354) and with a greater incidence than in the placebo group (n = 356) were increased hematocrit (2.5% vs 0.6%, P = .04), upper respiratory tract infection (2.3% vs 1.7%, P = .60), arthralgia (2.3% vs 1.1%, P = .26), burning sensation (1.7% vs 1.1%, P = .55), fatigue (1.1% vs 0.0%, P = .06), increased prostate-specific antigen (1.1% vs 0.6%, P = .45), erythema (1.1% vs 0.0%, P = .06), and cough (1.1% vs 0.6%, P = .45).
Eleven patients (3.09%) in the placebo group and seven patients (1.98%) in the testosterone group experienced at least one adverse event leading to discontinuation during the double-blinded treatment period (P = .475).
There were no deaths during the double-blinded treatment phase of this study. Serious adverse events other than death were experienced by eight patients (2.25%) in the placebo group and four patients (1.13%) in the testosterone group (P = .384). Serious adverse events experienced by patients in the testosterone group were cholangiocarcinoma, convulsion, malignant neoplasm of the lung, viral meningitis, overdose, and suicide attempt. Serious adverse events experienced by patients in the placebo group were unstable angina, neck pain, chest injury, craniocerebral injury, foreign body, traumatic hematuria, ischemic stroke, lentigo maligna, pulmonary embolism, and venous thrombosis (limb).
Approximately 3% of testosterone-treated patients and 1% of placebo-treated patients experienced at least one adverse event of special interest. Adverse events of special interest reported in patients treated with testosterone were elevated hematocrit (defined as an increase > 54%; testosterone = 1.4%, placebo = 0.3%) and elevated prostate-specific antigen (defined as an increase > 4 ng/dL; testosterone = 1.4%, placebo = 0.6%).
Excluding the increase in hematocrit levels, no other clinically adverse changes were observed in laboratory parameters or vital signs in testosterone-treated patients compared with placebo-treated patients.
Discussion
The goal of testosterone replacement therapy is to restore serum total testosterone levels to the normal range, with subsequent improvement in the signs and symptoms of hypogonadism. However, there is little agreement as to the appropriate clinical end points to study (other than the serum total testosterone level), which validated patient-reported outcome to use, whether a validated patient-reported outcome for hypogonadism exists, or even if there is satisfactory evidence that testosterone replacement therapy is safe and effective.14
Brock et al11 reported that 12 weeks of daily treatment with testosterone solution 2% in hypogonadal men resulted in normal circulating testosterone levels compared with placebo (73% vs 15%; P < .001) and in significant improvement in all domains of sexual function as assessed by the IIEF (erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction). The results presented in this report extend those previous findings to ejaculatory function as measured by the MSHQ-EjD-SF, although the observed improvement was not accompanied by a decrease in the extent to which patients were bothered by their ejaculatory dysfunction. This apparent lack of improvement in bother scores could be explained by the low baseline values for the bother item, which left very little room for improvement. Moreover, it is important to consider that the MSHQ-EjD-SF was designed and validated to measure ejaculatory dysfunction and bother in men who were being treated with α-blockers. Therefore, performance characteristics of the MSHQ-EjD-SF in men with hypogonadism have not been established or validated. Better tools might be needed to measure bother score changes in non-erectile sexual dysfunction.
Little information exists in the published literature on the impact of testosterone replacement on ejaculatory dysfunction. In a previous investigation into the treatment of ejaculatory dysfunction with testosterone solution, Paduch et al15 found no statistically significant effect of testosterone solution on MSHQ-EjD-SF scores or on the IIEF orgasmic function domain. However, post hoc analysis of those patients in the testosterone arm who achieved testosterone levels of at least 300 ng/dL showed a statistically significant increase in the ejaculatory function score of the MSHQ-EjD-SF.16
Limitations
First, the most important limitation of this analysis is that ejaculatory function was not an inclusion criterion for this study. However, ejaculatory dysfunction is often seen in patients with hypogonadism, and scores on the MSHQ-EjD-SF indicate the presence of at least some degree of ejaculatory dysfunction. Second, the short duration of this clinical trial does not allow for the evaluation of long-term outcomes. Third, none of the currently available questionnaires, including the MSHQ-EjD-SF, is validated to study changes in orgasmic and ejaculatory function in hypogonadal men. Additional studies might be needed to develop and validate patient responses for this population. Fourth, it should be noted that the use of α1-adrenergic antagonists was not an exclusion criterion for this clinical trial; therefore, some ejaculatory dysfunction seen in the men in this study might have been due in part to the use of this class of agents.
Conclusions
In this multicenter, double-blinded, placebo-controlled study of testosterone replacement therapy, testosterone solution 2% effectively improved ejaculatory function in androgen-deficient men, although there was no statistically significant difference in bother. The safety findings were consistent with those of prior studies of testosterone solution 2% in hypogonadal men; no new safety signals were identified.
Statement of authorship
Category 1
- (a)
Conception and Design
Mario Maggi; Darell Heiselman; Jack Knorr
- (b)
Acquisition of Data
Mario Maggi
- (c)
Analysis and Interpretation of Data
Mario Maggi; Darell Heiselman; Jack Knorr; Smriti Iyengar; Darius A. Paduch; Craig Donatucci
Category 2
- (a)
Drafting the Article
Darell Heiselman; Smriti Iyengar
- (b)
Revising It for Intellectual Content
Mario Maggi; Darell Heiselman; Jack Knorr; Smriti Iyengar; Darius A. Paduch; Craig Donatucci
Category 3
- (a)
Final Approval of the Completed Article
Mario Maggi; Darell Heiselman; Jack Knorr; Smriti Iyengar; Darius A. Paduch; Craig Donatucci
Funding
This study was funded by Eli Lilly and Company.
Acknowledgments
Assistance with the drafting and editing of this report was provided by inVentiv Health Clinical (funded by Eli Lilly and Company).
References
Author notes
Conflicts of Interest: Dr Maggi is a consultant and speaker for Bayer, Eli Lilly, Menarini, Prostrakan, and Intercept. Dr Paduch receives grant support and speaker fees from Eli Lilly and Company. Drs Heiselman, Knorr, Iyengar, and Donatucci are employees and minor shareholders of Eli Lilly and Company.



