-
PDF
- Split View
-
Views
-
Cite
Cite
Karoline Ehlert, Johannes H Schulte, Jörn-Sven Kühl, Peter Lang, Angelika Eggert, Sebastian Voigt, Efficacy of Brincidofovir in Pediatric Stem Cell Transplant Recipients With Adenovirus Infections, Journal of the Pediatric Infectious Diseases Society, Volume 10, Issue 11, November 2021, Pages 987–993, https://doi.org/10.1093/jpids/piab072
Close - Share Icon Share
Abstract
Adenovirus (AdV) infections are of particular concern in pediatric hematopoietic stem cell transplantation (HSCT) recipients as therapeutic options are limited. Brincidofovir (BCV) is the lipid-conjugated pro-drug of cidofovir (CDV) with oral bioavailability and higher intracellular concentrations of the active drug.
In this retrospective, single-center analysis, we included allogeneic pediatric HSCT recipients with refractory AdV infections because of contraindications or insufficient response to CDV. Common posttransplant viruses were monitored at least weekly by PCR in blood, stool, and urine.
Each of the 8 patients received 6 to 12 doses of BCV. BCV treatment was initiated between days +5 and +77. AdV DNAemia and intestinal AdV infection disappeared completely in 6/8 patients. Early AdV DNAemia before day +21 did not result in increased mortality. One patient with a systemic, acyclovir-resistant HSV-1 infection responded rapidly to BCV. Four patients did not survive. AdV infection-related death in 2 patients was accompanied by >1 × 109/mL AdV copy numbers in the blood. Two more patients died of graft-vs-host disease and acute respiratory distress syndrome, respectively, both not related to AdV.
AdV DNAemia and intestinal infection subsided completely in 75% of pediatric HSCT recipients treated with BCV. AdV DNAemia exceeding 1 × 109/mL and a poor lymphocyte recovery of <250/µL were associated with high mortality. Early AdV DNAemia before day +21, however, did not result in a worse outcome. Although access to BCV is currently suspended, further clinical trials are needed to clarify the role of BCV in HSCT recipients with AdV infections and its potential benefit in preventing AdV DNAemia in immunocompromised patients.
Viral infections after allogeneic, hematopoietic stem cell transplantation (HSCT) are frequent, particularly after in vivo or in vitro T-cell depletion and in graft-vs-host disease (GvHD) with the need of prolonged pharmacologic immunosuppression [1]. Whereas therapies for stem cell transplant recipients with cytomegalovirus (CMV) infections are well established, options for children with adenovirus (AdV) infections are limited. AdV infections in immunocompromised HSCT recipients are either caused by endogenous reactivation or primary infection with a new AdV type [2–5]. There is a broad clinical spectrum of AdV infections ranging from asymptomatic patients to life-threatening diseases [3]. Reduction of immunosuppression, the use of off-label cidofovir (CDV), and AdV-specific T cells are currently the most frequent approaches [6]. However, treatment of patients with CDV may be complicated by renal insufficiency or hematologic toxicity. In the absence of donor-derived AdV-specific T cells, the generation and administration of T cells from a third-party donor is another therapeutic option for individual patients [7].
Brincidofovir (BCV) is a lipid-linked derivative of CDV with improved oral bioavailability and an increased intracellular concentration of the active drug. In contrast to CDV, the substance is not contraindicated in patients with renal insufficiency [8] and liver dysfunction. Therefore, it might represent an attractive alternative treatment option. In a retrospective multicenter trial involving children and adolescents with AdV DNAemia after HSCT, the results of 18 patients treated with BCV were compared to 23 patients treated with CDV. It was shown that BCV was superior to CDV with regard to antiviral activity and safety [9]. A randomized, placebo-controlled phase II trial in pediatric and adult allogeneic HSCT recipients demonstrated a rapid and sustained decline of AdV copies in plasma of patients treated with BCV compared to placebo [10]. In in vitro analyses, it was demonstrated that BCV had the broadest antiviral activity against double-stranded (ds) DNA viruses when compared with other antiviral substances [11].
Here, we report the results of a retrospective analysis on the use of BCV in a large pediatric center for allogeneic HSCT.
METHODS
In this retrospective, single-center case series, we included allogeneic pediatric HSCT recipients with malignant and nonmalignant diseases with AdV infections and insufficient response to CDV.
Common posttransplant viruses including AdV were monitored at least weekly by PCR in blood, stool, and urine. AdV in blood was quantitatively measured by PCR in EDTA plasma. All samples were assessed using a LightCycler 480 and Roche reagents. Patients received a weekly dose of CDV (5 mg/kg body weight [BW]) as soon as the AdV PCR in blood turned positive. When no reduction of AdV load was observed within 2 weeks after initiation of the antiviral treatment, or if side effects demanded CDV discontinuation, BCV was obtained through an emergency expanded access program. Written informed consent was obtained by parental custodians and additionally by patients where appropriate. BCV was applied according to the recommended dosing schedule provided by Chimerix Inc. Patients with concurrent treatment of cyclosporin A (CsA) received initially either 1.4 mg/kg BW (BW <48 kg) or 70 mg (BW >/=48 kg) twice weekly and for consolidation the double dose once weekly. Patients without concurrent treatment of CsA received initially either 2 mg/kg BW (BW <48 kg) or 100 mg (BW >/=48 kg) twice weekly and for consolidation the double dose once weekly. BCV was started 48 hours after the last dose of CDV at the earliest.
Treatment-related data of the respective patients were collected from their individual charts and the clinical electronic information system. Renal and hepatic function was monitored before, during, and after treatment with BCV. Creatinine, bilirubin, and aspartate aminotransferase (AST) in plasma were measured mostly daily in a 2-week period before, during, and after treatment with BCV. Gastrointestinal symptoms, primarily diarrhea, are dose-limiting factors for BCV administration. Therefore, stool frequencies were assessed on a daily basis at the same time as defined above. Partial details of treatment in patient 1 were already published in 2016 [12]. The local ethics committee was consulted for each patient before receiving BCV.
RESULTS
Patient Characteristics and Transplant-Related Data
Eight patients, 4 girls and 4 boys, aged 1-18 years, were included in this retrospective case series. Seven of 8 patients received their allogeneic HSCT for hematologic diseases, mostly acute leukemia and myelodysplastic syndrome (MDS), and 1 patient for neuroblastoma. Stem cell donors were HLA-identical siblings in 2 patients, matched unrelated donors in 5 patients, and a haploidentical parent in 1 patient. The use of bone marrow and peripheral blood stem cells as stem cell sources was equally distributed. Six patients were treated with either anti-thymocyte globulin or alemtuzumab as part of the preparative regimen for GvHD prophylaxis and/or graft rejection (Table 1). Four patients died of transplant-related adverse events between days +64 and +161 after their respective transplant procedures.
| Patient Number, Sex, Ethnicity . | Age at Transplant in Years . | Diagnosis . | Donor, Stem Cell Source . | Preparative Regimen . | Outcome . |
|---|---|---|---|---|---|
| 1, Female, Caucasian | 5 | Myelodysplastic syndrome | MUD, BM | Fludarabine, TT, thymoglobulin, TBI 4Gy | Alive |
| 2, Female, Caucasian | 15 | Hodgkin lymphoma | MSD, BM | Fludarabine, melphalan | Deceased b/o GvHD; AdV-negative in tracheal samples |
| 3, Male, Caucasian | 7 | Acute lymphoblastic leukemia | MUD, PBSC | TBI 12Gy, etoposide, ATG | Alive |
| 4, Female, Caucasian | 18 | Myelodysplastic syndrome | MUD, PBSC | Fludarabine, treosulfan, TT, ATG | Alive |
| 5, Male, Caucasian | 3 | Neuroblastoma | MMRD, PBSC (haploidentical) | Fludarabine, TT, melphalan, ATG | Alive |
| 6, Female, Caucasian | 17 | Fanconi anemia | MUD, BM | Busulfan, fludarabine, alemtuzumab | Deceased b/o AdV + refractory GvHD + MOF |
| 7, Male, Caucasian | 1 | Acute myeloid leukemia | MUD, PBSC | Busulfan, cyclophosphamide, melphalan, ATG | Deceased b/o ARDS, AdV |
| 8, Male, Caucasian | 12 | Acute lymphoblastic leukemia | MSD, BM | TBI 12Gy, etoposide | Deceased b/o ARDS; AdV negative in tracheal samples |
| Patient Number, Sex, Ethnicity . | Age at Transplant in Years . | Diagnosis . | Donor, Stem Cell Source . | Preparative Regimen . | Outcome . |
|---|---|---|---|---|---|
| 1, Female, Caucasian | 5 | Myelodysplastic syndrome | MUD, BM | Fludarabine, TT, thymoglobulin, TBI 4Gy | Alive |
| 2, Female, Caucasian | 15 | Hodgkin lymphoma | MSD, BM | Fludarabine, melphalan | Deceased b/o GvHD; AdV-negative in tracheal samples |
| 3, Male, Caucasian | 7 | Acute lymphoblastic leukemia | MUD, PBSC | TBI 12Gy, etoposide, ATG | Alive |
| 4, Female, Caucasian | 18 | Myelodysplastic syndrome | MUD, PBSC | Fludarabine, treosulfan, TT, ATG | Alive |
| 5, Male, Caucasian | 3 | Neuroblastoma | MMRD, PBSC (haploidentical) | Fludarabine, TT, melphalan, ATG | Alive |
| 6, Female, Caucasian | 17 | Fanconi anemia | MUD, BM | Busulfan, fludarabine, alemtuzumab | Deceased b/o AdV + refractory GvHD + MOF |
| 7, Male, Caucasian | 1 | Acute myeloid leukemia | MUD, PBSC | Busulfan, cyclophosphamide, melphalan, ATG | Deceased b/o ARDS, AdV |
| 8, Male, Caucasian | 12 | Acute lymphoblastic leukemia | MSD, BM | TBI 12Gy, etoposide | Deceased b/o ARDS; AdV negative in tracheal samples |
Abbreviations: AdV, adenovirus; ARDS, acute respiratory distress syndrome; ATG, anti-thymocyte globulin; b/o, because of; BM, bone marrow; GvHD, graft-vs-host disease; Gy, gray; MMRD, mismatched related donor; MOF, multi-organ failure; MSD, matched sibling donor; MUD, matched unrelated donor; PBSC, peripheral blood stem cells; TBI, total body irradiation; TT, thiotepa.
| Patient Number, Sex, Ethnicity . | Age at Transplant in Years . | Diagnosis . | Donor, Stem Cell Source . | Preparative Regimen . | Outcome . |
|---|---|---|---|---|---|
| 1, Female, Caucasian | 5 | Myelodysplastic syndrome | MUD, BM | Fludarabine, TT, thymoglobulin, TBI 4Gy | Alive |
| 2, Female, Caucasian | 15 | Hodgkin lymphoma | MSD, BM | Fludarabine, melphalan | Deceased b/o GvHD; AdV-negative in tracheal samples |
| 3, Male, Caucasian | 7 | Acute lymphoblastic leukemia | MUD, PBSC | TBI 12Gy, etoposide, ATG | Alive |
| 4, Female, Caucasian | 18 | Myelodysplastic syndrome | MUD, PBSC | Fludarabine, treosulfan, TT, ATG | Alive |
| 5, Male, Caucasian | 3 | Neuroblastoma | MMRD, PBSC (haploidentical) | Fludarabine, TT, melphalan, ATG | Alive |
| 6, Female, Caucasian | 17 | Fanconi anemia | MUD, BM | Busulfan, fludarabine, alemtuzumab | Deceased b/o AdV + refractory GvHD + MOF |
| 7, Male, Caucasian | 1 | Acute myeloid leukemia | MUD, PBSC | Busulfan, cyclophosphamide, melphalan, ATG | Deceased b/o ARDS, AdV |
| 8, Male, Caucasian | 12 | Acute lymphoblastic leukemia | MSD, BM | TBI 12Gy, etoposide | Deceased b/o ARDS; AdV negative in tracheal samples |
| Patient Number, Sex, Ethnicity . | Age at Transplant in Years . | Diagnosis . | Donor, Stem Cell Source . | Preparative Regimen . | Outcome . |
|---|---|---|---|---|---|
| 1, Female, Caucasian | 5 | Myelodysplastic syndrome | MUD, BM | Fludarabine, TT, thymoglobulin, TBI 4Gy | Alive |
| 2, Female, Caucasian | 15 | Hodgkin lymphoma | MSD, BM | Fludarabine, melphalan | Deceased b/o GvHD; AdV-negative in tracheal samples |
| 3, Male, Caucasian | 7 | Acute lymphoblastic leukemia | MUD, PBSC | TBI 12Gy, etoposide, ATG | Alive |
| 4, Female, Caucasian | 18 | Myelodysplastic syndrome | MUD, PBSC | Fludarabine, treosulfan, TT, ATG | Alive |
| 5, Male, Caucasian | 3 | Neuroblastoma | MMRD, PBSC (haploidentical) | Fludarabine, TT, melphalan, ATG | Alive |
| 6, Female, Caucasian | 17 | Fanconi anemia | MUD, BM | Busulfan, fludarabine, alemtuzumab | Deceased b/o AdV + refractory GvHD + MOF |
| 7, Male, Caucasian | 1 | Acute myeloid leukemia | MUD, PBSC | Busulfan, cyclophosphamide, melphalan, ATG | Deceased b/o ARDS, AdV |
| 8, Male, Caucasian | 12 | Acute lymphoblastic leukemia | MSD, BM | TBI 12Gy, etoposide | Deceased b/o ARDS; AdV negative in tracheal samples |
Abbreviations: AdV, adenovirus; ARDS, acute respiratory distress syndrome; ATG, anti-thymocyte globulin; b/o, because of; BM, bone marrow; GvHD, graft-vs-host disease; Gy, gray; MMRD, mismatched related donor; MOF, multi-organ failure; MSD, matched sibling donor; MUD, matched unrelated donor; PBSC, peripheral blood stem cells; TBI, total body irradiation; TT, thiotepa.
AdV Infection-Related Data
In 7 of the 8 patients, AdV DNAemia was first detected between days +13 and +75 posttransplant and in 1 patient (patient 1) already on day −25 before her third transplant. This patient, a girl with MDS, had her second allogeneic HSCT 3.5 months earlier and suffered from a second graft rejection. Between her transplant procedures, she displayed severe lymphopenia. In addition to AdV DNAemia, all patients were also diagnosed with intestinal AdV infection, as shown by positive PCR results in stool, mostly 1-3 weeks before AdV DNAemia. A single AdV species type C was detected in 5, non-C in 2 patients. Patient 7 had both C and non-C AdV infection (Table 2).
| Patient Number . | AdV Species . | AdV in Stools, Day . | AdV in Blood, Day . | AdV Maximum Copy Number in Blood, Day . | Treatment Before BCV . | AdV Copy Number at Start of BCV, Day . | BCV Doses . | Steroids at Start of BCV . | AdV Infection Resolved . | Further Viruses in the Transplant Period . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C | −83 to +26 | −25 to +26 | 4.43 × 106 +10 | CDV | 1.12 × 106 +8 | 12 | No | Yes | EBV (blood, d +34 to d +55), HSV (blood, oral, resistant to aciclovir), BK (urine, very weak in blood), HHV-6 (blood, d −15 to d −1) | Alive |
| 2 | C | +50 to +78 | +61 to +79 | 13 500 +64 | CDV | 2330, +76 | 5 | Yes | Yes | BK (urine) | Deceased +91 |
| 3 | C | +21 to +56 | +16 to +42 | 410 000 +28 | CDV | 410 000 +28 | 12 | Yes | Yes | BK (urine), EBV (resolved after rituximab) | Alive |
| 4 | Non-C | +13 to +97 | +13 to +67 | 802 000 +34 | CDV | 44 200 +51 | 4 | No | Yes | BK (urine), EBV (very weak, no specific treatment) | Alive |
| 5 | Non-C: A31 | +14 to +98 | +21 to +41 +63 to +93 | 44 100 +73 | CDV | 35 100 +27 | 6 | No | Yes | None | Alive |
| 6 | C | +14 to +38 | +21 to +84 | 1.37 × 109 +84 | CDV Ribavirin | 1.15 × 108 +51 | 6 | Yes | No | BK (urine) | Deceased +85 |
| 7 | C + non-C | +7 to +51 | +27 to +64 | 3.07 × 109 +47 | CDV, AdV-specific T cells | 2.98 × 109 +46 | 6 | No | No | EBV (blood, d +42 to d +51), HHV-6 (blood, d +34 to d +49) | Deceased +64 |
| 8 | C | +68 to +89 | +75 to +82 | 289 000 +75 | No CDV | 101 000 +77 | 10 | Yes | Yes | CMV (blood, d +19 to d +22), BK (urine) | Deceased +161 |
| Patient Number . | AdV Species . | AdV in Stools, Day . | AdV in Blood, Day . | AdV Maximum Copy Number in Blood, Day . | Treatment Before BCV . | AdV Copy Number at Start of BCV, Day . | BCV Doses . | Steroids at Start of BCV . | AdV Infection Resolved . | Further Viruses in the Transplant Period . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C | −83 to +26 | −25 to +26 | 4.43 × 106 +10 | CDV | 1.12 × 106 +8 | 12 | No | Yes | EBV (blood, d +34 to d +55), HSV (blood, oral, resistant to aciclovir), BK (urine, very weak in blood), HHV-6 (blood, d −15 to d −1) | Alive |
| 2 | C | +50 to +78 | +61 to +79 | 13 500 +64 | CDV | 2330, +76 | 5 | Yes | Yes | BK (urine) | Deceased +91 |
| 3 | C | +21 to +56 | +16 to +42 | 410 000 +28 | CDV | 410 000 +28 | 12 | Yes | Yes | BK (urine), EBV (resolved after rituximab) | Alive |
| 4 | Non-C | +13 to +97 | +13 to +67 | 802 000 +34 | CDV | 44 200 +51 | 4 | No | Yes | BK (urine), EBV (very weak, no specific treatment) | Alive |
| 5 | Non-C: A31 | +14 to +98 | +21 to +41 +63 to +93 | 44 100 +73 | CDV | 35 100 +27 | 6 | No | Yes | None | Alive |
| 6 | C | +14 to +38 | +21 to +84 | 1.37 × 109 +84 | CDV Ribavirin | 1.15 × 108 +51 | 6 | Yes | No | BK (urine) | Deceased +85 |
| 7 | C + non-C | +7 to +51 | +27 to +64 | 3.07 × 109 +47 | CDV, AdV-specific T cells | 2.98 × 109 +46 | 6 | No | No | EBV (blood, d +42 to d +51), HHV-6 (blood, d +34 to d +49) | Deceased +64 |
| 8 | C | +68 to +89 | +75 to +82 | 289 000 +75 | No CDV | 101 000 +77 | 10 | Yes | Yes | CMV (blood, d +19 to d +22), BK (urine) | Deceased +161 |
Abbreviations: BCV, brincidofovir; BK, human polyomavirus 1; CDV, cidofovir; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV-6, human herpesvirus-6; HSV, herpes simplex virus.
| Patient Number . | AdV Species . | AdV in Stools, Day . | AdV in Blood, Day . | AdV Maximum Copy Number in Blood, Day . | Treatment Before BCV . | AdV Copy Number at Start of BCV, Day . | BCV Doses . | Steroids at Start of BCV . | AdV Infection Resolved . | Further Viruses in the Transplant Period . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C | −83 to +26 | −25 to +26 | 4.43 × 106 +10 | CDV | 1.12 × 106 +8 | 12 | No | Yes | EBV (blood, d +34 to d +55), HSV (blood, oral, resistant to aciclovir), BK (urine, very weak in blood), HHV-6 (blood, d −15 to d −1) | Alive |
| 2 | C | +50 to +78 | +61 to +79 | 13 500 +64 | CDV | 2330, +76 | 5 | Yes | Yes | BK (urine) | Deceased +91 |
| 3 | C | +21 to +56 | +16 to +42 | 410 000 +28 | CDV | 410 000 +28 | 12 | Yes | Yes | BK (urine), EBV (resolved after rituximab) | Alive |
| 4 | Non-C | +13 to +97 | +13 to +67 | 802 000 +34 | CDV | 44 200 +51 | 4 | No | Yes | BK (urine), EBV (very weak, no specific treatment) | Alive |
| 5 | Non-C: A31 | +14 to +98 | +21 to +41 +63 to +93 | 44 100 +73 | CDV | 35 100 +27 | 6 | No | Yes | None | Alive |
| 6 | C | +14 to +38 | +21 to +84 | 1.37 × 109 +84 | CDV Ribavirin | 1.15 × 108 +51 | 6 | Yes | No | BK (urine) | Deceased +85 |
| 7 | C + non-C | +7 to +51 | +27 to +64 | 3.07 × 109 +47 | CDV, AdV-specific T cells | 2.98 × 109 +46 | 6 | No | No | EBV (blood, d +42 to d +51), HHV-6 (blood, d +34 to d +49) | Deceased +64 |
| 8 | C | +68 to +89 | +75 to +82 | 289 000 +75 | No CDV | 101 000 +77 | 10 | Yes | Yes | CMV (blood, d +19 to d +22), BK (urine) | Deceased +161 |
| Patient Number . | AdV Species . | AdV in Stools, Day . | AdV in Blood, Day . | AdV Maximum Copy Number in Blood, Day . | Treatment Before BCV . | AdV Copy Number at Start of BCV, Day . | BCV Doses . | Steroids at Start of BCV . | AdV Infection Resolved . | Further Viruses in the Transplant Period . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C | −83 to +26 | −25 to +26 | 4.43 × 106 +10 | CDV | 1.12 × 106 +8 | 12 | No | Yes | EBV (blood, d +34 to d +55), HSV (blood, oral, resistant to aciclovir), BK (urine, very weak in blood), HHV-6 (blood, d −15 to d −1) | Alive |
| 2 | C | +50 to +78 | +61 to +79 | 13 500 +64 | CDV | 2330, +76 | 5 | Yes | Yes | BK (urine) | Deceased +91 |
| 3 | C | +21 to +56 | +16 to +42 | 410 000 +28 | CDV | 410 000 +28 | 12 | Yes | Yes | BK (urine), EBV (resolved after rituximab) | Alive |
| 4 | Non-C | +13 to +97 | +13 to +67 | 802 000 +34 | CDV | 44 200 +51 | 4 | No | Yes | BK (urine), EBV (very weak, no specific treatment) | Alive |
| 5 | Non-C: A31 | +14 to +98 | +21 to +41 +63 to +93 | 44 100 +73 | CDV | 35 100 +27 | 6 | No | Yes | None | Alive |
| 6 | C | +14 to +38 | +21 to +84 | 1.37 × 109 +84 | CDV Ribavirin | 1.15 × 108 +51 | 6 | Yes | No | BK (urine) | Deceased +85 |
| 7 | C + non-C | +7 to +51 | +27 to +64 | 3.07 × 109 +47 | CDV, AdV-specific T cells | 2.98 × 109 +46 | 6 | No | No | EBV (blood, d +42 to d +51), HHV-6 (blood, d +34 to d +49) | Deceased +64 |
| 8 | C | +68 to +89 | +75 to +82 | 289 000 +75 | No CDV | 101 000 +77 | 10 | Yes | Yes | CMV (blood, d +19 to d +22), BK (urine) | Deceased +161 |
Abbreviations: BCV, brincidofovir; BK, human polyomavirus 1; CDV, cidofovir; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV-6, human herpesvirus-6; HSV, herpes simplex virus.
Six patients initially received CDV. However, a persistent decrease in AdV load >1 log level could not be observed in any patient after a 2-week administration. Antiviral treatment in patient 2 was switched to BCV because of kidney failure after CDV administration. Patient 5 had 2 episodes of AdV DNAemia, but received BCV only during the first one. AdV was permanently present in the feces of this patient and considered the source of the second episode of AdV DNAemia 22 days later. This second course of AdV DNAemia was controlled by the use of CDV. Patient 6, a girl with Fanconi anemia and advanced MDS with monosomy 7, developed repeated episodes of severe intestinal bleeding on day +23 when AdV DNAemia was diagnosed. Her condition worsened and she presented with complete paralytic ileus on day +39. Treatment with BCV was initiated on day +51 after her AdV DNAemia had rapidly increased. Conservative approaches to reestablish bowel function were not successful. The placement of a jejunostoma and an ileostoma on day +59 was required. Two small segments of the bowel, 10 and 15 mm, respectively, were resected and exhibited an advanced destruction of the mucosa, ulcerations, villous atrophy, and a high number of apoptoses consistent with severe acute GvHD. This patient also had surgery for correction of duodenal atresia at the age of 3 years. Patient 8 received first-line BCV because of impaired renal function.
Details of AdV infection and treatment are summarized in Table 2. Within 3 to 18 days after the first administration of BCV, AdV DNAemia resolved completely in 6 of 8 patients. The presence of AdV in stool samples preceded AdV DNAemia by 1-3 weeks in 7/8 HSCT recipients. The only exception was noticed in patient 1, in whom AdV was found in stool samples from days −83 until day +26. All patients with AdV clearance in blood also showed viral clearance in the gastrointestinal tract. Two to 71 days after the first administration of BCV, AdV was no longer detectable in stool specimens.
AdV DNAemia developed as early as pretransplant or before day +21 in 3 patients, who are all alive and well (patients 1, 3, and 4).
Additional Viral Infections in the Posttransplant Period
Apart from patient 7, additional viral infections were detected in all other HSCT recipients. In patient 1, oral and systemic infection with an acyclovir-resistant HSV-1 strain resolved rapidly after the onset of treatment with BCV. However, with regard to all other viral infections in the remaining 7 patients, a preventive or therapeutic effect of BCV could not be observed.
Adverse Effects During Treatment With BCV
Table 3 summarizes laboratory parameters and stool frequencies before, during, and after BCV treatment. The most pronounced findings were detected in patients 2 and 6. Patient 2 had a 7-fold increase in bilirubin during treatment with BCV and died of treatment-refractory GvHD with severe liver affection. Patient 6 had a 2- to 5-fold increase in plasma creatinine, bilirubin, and ASAT while on treatment with BCV, presumably due to uncontrolled AdV infection. She required respiratory support and hemodialysis but finally died of multi-organ failure (MOF). In the remaining 6 patients, mild changes in laboratory parameters were noticed. There was no clear trend among the patients regarding their stool frequencies. No interruption of BCV therapy was necessary for any patient.
Laboratory Results and Clinical Parameters Before, During, and After Treatment With BCV
| Patient Number . | Plasma Creatinine, mg/dL (Age-Adjusted Reference Range) . | Plasma Bilirubin, mg/dL (Age-Adjusted Reference Range) . | ASAT, Units/L (Age-Adjusted Reference Range) . | Stool Frequency, Numbers/Day . | Comments . |
|---|---|---|---|---|---|
| 1 | 0.2-0.4-0.4 (0.5-0.8) | 0.37-0.62-0.85 (0.2-1.0) | 24-97-55 (<53) | 2.7-3.7-3.9 | None |
| 2 | 0.8-0.7-no data (0.8-1.4) | 3.5-25.9-NA (0.2-1.0) | 39-88-NA (<46) | 11.7-7.9-NA | Patient received BCV until the day of her death |
| 3 | 0.7-0.4-0.5 (0.6-0.9) | 0.7-2.0-0.7 (0.2-1.0) | 94-118-129 (<50) | 4.7-4.7-2.3 | None |
| 4 | 0.5-0.5-0.6 (0.8-1.4) | 14.6-7.4-5.2 (0.2-1.0) | 50-50-40 (<46) | 3.1-5-5 | The third period included only 7 d |
| 5 | 0.2-0.3-0.5 (0.4-0.7) | 0.2-0.3-0.2 (0.2-1.0) | 71-58-50 (<96) | 5.6-5.1-8.2 | None |
| 6 | 0.9-2.4-no data (0.8-1.4) | 1.1-6.3-11.1 (0.2-1.0) | 138-720-581 (<46) | 5.9-2.1-blood/mucus | Due to her death, the third period for ASAT and bilirubin included only 5 d. Because of hemodialysis, plasma creatinine was not informative in the third period |
| 7 | 0.3-0.3-0.3 (0.4-0.7) | 0.5-1.5-4.4 (0.2-1.0) | 39-28-16 (<96) | 2.1-3.1-4.7 | Due to this death, the third period included only 3 d |
| 8 | 0.5-0.4-0.4 (0.6-1.0) | 0.4-0.3-0.4 (0.2-1.0) | 46-38-59 (<50) | 4.3-4.6-3.1 | None |
| Patient Number . | Plasma Creatinine, mg/dL (Age-Adjusted Reference Range) . | Plasma Bilirubin, mg/dL (Age-Adjusted Reference Range) . | ASAT, Units/L (Age-Adjusted Reference Range) . | Stool Frequency, Numbers/Day . | Comments . |
|---|---|---|---|---|---|
| 1 | 0.2-0.4-0.4 (0.5-0.8) | 0.37-0.62-0.85 (0.2-1.0) | 24-97-55 (<53) | 2.7-3.7-3.9 | None |
| 2 | 0.8-0.7-no data (0.8-1.4) | 3.5-25.9-NA (0.2-1.0) | 39-88-NA (<46) | 11.7-7.9-NA | Patient received BCV until the day of her death |
| 3 | 0.7-0.4-0.5 (0.6-0.9) | 0.7-2.0-0.7 (0.2-1.0) | 94-118-129 (<50) | 4.7-4.7-2.3 | None |
| 4 | 0.5-0.5-0.6 (0.8-1.4) | 14.6-7.4-5.2 (0.2-1.0) | 50-50-40 (<46) | 3.1-5-5 | The third period included only 7 d |
| 5 | 0.2-0.3-0.5 (0.4-0.7) | 0.2-0.3-0.2 (0.2-1.0) | 71-58-50 (<96) | 5.6-5.1-8.2 | None |
| 6 | 0.9-2.4-no data (0.8-1.4) | 1.1-6.3-11.1 (0.2-1.0) | 138-720-581 (<46) | 5.9-2.1-blood/mucus | Due to her death, the third period for ASAT and bilirubin included only 5 d. Because of hemodialysis, plasma creatinine was not informative in the third period |
| 7 | 0.3-0.3-0.3 (0.4-0.7) | 0.5-1.5-4.4 (0.2-1.0) | 39-28-16 (<96) | 2.1-3.1-4.7 | Due to this death, the third period included only 3 d |
| 8 | 0.5-0.4-0.4 (0.6-1.0) | 0.4-0.3-0.4 (0.2-1.0) | 46-38-59 (<50) | 4.3-4.6-3.1 | None |
Abbreviations: ASAT, aspartate aminotransferase; BCV, brincidofovir; NA, not applicable.
For each laboratory result and stool frequency as a clinical parameter, the respective values are given as mean numbers per day in the 2 weeks before administration of BCV (first period), during treatment with BCV (second period), and in the 2 weeks after administration of BCV (third period).
Laboratory Results and Clinical Parameters Before, During, and After Treatment With BCV
| Patient Number . | Plasma Creatinine, mg/dL (Age-Adjusted Reference Range) . | Plasma Bilirubin, mg/dL (Age-Adjusted Reference Range) . | ASAT, Units/L (Age-Adjusted Reference Range) . | Stool Frequency, Numbers/Day . | Comments . |
|---|---|---|---|---|---|
| 1 | 0.2-0.4-0.4 (0.5-0.8) | 0.37-0.62-0.85 (0.2-1.0) | 24-97-55 (<53) | 2.7-3.7-3.9 | None |
| 2 | 0.8-0.7-no data (0.8-1.4) | 3.5-25.9-NA (0.2-1.0) | 39-88-NA (<46) | 11.7-7.9-NA | Patient received BCV until the day of her death |
| 3 | 0.7-0.4-0.5 (0.6-0.9) | 0.7-2.0-0.7 (0.2-1.0) | 94-118-129 (<50) | 4.7-4.7-2.3 | None |
| 4 | 0.5-0.5-0.6 (0.8-1.4) | 14.6-7.4-5.2 (0.2-1.0) | 50-50-40 (<46) | 3.1-5-5 | The third period included only 7 d |
| 5 | 0.2-0.3-0.5 (0.4-0.7) | 0.2-0.3-0.2 (0.2-1.0) | 71-58-50 (<96) | 5.6-5.1-8.2 | None |
| 6 | 0.9-2.4-no data (0.8-1.4) | 1.1-6.3-11.1 (0.2-1.0) | 138-720-581 (<46) | 5.9-2.1-blood/mucus | Due to her death, the third period for ASAT and bilirubin included only 5 d. Because of hemodialysis, plasma creatinine was not informative in the third period |
| 7 | 0.3-0.3-0.3 (0.4-0.7) | 0.5-1.5-4.4 (0.2-1.0) | 39-28-16 (<96) | 2.1-3.1-4.7 | Due to this death, the third period included only 3 d |
| 8 | 0.5-0.4-0.4 (0.6-1.0) | 0.4-0.3-0.4 (0.2-1.0) | 46-38-59 (<50) | 4.3-4.6-3.1 | None |
| Patient Number . | Plasma Creatinine, mg/dL (Age-Adjusted Reference Range) . | Plasma Bilirubin, mg/dL (Age-Adjusted Reference Range) . | ASAT, Units/L (Age-Adjusted Reference Range) . | Stool Frequency, Numbers/Day . | Comments . |
|---|---|---|---|---|---|
| 1 | 0.2-0.4-0.4 (0.5-0.8) | 0.37-0.62-0.85 (0.2-1.0) | 24-97-55 (<53) | 2.7-3.7-3.9 | None |
| 2 | 0.8-0.7-no data (0.8-1.4) | 3.5-25.9-NA (0.2-1.0) | 39-88-NA (<46) | 11.7-7.9-NA | Patient received BCV until the day of her death |
| 3 | 0.7-0.4-0.5 (0.6-0.9) | 0.7-2.0-0.7 (0.2-1.0) | 94-118-129 (<50) | 4.7-4.7-2.3 | None |
| 4 | 0.5-0.5-0.6 (0.8-1.4) | 14.6-7.4-5.2 (0.2-1.0) | 50-50-40 (<46) | 3.1-5-5 | The third period included only 7 d |
| 5 | 0.2-0.3-0.5 (0.4-0.7) | 0.2-0.3-0.2 (0.2-1.0) | 71-58-50 (<96) | 5.6-5.1-8.2 | None |
| 6 | 0.9-2.4-no data (0.8-1.4) | 1.1-6.3-11.1 (0.2-1.0) | 138-720-581 (<46) | 5.9-2.1-blood/mucus | Due to her death, the third period for ASAT and bilirubin included only 5 d. Because of hemodialysis, plasma creatinine was not informative in the third period |
| 7 | 0.3-0.3-0.3 (0.4-0.7) | 0.5-1.5-4.4 (0.2-1.0) | 39-28-16 (<96) | 2.1-3.1-4.7 | Due to this death, the third period included only 3 d |
| 8 | 0.5-0.4-0.4 (0.6-1.0) | 0.4-0.3-0.4 (0.2-1.0) | 46-38-59 (<50) | 4.3-4.6-3.1 | None |
Abbreviations: ASAT, aspartate aminotransferase; BCV, brincidofovir; NA, not applicable.
For each laboratory result and stool frequency as a clinical parameter, the respective values are given as mean numbers per day in the 2 weeks before administration of BCV (first period), during treatment with BCV (second period), and in the 2 weeks after administration of BCV (third period).
Pharmacological Immunosuppressive Treatment During AdV Infection
Pharmacologic immunosuppressive treatment is summarized in Table 4. As AdV DNAemia was already observed pretransplant in patient 1, she did not receive GvHD prophylaxis at that time point. In patient 7, GvHD prophylaxis had already been terminated, when BCV treatment was initiated. Pharmacologic immunosuppression in patients 1-6 and 8 comprised of CsA, everolimus, MMF (mycophenolate mofetil), and prednisone, and included an additional methylprednisolone pulse in patient 2. At the end of BCV treatment, 3 patients (5, 6, and 7) were without systemic immunosuppression. Patient 5 had finished a 30-day course of MMF according to protocol. Although intestinal histopathology indicated the presence of GvHD in patient 6, CsA and prednisone were discontinued in the attempt to mount a host response.
Pharmacologic Immunosuppression at Start of AdV DNAemia, at Start and at the End of Treatment With BCV
| Patient Number . | Pharmacologic Immunosuppression . | . | . |
|---|---|---|---|
| . | At Start of AdV DNAemia . | At Start of Treatment With BCV . | At the End of Treatment With BCV . |
| 1 | None (already positive pretransplant) | CsA (trough 115 µg/L) | CsA (trough 97 µg/L), prednisone |
| 2 | CsA (trough 130 µg/L), MMF, budesonide, prednisone | CsA (trough 87 µg/L), MMF, prednisone, budesonide, methylprednisolone pulse (first of 3 consecutive days) | CsA (trough 67 µg/L), MMF, prednisone, budesonide |
| 3 | CsA (trough 84 µg/L), MMF | everolimus (trough 7.3 µg/L), MMF, prednisone | CsA (trough, 46 µg/L), MMF, prednisone |
| 4 | CsA (trough 103 µg/L) | CsA (trough 46 µg/L) | CsA (trough 46 µg/L) |
| 5 | MMF | MMF | None |
| 6 | CsA (trough 140 µg/L), MMF, prednisone | CsA (trough 101 µg/L), prednisone | None |
| 7 | Everolimus (trough 4.8 µg/L), MMF | None | None |
| 8 | Everolimus (trough 5.4 µg/L), MMF, prednisone | Everolimus (trough 4.3 µg/L), MMF, prednisone | Everolimus (trough 2.4 µg/L), MMF, prednisone, methylprednisolone pulse (second of 3 consecutive days), ruxolitinib |
| Patient Number . | Pharmacologic Immunosuppression . | . | . |
|---|---|---|---|
| . | At Start of AdV DNAemia . | At Start of Treatment With BCV . | At the End of Treatment With BCV . |
| 1 | None (already positive pretransplant) | CsA (trough 115 µg/L) | CsA (trough 97 µg/L), prednisone |
| 2 | CsA (trough 130 µg/L), MMF, budesonide, prednisone | CsA (trough 87 µg/L), MMF, prednisone, budesonide, methylprednisolone pulse (first of 3 consecutive days) | CsA (trough 67 µg/L), MMF, prednisone, budesonide |
| 3 | CsA (trough 84 µg/L), MMF | everolimus (trough 7.3 µg/L), MMF, prednisone | CsA (trough, 46 µg/L), MMF, prednisone |
| 4 | CsA (trough 103 µg/L) | CsA (trough 46 µg/L) | CsA (trough 46 µg/L) |
| 5 | MMF | MMF | None |
| 6 | CsA (trough 140 µg/L), MMF, prednisone | CsA (trough 101 µg/L), prednisone | None |
| 7 | Everolimus (trough 4.8 µg/L), MMF | None | None |
| 8 | Everolimus (trough 5.4 µg/L), MMF, prednisone | Everolimus (trough 4.3 µg/L), MMF, prednisone | Everolimus (trough 2.4 µg/L), MMF, prednisone, methylprednisolone pulse (second of 3 consecutive days), ruxolitinib |
Abbreviations: AdV, adenovirus; BCV, brincidofovir; CsA, cyclosporin A; MMF, mycophenolate mofetil.
Pharmacologic Immunosuppression at Start of AdV DNAemia, at Start and at the End of Treatment With BCV
| Patient Number . | Pharmacologic Immunosuppression . | . | . |
|---|---|---|---|
| . | At Start of AdV DNAemia . | At Start of Treatment With BCV . | At the End of Treatment With BCV . |
| 1 | None (already positive pretransplant) | CsA (trough 115 µg/L) | CsA (trough 97 µg/L), prednisone |
| 2 | CsA (trough 130 µg/L), MMF, budesonide, prednisone | CsA (trough 87 µg/L), MMF, prednisone, budesonide, methylprednisolone pulse (first of 3 consecutive days) | CsA (trough 67 µg/L), MMF, prednisone, budesonide |
| 3 | CsA (trough 84 µg/L), MMF | everolimus (trough 7.3 µg/L), MMF, prednisone | CsA (trough, 46 µg/L), MMF, prednisone |
| 4 | CsA (trough 103 µg/L) | CsA (trough 46 µg/L) | CsA (trough 46 µg/L) |
| 5 | MMF | MMF | None |
| 6 | CsA (trough 140 µg/L), MMF, prednisone | CsA (trough 101 µg/L), prednisone | None |
| 7 | Everolimus (trough 4.8 µg/L), MMF | None | None |
| 8 | Everolimus (trough 5.4 µg/L), MMF, prednisone | Everolimus (trough 4.3 µg/L), MMF, prednisone | Everolimus (trough 2.4 µg/L), MMF, prednisone, methylprednisolone pulse (second of 3 consecutive days), ruxolitinib |
| Patient Number . | Pharmacologic Immunosuppression . | . | . |
|---|---|---|---|
| . | At Start of AdV DNAemia . | At Start of Treatment With BCV . | At the End of Treatment With BCV . |
| 1 | None (already positive pretransplant) | CsA (trough 115 µg/L) | CsA (trough 97 µg/L), prednisone |
| 2 | CsA (trough 130 µg/L), MMF, budesonide, prednisone | CsA (trough 87 µg/L), MMF, prednisone, budesonide, methylprednisolone pulse (first of 3 consecutive days) | CsA (trough 67 µg/L), MMF, prednisone, budesonide |
| 3 | CsA (trough 84 µg/L), MMF | everolimus (trough 7.3 µg/L), MMF, prednisone | CsA (trough, 46 µg/L), MMF, prednisone |
| 4 | CsA (trough 103 µg/L) | CsA (trough 46 µg/L) | CsA (trough 46 µg/L) |
| 5 | MMF | MMF | None |
| 6 | CsA (trough 140 µg/L), MMF, prednisone | CsA (trough 101 µg/L), prednisone | None |
| 7 | Everolimus (trough 4.8 µg/L), MMF | None | None |
| 8 | Everolimus (trough 5.4 µg/L), MMF, prednisone | Everolimus (trough 4.3 µg/L), MMF, prednisone | Everolimus (trough 2.4 µg/L), MMF, prednisone, methylprednisolone pulse (second of 3 consecutive days), ruxolitinib |
Abbreviations: AdV, adenovirus; BCV, brincidofovir; CsA, cyclosporin A; MMF, mycophenolate mofetil.
At the time of their respective deaths, 3 of 4 patients had an absolute lymphocyte count below 250/µL (patients 2, 6, and 7). Patient 8 had an absolute lymphocyte count of >800/µL at death but died of acute respiratory distress syndrome (ARDS) not related to AdV infection (Figure 1).
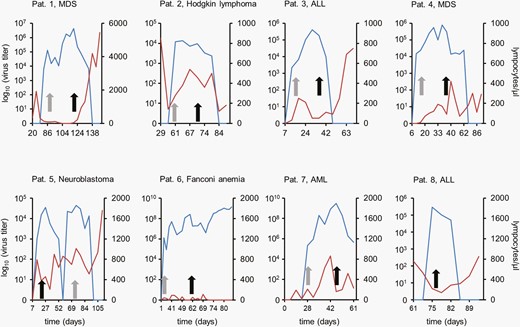
AdV load and lymphocyte counts in blood during antiviral treatment. AdV load is given in blue, the lymphocyte counts are in red. Gray arrows indicate the start of treatment with CDV, black arrows with BCV. Abbreviations: AdV, adenovirus; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCV, brincidofovir; CDV, cidofovir; MDS, myelodysplastic syndrome.
Survival
Four patients died of transplant-related adverse events between days +64 and +161 after their respective transplant procedures. Patient 2 developed only mild AdV symptoms but suffered from treatment-refractory GvHD. AdV was not detected in her tracheal samples, when she deceased on day +91. Acute respiratory failure was the reason for death in patient 8 on day +161 posttransplant and thus 79 days after the resolution of AdV DNAemia. Again, AdV was not detected in his tracheal samples. In 2 patients (6 and 7), fatal MOF and ARDS were presumably consequences of AdV infection. These 2 patients were also those with exceedingly high AdV DNAemia > 1 × 109/mL. Early AdV DNAemia before day +21 posttransplant did not result in increased mortality.
DISCUSSION
Viral infections are a major factor for treatment-related mortality in pediatric HSCT recipients. The outcome in affected patients depends on the type of virus identified, the degree of immune reconstitution, and risk factors such as the presence of GvHD, organ function impairment induced by the specific pathogen, [13, 14] or additional preexisting and transplant-related comorbidities. In particular, AdV infections have shown to be distinct risk factors for treatment-related mortality in pediatric and adult allogeneic HSCT [15–17]. Whereas therapies for patients with CMV infections are well established, systemic AdV infections pose a major threat after allogeneic HSCT due to the scarcity of therapeutic options [2, 3]. CDV is the most frequently used off-label drug for immunocompromised patients with systemic AdV infections. In pediatric HSCT recipients, the development of acute and chronic kidney disease has been described during CDV treatment of patients with AdV infections [14]. Additional problems in the use of CDV are its limited efficacy and the necessity to swallow probenecid for the protection of kidney function. Particularly, early after transplant and in small children, this may pose an obstacle to the administration of CDV.
In the past few years, the use of BCV has gained much interest with response rates of 70%-80% even in CDV-unresponsive patients [5, 9, 10, 18]. This result is supported in our series of pediatric HSCT recipients with the clearance of AdV DNAemia in 6/8 (75%) patients 3-18 days after the first administration of BCV. Two patients with exceedingly high AdV copy numbers in blood >1 × 109/mL (patients 6 and 7) did not survive. Patient 6, a girl with Fanconi anemia and advanced MDS with monosomy 7, had severe, hemorrhagic, refractory gastrointestinal GvHD, and a history of duodenal atresia with a surgical procedure at the age of 3 years. For these reasons, it remained unclear if resorption of BCV in this girl was sufficient enough to exhibit a therapeutic effect. As the repeated episodes of intestinal bleeding and progression of inflammation resulting in a paralytic ileus were already observed 2-4 weeks before the onset of treatment with BCV, it appears unlikely that the findings in laparotomy and subsequent histopathologic assessment were induced by BCV. Unfavorable posttransplant outcomes in pediatric patients with an exceedingly high AdV load in plasma are in accordance with previously published work [19].
Although transient stabilization or even resolution of AdV DNAemia may be achieved by antiviral treatment [5], a permanent control of AdV infection is dependent on the presence of AdV-specific T cells [20–22]. Glucocorticosteroids are known to cause delays in immune reconstitution after HSCT and thus facilitate the occurrence of viral infections [1, 23]. Although 3 of 4 deceased patients were simultaneously treated with glucocorticosteroids and BCV, the number of patients is too small to assess a possible, general adverse effect of glucocorticosteroids.
As BCV is reported to have a broad antiviral, in vitro activity [11], concurrent viral infections in our patients were also investigated. A rapid response to BCV concerning EBV, CMV, AdV, and BK virus was reported in an adult patient after allogeneic HSCT [24]. In our study, we identified 1 patient whose systemic infection with an acyclovir-resistant HSV-1 strain resolved rapidly after introducing BCV [12]. In all other patients, we did not observe a correlation between treatment of patients with BCV and the onset or resolution of additional viral infections. However, it needs to be mentioned that additional viral infections in our study patients appeared either before or after treatment with BCV. A protective effect of BCV was not observed.
The majority of cases with AdV DNAemia arises from reactivation of the virus [25, 26] in the gastrointestinal tract. This is in accordance with our data showing that the presence of AdV in stool samples preceded systemic AdV DNAemia by 1-3 weeks in 7/8 HSCT recipients. All patients with AdV clearance in blood also showed clearance of the virus from their feces. Two to 71 days after the first administration of BCV, AdV was no longer detectable in stool specimens.
In 3 patients, all of whom are alive and well, AdV DNAemia was observed already before day +21 posttransplant. This observation might be a coincidence as the number of patients is small. Regarding the intensity of the preparative regimens and the recovery of the lymphocyte counts, these points, at least, do not explain their favorable outcome. The absence of further transplant-associated toxicities as GvHD or impaired organ functions in these patients might have played a role as well as the fact that AdV DNAemia was quite moderate in these patients as viral loads did not exceed 5 × 106/mL.
The 2 patients with the highest AdV load in blood were those with the poorest lymphocyte recovery. This probably illustrates the major importance of immunologic control of AdV infection by specific T cells. Therefore, if feasible, a reduction or discontinuation of immunosuppressive treatment is generally recommended in patients with systemic AdV infection. A rapid reduction, however, is often not possible or advisable as it carries the risk of GvHD in allogeneic HSCT recipients. In the patients reported here, pharmacologic immunosuppression was discontinued in patients 5 and 6 after the start of treatment with BCV. In all other patients, GvHD prophylaxis or treatment was maintained. While immune system recovery is a gradual process, we observed a rapid decrease of AdV DNAemia after the onset of treatment with BCV in most patients. This might be indicative for antiviral efficacy. A combined effect of CDV and BCV, however, cannot be excluded with certainty as the recommended interval between the last dose of CDV and the first dose of BCV was 48 hours, which is below the half-life of CDV. Whether patients with AdV DNAemia below 1 × 105/mL are generally able to control the virus by themselves, remains a point of discussion.
During treatment with BCV, renal and hepatic function deteriorated considerably in patients 2 and 6. This deterioration could be attributed to treatment-refractory GvHD in patient 2 and uncontrolled AdV infection with MOF in patient 6, respectively. A premature termination of treatment with BCV was not necessary for any patient.
Overall survival in our patients was 50%. Uncontrolled AdV infection was supposed to be the cause of death in 2 patients. Because of preexisting conditions and severe GvHD of the gastrointestinal tract, BCV bioavailability was probably strongly reduced in 1 of these 2 patients. Although AdV infections occur more frequently in younger children, fatal courses at least in the group of patients reported here occurred primarily in older children above the age of 10 years [4].
There may be a general agreement that BCV is able to reduce AdV DNAemia in HSCT and also in solid organ transplant recipients [9, 10, 27, 28]. Particularly, in a small cohort of 8 pediatric and 5 adult immunocompromised patients, mostly allogeneic HSCT recipients, those with a response to BCV treatment were reported to have a longer survival than those without a response [27]. A most recent retrospective study in 93 pediatric stem cell transplant recipients did not show an earlier resolution of AdV disease with antiviral treatment compared to patients, who received any type of antiviral treatment including BCV [29]. However, it was also observed that patients with AdV disease had a lower survival than those without AdV disease. Since the composition of the 2 patient groups was extremely different, the results are difficult to compare. A final evaluation in this setting is still pending.
There are limited data on the use of oral BCV in the antiviral prophylaxis after allogeneic HSCT, mainly from patients recruited into the SUPPRESS clinical trial (ClinicalTrials.gov NCT01769170). BCV recipients developed CMV viremia less frequently than placebo recipients. However, it was also observed that these patients had a higher rate of GvHD, serious adverse events, and mortality [30]. Similarly, the prophylactic use of BCV decreased the incidence of HHV-6B reactivation in these adult patients [31]. Although data on the use of prophylactic oral BCV to prevent AdV infection and disease in allogeneic HSCT recipients are still lacking, this approach might be worth exploring.
The major limitation of our analyses is the small sample size that prevents drawing definite conclusions or identifying clear risk factors for unfavorable outcomes in patients treated with BCV. Despite the frequent resolution of AdV DNAemia in stem cell transplant recipients, the final role of BCV in immunocompromised patients is still in discussion. Particularly, it remains unclear if the preemptive use of BCV in patients with an AdV infection limited to the gastrointestinal tract is able to prevent AdV DNAemia and thus reduce AdV-related death.
CONCLUSIONS
AdV viremia resolved in 75% of pediatric allogeneic HSCT recipients being treated with BCV. Exceedingly high AdV copy numbers in the blood of more than 1 × 109/mL and a poor lymphocyte recovery below <250/µL were associated with a poor survival in our patients. Although access to BCV is currently suspended, further clinical trials are needed to clarify the role of BCV in allogeneic HSCT recipients with AdV disease and its potential benefit in the prevention of AdV DNAemia in immunocompromised patients.
Notes
Acknowledgments. The authors wish to thank Sascha Troschke-Meurer and Georg Hermsdorf for their technical support.
Financial support. The authors did not receive any funding.
Potential conflict of interests. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.



