-
PDF
- Split View
-
Views
-
Cite
Cite
Han Som Choi, Jong Gyun Ahn, Hoon-Chul Kang, Reflections on a 38-Day-Old Japanese Encephalitis Patient Born to a Pregnant Traveler in Endemic Area: Strategies for Prevention, Journal of the Pediatric Infectious Diseases Society, Volume 10, Issue 11, November 2021, Pages 1037–1039, https://doi.org/10.1093/jpids/piab022
Close - Share Icon Share
Abstract
Vaccination against Japanese encephalitis is recommended for long-term travelers to endemic areas; however, the recommendation for pregnant women is controversial. An infant was diagnosed with Japanese encephalitis whose mother moved to an endemic area while pregnant. Preventive strategies are discussed.
An infant was diagnosed with Japanese encephalitis whose mother travelled to endemic area while pregnant. As preventive strategies, we suggest vaccination of mother before or during pregnancy before the travel, or application of physical barriers before the child’s vaccination.
Japanese encephalitis (JE) is the most common encephalitis in Asia. It is caused by the JE virus (JEV), which is transmitted by Culex mosquitoes [1]. Since unvaccinated people are at high risk for JE, the US Centers for Disease Control and Prevention (CDC) recommends vaccination for travelers with long-term or frequent visits to endemic areas [2]. However, vaccination is not routinely recommended for pregnant travelers because the risk of vaccination to the fetus is unknown. In this regard, we present the case of an American infant whose family moved to Korea while the mother was pregnant and had not been vaccinated against JEV.
CASE PRESENTATION
A 38-day-old infant boy visited a hospital after having a fever and suspicious seizures for 1 day. Upon arrival, he had a 39.0°C fever and occasional “twitching” movements of his extremities.
The patient was born in Seoul, Korea, at term with no perinatal, familial, or medical history. His mother moved to Seoul from the United States a few months before while pregnant with the child, and she had not been vaccinated against JEV.
The patient’s leukocyte count was 14 100/μL (reference range 4 500-13 000/μL), hemoglobin was 11.9 g/dL (reference 13.0-16.5 g/dL), and platelet count was 463 000 /μL (reference 153 000-339 000/μL). Serum chemistry, gas, and urine studies were nonspecific. Cerebrospinal fluid (CSF) showed a leukocyte count of 190 cells/μL (reference 0-30 cells/μL) with a neutrophil ratio of 40% (reference 0%-8%), lymphocyte ratio of 58% (reference 5%-35%), protein 125 mg/dL (reference 12-60 mg/dL), and glucose 49 mg/dL (reference 40-70 mg/dL). CSF was sent for bacterial culture, herpes simplex virus polymerase chain reaction (PCR), and Mycobacterium tuberculosis PCR. Ampicillin and ceftriaxone were started empirically.
The patient was transferred to a tertiary center for suspected encephalitis. On admission, he had 10 episodes of focal clonic seizures, requiring levetiracetam, phenobarbital, and topiramate. Antibiotics were escalated to vancomycin, cefotaxime, with the addition of acyclovir, and fluconazole. Mannitol and dexamethasone were administered for control of elevated intracranial pressure. Intravenous immunoglobulin (Ig) at 2 g/kg was administered for 3 days.
Brain magnetic resonance imaging (MRI) on hospital day 2 showed multifocal increased signal intensities bilaterally in the centrum semiovale and striatocapsular areas on diffusion-weighted images. An electroencephalogram (EEG) showed no subclinical seizures. CSF culture and PCR studies, an epilepsy gene panel, and metabolic screening yielded negative findings.
Follow-up EEG on day 4 showed frequent multifocal subclinical seizures, which continued despite fosphenytoin and midazolam infusion up to 30 μg/kg/min, and trials of pyridoxine, biotin, and pyridoxal-5′-phosphate. Ketamine suppressed subclinical seizures at 3 mg/kg/h on day 10.
While reviewing the patient’s history, the parents recalled the infant having a mosquito bite a few days before admission. The patient’s serum was sent to the Korea Center for Disease Control and Prevention for an enzyme-linked immunosorbent assay. On day 14, the patient’s serum was reported positive for JEV IgM. On day 20, his serum JEV titer was reported to be 1:256 by indirect immune-fluorescent antibody titration, confirming JE as the etiology of the patient’s status epilepticus.
The last EEG done on day 31 showed diffusely suppressed background rhythms with no epileptiform discharges. The patient has had no reported seizure since then. A brain MRI 4 months after the event showed diffuse encephalomalacia. Three years later, the patient’s parents reported that the patient’s motor skills stagnated at the level of a 4 month old.
Discussion
To our knowledge, this is the youngest case of JE reported [2]. Healthcare providers should be aware of the potential for JE onset as early as 1 month of age. Analyzing this case, we devised several strategies to prevent early-onset JE in children born to women traveling or moving to endemic areas (Figure 1).
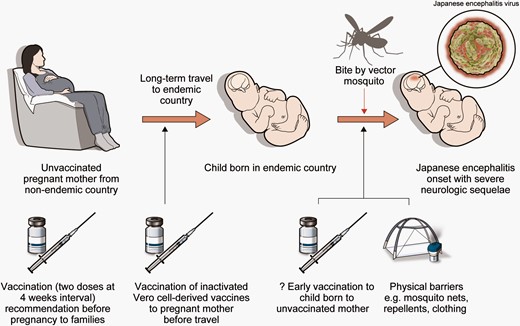
Sequence of Japanese encephalitis virus (JEV) infection in infant of traveling pregnant mother and preventive strategies. The mother was unvaccinated against JEV before moving to an endemic area. The infant was born without preventive antibodies. Bite by vector mosquito caused Japanese encephalitis in the infant. To stop the infection sequence, JEV vaccination could be done to mother before pregnancy. Also, the mother could be vaccinated during pregnancy before the travel, especially when traveling to areas of high risk. If the mother is unvaccinated until the child is born, physical barriers are recommended until the child gets routine JEV vaccination. Early vaccination may be an option but requires safety evidence in infants under 2 months old.
The early onset of JE in our case could be due to multiple factors: non-vaccination of the mother, non-vaccination of the infant, and inadequate physical barriers. Correction of each step could prevent JE in infants whose families move to endemic areas.
The optimal way of preventing JE in infants is vaccination. Non-vaccinated pregnant mothers could delay any visits to endemic countries until the infant is born and has been vaccinated against JE. Vaccination can start as early as 2 months old, which is the minimum age approved by the US Food and Drug Administration (FDA) [3].
Vaccination of pregnant women could be another option. The WHO recommends vaccination to pregnant women if JE risk is high [1]. The CDC cautiously recommends that pregnant travelers to high-risk areas be vaccinated, but only after weighing the benefits and risks of vaccination. Also, the CDC recommends against vaccination of pregnant women if there is a low risk of acquiring JE due to the theoretical risk of vaccination to the fetus [2]. The European Medicines Agency advises against vaccination of pregnant women because of limited safety data [4].
Although the safety of vaccination during pregnancy has not been confirmed in human studies, vaccinating inactivated vaccines to pregnant women against JE should be carefully considered, if the travel cannot be deferred and the risk of JE is present in the traveling areas. The FDA mentions that there are no reported side effects related to vaccination [3]. Also, the only evidence relevant to safety in human pregnancy is that 24 pregnant women who were inadvertently vaccinated during clinical studies by inactivated Vero cell-derived JE vaccines reported no adverse effects [1]. Further, there is no evidence that vaccination of inactivated virus causes harm to the fetus. In the United States, tetanus, diphtheria, acellular pertussis, and inactivated influenza vaccinations are recommended to the pregnant women because the benefit of maternal vaccination outweighs the vaccine’s risk to the fetus [5]. As the risk of infection of either the mother or newborn is substantial as in this case, vaccination of pregnant populations could be recommended while reassuring evidence on the safety of the vaccine for pregnant women and their fetuses accumulates.
Another option that needs further study is the vaccination of women before pregnancy, especially those with a high likelihood of traveling to endemic areas (eg, soldiers, diplomats, or their family). In South Korea, national vaccination of children starts at 12 months, yet no cases of JE in younger infants have been reported. It is possible that neutralizing antibodies may be transmitted from the vaccinated mother to the fetus and last until routine vaccination begins. The influenza and pertussis vaccinations in pregnant women are assumed to elicit the production of antibodies that are transmitted to the fetus and prevent infection of their infants [6]. The FDA reports that the adults who were vaccinated against JEV had a seroprotection rate of over 86% at 1 year after the vaccination. Another study showed a seroprotection rate of 69% 14 months after primary vaccination, before booster injection. This was further increased to 98.5% at 12 months after the booster vaccination [3]. This high seroprotection rate at 1 year after primary or booster vaccination may be a hopeful sign for both the pregnant woman and her fetus, assuming mother-to-fetus antibody transmission. If JE vaccination done before pregnancy can protect the child between birth and routine immunization, safety concerns regarding vaccination during pregnancy may be less relevant. Further studies on mother-to-child transmission of neutralizing antibodies against JEV, and the proper timing of vaccination to ensure the safety of the fetus, would inform immunization guidance for travelers intending to become pregnant.
Physical barriers such as mosquito nets, repellents, and adequate clothing may also be effective at reducing infection in infants. Although the WHO shows that barriers are not as effective as vaccination, both the WHO and the CDC recommend them nonetheless [1, 2]. As Culex species are active in the evening and at nighttime [1], screens and bed nets could be useful especially at those times.
Early vaccination in infants is another option. There are no studies addressing the safety and efficacy of vaccination of infants younger than 2 months of age [3]. Until further studies demonstrate that JEV vaccination of young infants is safe, other methods should be adopted to prevent infants younger than 2 months from becoming infected.
Our case shows the severe impact of JE on an infant of an unvaccinated family. Guidelines for those anticipating long-term travel to endemic areas should be revised to protect children from JEV from birth.
Notes
Acknowledgment. The authors thank Medical Illustration & Design, in Yonsei University College of Medicine, for all artistic support related to this work.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.



