-
PDF
- Split View
-
Views
-
Cite
Cite
Ivan Berka, Peter Korček, Zbyněk Straňák, C-Reactive Protein, Interleukin-6, and Procalcitonin in Diagnosis of Late-Onset Bloodstream Infection in Very Preterm Infants, Journal of the Pediatric Infectious Diseases Society, Volume 10, Issue 11, November 2021, Pages 1004–1008, https://doi.org/10.1093/jpids/piab071
Close - Share Icon Share
Abstract
Late-onset bloodstream infection (LOBSI) is common in very preterm infants. Early and accurate diagnosis is crucial for prognosis and outcome. We aimed to analyze the accuracy of routinely used inflammatory biomarkers in the diagnosis of LOBSI as compared to uninfected controls.
In this single-center, retrospective case-control study, interleukin-6 (IL-6), procalcitonin (PCT), and C-reactive protein (CRP) were routinely measured, when infection was clinically suspected. The definition of LOBSI was based on positive blood culture, clinical signs of infection, and onset more than 72 hours after birth.
Among 285 enrolled infants, 66 developed LOBSI. IL-6 was superior to other markers, and levels greater than 100 ng/L had a sensitivity of 94% and a specificity of 99% for the presence of LOBSI. Receiver operating characteristic curve of IL-6 had area under the curve of 0.988 (95% CI = 0.975-1.00, P < .001). The negative predictive value of IL-6, CRP, and PCT for optimal cutoff values was 99%, 95%, and 93%, respectively. The logistic regression model of IL-6 > 100 ng/L or CRP > 10 mg/L were successfully predicted LOBSI in 97.9% of cases.
The combination of IL-6 and CRP seems to have great potential in routine rapid diagnosis of LOBSI development. High negative predictive value of all tested markers could encourage the early discontinuation of antibiotic treatment.
Neonatal late-onset sepsis (LOS) in very preterm infants is defined by positive blood culture and the onset of symptoms more than 72 hours after birth [1]. LOS is associated with significantly increased mortality and morbidity [2]. It is generally considered to be caused by pathogens acquired from the environment [3]. However, in some very preterm and high-risk neonates, LOS can also be caused by microorganisms obtained at birth [1].
Conventional rapid diagnosis of LOS is based on clinical signs, serum inflammatory markers, and blood count changes due to delayed availability of microbiological assessment [4]. Clinical symptoms are regarded as nonspecific and blood count changes suffer from low sensitivity [4]. C-reactive protein (CRP) and procalcitonin (PCT) represent inflammatory markers widely adopted for clinical use [5]. Their diagnostic utility varies according to the time of sepsis onset and appears to be higher in LOS as compared to early-onset neonatal sepsis (EOS) [6]. The main limit of CRP is a delayed rise in serum levels after the onset of infection [7]. PCT has a sharper rise in serum levels in an infectious event, and a reasonable ability to detect LOS was reported [8]. The crucial limit of PCT to diagnose neonatal sepsis is the relatively low sensitivity [5]. Cytokines such as interleukin-6 (IL-6) are documented to offer additional diagnostic value, however, their availability for routine clinical use seemed to be limited outside of research settings due to extended laboratory turnaround [6]. Nevertheless, immunoassays with a shorter time to provide results are available [9, 10]. Many authors recommend a combination of markers as superior to individual inflammatory markers in LOS development prediction, but which would be the optimal one remains unclear [3, 4, 9, 10].
The objective of this retrospective case-control study was to analyze the accuracy of routinely used inflammatory biomarkers in the diagnosis of late-onset bloodstream infection (LOBSI) as compared to controls without confirmed bacterial bloodstream infection.
MATERIALS AND METHODS
Subjects
This retrospective case-control study was conducted in a single tertiary neonatal intensive care unit (NICU). Inclusion criteria were: preterm delivery ≤32 weeks of gestation, inborn neonates, and completed blood inflammatory markers measurements due to suspected sepsis development more than 72 hours after delivery as part of the standard care. All infants with incomplete laboratory records and/or unknown outcomes regarding LOS diagnosis were excluded. Data from the period of January 2015 to December 2018 were analyzed. The study protocol was approved by the Local Ethics Committee (03/2014). Written informed consent for anonymous use of clinical data was obtained from parents of each infant.
Blood Sampling and Test Timing
Blood samples were taken from arterial or venous catheters in all infants when infection development was clinically suspected. IL-6, PCT, and CRP were routinely measured. Serum IL-6 level was assessed by electrochemiluminescence immunoassay (Cobas 6000, e601 module, Roche Diagnostics, Mannheim, Germany). The upper detection limit of IL-6 was 5000 ng/L. Immunoluminometric assay (Lumitest PCT, Brahms, Germany) was used for PCT analysis. Luminescence was measured automatically in a Berilux Analyzer (Behring Diagnostics, Germany). The upper detection limit of PCT was 100 µg/L. CRP was measured by immunoturbidimetry (Cobas 6000, c501 module, Roche Diagnostics, Mannheim, Germany). The overall volume of blood required was 0.6 mL. The time taken to obtain results was 90 minutes.
Blood Culture
Blood cultures with a volume of at least 1 mL and samples for inflammatory biomarkers were collected at the same time or immediately after positive results were obtained. The BacT/Alert automated blood culture monitoring system (BacT/Alert, BioMerieux, USA) was used. Specimens were transported to the laboratory immediately after collection. After insertion into the BacT/Alert culture instrument, samples were incubated for a standard period of 5 days (120 hours) before being marked as negative. Bottles marked as positive were subcultured on Columbia agar + 5% sheep blood (BioMerieux), UriSelect™ 4 Medium (Bio-Rad), and Schaedler agar +5% sheep blood (BioMerieux) plates, and a microscopic slide for Gram stain was prepared. After 4-6 hours of incubation, the streaked agar plates were inspected and in case of a detectable growth, the visible colonies were identified using the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) on a MicroFlex LT/SH smart platform (Bruker Daltonik GmbH). The clinical team was informed directly in the case of a positive finding. Blood culture contamination was considered when any microbial finding occurred 72 hours or later after sampling.
Definitions
LOBSI was based on positive blood culture and clinical signs of infection with onset more than 72 hours after birth. Clinical signs of late-onset infection included: hypothermia/body temperature instability, respiratory signs (apnea, dyspnea, desaturations, sudden respiratory impairment), cardiac (tachycardia, bradycardia, poor peripheral perfusion, blood pressure instability), and neurological (lethargy, irritability, hypotonia, hypertonia, suspected seizures) symptoms and feeding difficulties [11].
Clinical sepsis (culture-negative sepsis-like syndrome [CNSLS]) was defined as the presence of clinical signs of infection, positive inflammatory biomarker, and negative blood culture.
Nonbacterial sepsis was considered when a known nonbacterial pathogen was identified and clinical signs of infection occurred.
The control group consisted of all cases not fulfilling the LOBSI criteria and without other known causes of clinical impairment and/or systemic inflammatory response to avoid selection bias.
Patients with other causes of clinical worsening and possible systemic inflammatory response were excluded from the primary analysis. Those included: necrotizing enterocolitis (NEC) ≥IIb according to modified Bell criteria, nonbacterial sepsis, CNSLS, system-specific infections (pneumonia, urinary tract infection, phlebitis, skin lesions of infectious origin, enteritis, conjunctivitis), and surgery. Complementary analysis including patients with clinical sepsis in the control group was performed as well.
Antibiotic Treatment
Antibiotic treatment was always initiated in all cases with clinical signs of infection and positive inflammatory biomarkers after blood culture specimen collection. Duration of antibiotic therapy depended on blood culture results. LOBSI positive infants were treated at least 5 days after negative blood culture control. In infants with clinical sepsis, the blood culture was not repeated, and antibiotics were administered for ≥5 days according to clinical judgment.
Statistical Analysis
Data analysis was performed using the IBM SPSS Statistics 23.0.0.0 software (IBM Corp., Armonk, NY). Patient demographics and clinical characteristics are presented as medians and interquartile ranges (IQRs) or as means and standard deviations for continuous variables and for categorical variables as counts and category percentages. Chi-square, Kruskal-Wallis, and Mann-Whitney U tests were used to compare non-normally distributed variables. Two-sided P values <.05 were considered statistically significant. Receiver operating characteristic curves (ROC) and area under the curves (AUC) were analyzed to identify proper cutoff values. Logistic regression model was used to explore combinations of markers.
Results
Out of 669 infants eligible for the study, 312 were excluded for incomplete laboratory records and/or unknown outcome. Thirteen patients were excluded for NEC, 1 for viral sepsis (HSV-2), 27 for system-specific infections, and 5 for laboratory values obtained after surgery. Twenty-six patients were excluded from the primary analysis for CNSLS. Of the 285 remaining infants, 66 fulfilled the LOBSI criteria, and 219 were classified as uninfected controls. The data of both groups are presented in Table 1.
| Variable . | LOBSI (N = 66) . | Control (N = 219) . | P Value . |
|---|---|---|---|
| Gestational age, weeks | 27 (25-29) | 28 (26-30) | .094 |
| Birth weight, g | 970 (740-1230) | 995 (820-1290) | .200 |
| Male sex | 42 (64) | 133 (61) | .773 |
| Multiple gestation | 33 (50) | 94 (43) | .326 |
| ANS (none/incomplete/complete) | 7(11)/16(24)/43(65) | 22(10)/50(23)/147(67) | .480 |
| Mode of delivery | |||
| C-section | 54 (82) | 182 (83) | .853 |
| Vaginal | 12 (18) | 37 (17) | |
| Small for gestational age | 5 (7.6) | 34 (15.5) | .107 |
| CVC, days | 13 (2-27) | 6 (0-8.5) | <.001 |
| Mortality | 6 (9.1) | 9 (4.1) | .161 |
| Variable . | LOBSI (N = 66) . | Control (N = 219) . | P Value . |
|---|---|---|---|
| Gestational age, weeks | 27 (25-29) | 28 (26-30) | .094 |
| Birth weight, g | 970 (740-1230) | 995 (820-1290) | .200 |
| Male sex | 42 (64) | 133 (61) | .773 |
| Multiple gestation | 33 (50) | 94 (43) | .326 |
| ANS (none/incomplete/complete) | 7(11)/16(24)/43(65) | 22(10)/50(23)/147(67) | .480 |
| Mode of delivery | |||
| C-section | 54 (82) | 182 (83) | .853 |
| Vaginal | 12 (18) | 37 (17) | |
| Small for gestational age | 5 (7.6) | 34 (15.5) | .107 |
| CVC, days | 13 (2-27) | 6 (0-8.5) | <.001 |
| Mortality | 6 (9.1) | 9 (4.1) | .161 |
Abbreviations: ANS, antenatal steroids; CVC, central venous catheter; LOBSI, late-onset bloodstream infection.
Continuous data are presented as median and interquartile range (IQR), categorical variables are presented as number (percentage). Mann-Whitney U test was used to compare medians, and chi-square test was used for proportions.
| Variable . | LOBSI (N = 66) . | Control (N = 219) . | P Value . |
|---|---|---|---|
| Gestational age, weeks | 27 (25-29) | 28 (26-30) | .094 |
| Birth weight, g | 970 (740-1230) | 995 (820-1290) | .200 |
| Male sex | 42 (64) | 133 (61) | .773 |
| Multiple gestation | 33 (50) | 94 (43) | .326 |
| ANS (none/incomplete/complete) | 7(11)/16(24)/43(65) | 22(10)/50(23)/147(67) | .480 |
| Mode of delivery | |||
| C-section | 54 (82) | 182 (83) | .853 |
| Vaginal | 12 (18) | 37 (17) | |
| Small for gestational age | 5 (7.6) | 34 (15.5) | .107 |
| CVC, days | 13 (2-27) | 6 (0-8.5) | <.001 |
| Mortality | 6 (9.1) | 9 (4.1) | .161 |
| Variable . | LOBSI (N = 66) . | Control (N = 219) . | P Value . |
|---|---|---|---|
| Gestational age, weeks | 27 (25-29) | 28 (26-30) | .094 |
| Birth weight, g | 970 (740-1230) | 995 (820-1290) | .200 |
| Male sex | 42 (64) | 133 (61) | .773 |
| Multiple gestation | 33 (50) | 94 (43) | .326 |
| ANS (none/incomplete/complete) | 7(11)/16(24)/43(65) | 22(10)/50(23)/147(67) | .480 |
| Mode of delivery | |||
| C-section | 54 (82) | 182 (83) | .853 |
| Vaginal | 12 (18) | 37 (17) | |
| Small for gestational age | 5 (7.6) | 34 (15.5) | .107 |
| CVC, days | 13 (2-27) | 6 (0-8.5) | <.001 |
| Mortality | 6 (9.1) | 9 (4.1) | .161 |
Abbreviations: ANS, antenatal steroids; CVC, central venous catheter; LOBSI, late-onset bloodstream infection.
Continuous data are presented as median and interquartile range (IQR), categorical variables are presented as number (percentage). Mann-Whitney U test was used to compare medians, and chi-square test was used for proportions.
Late-Onset Bloodstream Infection and Control Group
Forty-nine infants in the BSI group were infected by gram-positive bacteria, 17 by gram-negative bacteria. No blood culture contamination was found throughout the study. The list of pathogens identified by blood cultures is shown in Table 2. IL-6 and CRP levels were significantly higher in LOBSI group in comparison to control group (median 468 ng/L, IQR 235-1627 vs 13 ng/L, IQR 6-26, P < .0001 and 23.9 mg/L, IQR 10.5-53.8 vs 0.6 mg/L, IQR 0.3-2.5, P < .0001). PCT values were also elevated in LOBSI group in comparison to control group (median 6.3 µg/L, IQR 1.73-25.5 vs 0.47 µg/L, IQR 0.27-0.96, P < .0001). Patients infected by gram-negative bacteria had higher diagnostic serum levels of IL-6 and PCT in comparison to patients infected by gram-positive bacteria (median 2718 ng/L, IQR 479-5000 vs 446 ng/L, IQR 226-1201, P < .0001 and median 21 µg/L, IQR 4.1-42.14 vs 4.8 µg/L, IQR 1.46-20.73, P < .0001, respectively). The median postnatal age at sampling was 12 days in LOBSI group and 14 days in control group.
| Bacterial Strain . | Number of Cases . |
|---|---|
| Staphylococcus aureus | 24 |
| CoNS | 20 |
| Enterococcus faecalis | 2 |
| Bacillus licheniformis | 1 |
| Streptococcus agalactiae | 1 |
| Lactobacillus rhamnosus | 1 |
| Escherichia coli | 13 |
| Acinetobacter baumanii | 2 |
| Enterobacter cloacae | 1 |
| Klebsiella oxytoca | 1 |
| Bacterial Strain . | Number of Cases . |
|---|---|
| Staphylococcus aureus | 24 |
| CoNS | 20 |
| Enterococcus faecalis | 2 |
| Bacillus licheniformis | 1 |
| Streptococcus agalactiae | 1 |
| Lactobacillus rhamnosus | 1 |
| Escherichia coli | 13 |
| Acinetobacter baumanii | 2 |
| Enterobacter cloacae | 1 |
| Klebsiella oxytoca | 1 |
Abbreviation: CoNS, coagulase-negative staphylococci (Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus capitis, and Staphylococcus haemolyticus).
| Bacterial Strain . | Number of Cases . |
|---|---|
| Staphylococcus aureus | 24 |
| CoNS | 20 |
| Enterococcus faecalis | 2 |
| Bacillus licheniformis | 1 |
| Streptococcus agalactiae | 1 |
| Lactobacillus rhamnosus | 1 |
| Escherichia coli | 13 |
| Acinetobacter baumanii | 2 |
| Enterobacter cloacae | 1 |
| Klebsiella oxytoca | 1 |
| Bacterial Strain . | Number of Cases . |
|---|---|
| Staphylococcus aureus | 24 |
| CoNS | 20 |
| Enterococcus faecalis | 2 |
| Bacillus licheniformis | 1 |
| Streptococcus agalactiae | 1 |
| Lactobacillus rhamnosus | 1 |
| Escherichia coli | 13 |
| Acinetobacter baumanii | 2 |
| Enterobacter cloacae | 1 |
| Klebsiella oxytoca | 1 |
Abbreviation: CoNS, coagulase-negative staphylococci (Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus capitis, and Staphylococcus haemolyticus).
Analysis of Diagnostic Properties of Inflammatory Markers
The ROC for LOBSI detection by IL-6 (Figure 1) had an AUC of 0.988 (95% CI = 0.975-1.00, P < .0001). The IL-6 level of 100 ng/L had a sensitivity of 94% and a specificity of 99% for the presence of LOBSI, with a positive predictive value (PPV) of 97% and a negative predictive value (NPV) of 98%. The accuracy of this IL-6 serum level to diagnose LOBSI was 98%. The ROC for LOBSI detection by CRP (Figure 1) had AUC of 0.947 (95% CI = 0.918-0.975, P < .0001). The CRP level of 9.0 mg/L had a sensitivity of 80% and a specificity of 95% for the presence of LOBSI, with a PPV of 83% and a NPV of 94%. The accuracy of this CRP serum level was 92%. The ROC for LOBSI detection by PCT (Figure 1) had AUC of 0.898 (95% CI = 0.857-0.939, P < .0001). The PCT level of 1.2 µg/L had a sensitivity of 79% and a specificity of 79% for the presence of LOBSI, with a positive and NPV of 53% and 93%, respectively. The accuracy of this PCT level was 79%.
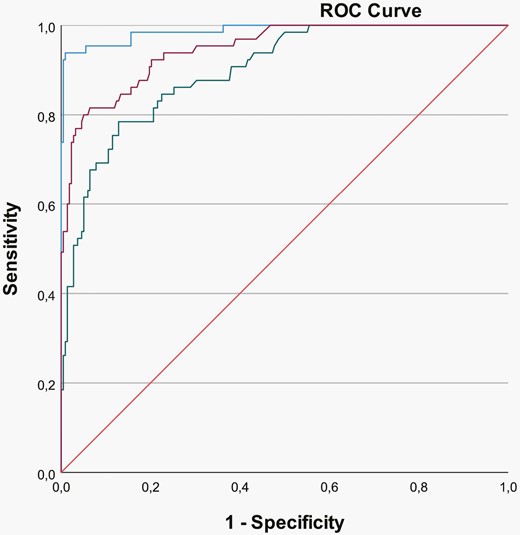
Receiver operating characteristic curves for LOBSI and IL-6 (blue), CRP (purple), and PCT (green).
The logistic regression model of IL-6 > 100 ng/L or CRP > 10 mg/L successfully predicted LOBSI in 97.9% of cases.
Complementary analysis including patients with clinical sepsis in the control group revealed sensitivity, specificity, PPV, and NPV: 94%, 91%, 74%, and 98% for IL-6, 80%, 88%, 65%, and 94% for CRP, 79%, 76%, 47%, and 93% for PCT. For complementary analysis identical cutoffs were used.
Patients with clinical sepsis had elevated diagnostic values of IL-6, PCT, and CRP as compared to uninfected controls (median 359 ng/L, IQR 117-1757, P < .0001, median 2.27 µg/L, IQR 0.72-6.27, P < .0001 and median 12.4, IQR 2.1-18.7, P < .0001, respectively).
Patients Excluded From Analysis of Diagnostic Properties
Other encountered causes of clinical deterioration and possible systemic inflammatory response are summarized in Table 3. Patients with NEC had the highest diagnostic values of IL-6 and CRP in comparison with the control group (median 3371 ng/L, IQR 626-5000, P < .0001 and median 45.4, IQR 4.9-119.6, P < .0001). Patients with system-specific infections and those sampled after surgery were represented by small sample sizes and their serum levels of inflammatory markers varied (Table 3).
| Clinical Condition . | Number of Cases . | Inflammatory Markers . | ||
|---|---|---|---|---|
| . | . | IL-6 (ng/L) . | PCT (µg/L) . | CRP (mg/L) . |
| Median (IQR) | ||||
| NEC | 13 | 3371 (626-5000) | 6.42 (3.72-53.94) | 45.4 (4.9-119.6) |
| Pneumonia | 11 | 151 (57-448) | 0.59 (0.25-4.23) | 12.4 (2.1-18.7) |
| Range | ||||
| UTI | 5 | 59.6-283.3 | 0.13-11.32 | 0.3-63.5 |
| Phlebitis | 4 | 9.6-80.3 | 0.63-6.18 | 12.5-72.66 |
| Infectious skin lesions | 3 | 19.4-562.4 | 0.19-2.11 | 0.4-9.0 |
| Enteritis | 3 | 276.3-2913 | 14.99-100 | 18.8-189.8 |
| Surgery (PDA, SIP) | 5 | 132.5-874.4 | 0.4-46.36 | 4.0-50.6 |
| Value | ||||
| Purulent conjunctivitis | 1 | 4.3 | 0.18 | 0.1 |
| Viral sepsis | 1 | 262.4 | 1.02 | 17.6 |
| Clinical Condition . | Number of Cases . | Inflammatory Markers . | ||
|---|---|---|---|---|
| . | . | IL-6 (ng/L) . | PCT (µg/L) . | CRP (mg/L) . |
| Median (IQR) | ||||
| NEC | 13 | 3371 (626-5000) | 6.42 (3.72-53.94) | 45.4 (4.9-119.6) |
| Pneumonia | 11 | 151 (57-448) | 0.59 (0.25-4.23) | 12.4 (2.1-18.7) |
| Range | ||||
| UTI | 5 | 59.6-283.3 | 0.13-11.32 | 0.3-63.5 |
| Phlebitis | 4 | 9.6-80.3 | 0.63-6.18 | 12.5-72.66 |
| Infectious skin lesions | 3 | 19.4-562.4 | 0.19-2.11 | 0.4-9.0 |
| Enteritis | 3 | 276.3-2913 | 14.99-100 | 18.8-189.8 |
| Surgery (PDA, SIP) | 5 | 132.5-874.4 | 0.4-46.36 | 4.0-50.6 |
| Value | ||||
| Purulent conjunctivitis | 1 | 4.3 | 0.18 | 0.1 |
| Viral sepsis | 1 | 262.4 | 1.02 | 17.6 |
Abbreviations: CRP, C-reactive protein; IL-6, interleukin-6; IQR, interquartile range; NEC, necrotizing enterocolitis; PCT, procalcitonin; PDA, persistent ductus arteriosus; SIP, spontaneous intestinal perforation; UTI, urinary tract infection.
| Clinical Condition . | Number of Cases . | Inflammatory Markers . | ||
|---|---|---|---|---|
| . | . | IL-6 (ng/L) . | PCT (µg/L) . | CRP (mg/L) . |
| Median (IQR) | ||||
| NEC | 13 | 3371 (626-5000) | 6.42 (3.72-53.94) | 45.4 (4.9-119.6) |
| Pneumonia | 11 | 151 (57-448) | 0.59 (0.25-4.23) | 12.4 (2.1-18.7) |
| Range | ||||
| UTI | 5 | 59.6-283.3 | 0.13-11.32 | 0.3-63.5 |
| Phlebitis | 4 | 9.6-80.3 | 0.63-6.18 | 12.5-72.66 |
| Infectious skin lesions | 3 | 19.4-562.4 | 0.19-2.11 | 0.4-9.0 |
| Enteritis | 3 | 276.3-2913 | 14.99-100 | 18.8-189.8 |
| Surgery (PDA, SIP) | 5 | 132.5-874.4 | 0.4-46.36 | 4.0-50.6 |
| Value | ||||
| Purulent conjunctivitis | 1 | 4.3 | 0.18 | 0.1 |
| Viral sepsis | 1 | 262.4 | 1.02 | 17.6 |
| Clinical Condition . | Number of Cases . | Inflammatory Markers . | ||
|---|---|---|---|---|
| . | . | IL-6 (ng/L) . | PCT (µg/L) . | CRP (mg/L) . |
| Median (IQR) | ||||
| NEC | 13 | 3371 (626-5000) | 6.42 (3.72-53.94) | 45.4 (4.9-119.6) |
| Pneumonia | 11 | 151 (57-448) | 0.59 (0.25-4.23) | 12.4 (2.1-18.7) |
| Range | ||||
| UTI | 5 | 59.6-283.3 | 0.13-11.32 | 0.3-63.5 |
| Phlebitis | 4 | 9.6-80.3 | 0.63-6.18 | 12.5-72.66 |
| Infectious skin lesions | 3 | 19.4-562.4 | 0.19-2.11 | 0.4-9.0 |
| Enteritis | 3 | 276.3-2913 | 14.99-100 | 18.8-189.8 |
| Surgery (PDA, SIP) | 5 | 132.5-874.4 | 0.4-46.36 | 4.0-50.6 |
| Value | ||||
| Purulent conjunctivitis | 1 | 4.3 | 0.18 | 0.1 |
| Viral sepsis | 1 | 262.4 | 1.02 | 17.6 |
Abbreviations: CRP, C-reactive protein; IL-6, interleukin-6; IQR, interquartile range; NEC, necrotizing enterocolitis; PCT, procalcitonin; PDA, persistent ductus arteriosus; SIP, spontaneous intestinal perforation; UTI, urinary tract infection.
DISCUSSION
Rapid diagnosis of LOS in the early stages of development remains clinically challenging with possible serious consequences [12]. This is especially true in the most affected population of very preterm neonates at NICU [13]. The optimal early diagnostic algorithm is unclear and despite intensive efforts, a consensual definition of neonatal sepsis based on organ dysfunction and host response is not available [14]. In clinical practice, the standard approach consists of the evaluation of risk factors, clinical symptoms, changes in blood counts, and a combination of serum inflammatory markers together with an emphasis on valid microbiological tests.
We compared the systemic inflammatory response quantified by a routine panel of markers in a study group of patients in whom these tests are most commonly used in clinical practice. The only statistically significant difference in clinical data between the groups was in the number of days spent with a central venous catheter in situ, which is a well-described risk factor for LOBSI [15]. Our study demonstrated that very preterm neonates with confirmed LOBSI had significantly increased serum markers of inflammation at the time of clinical suspicion compared to uninfected very preterm controls. Patients infected by gram-positive bacteria tended to have a less pronounced inflammatory response, as expressed by IL-6 and PCT, but not by CRP. This specific pattern was not reported previously. Considering the limited number of patients with gram-negative sepsis, this result may not be as convincing. LOBSI caused by gram-positive bacteria accounted for 74% of cases, a value similar to the reported 79% of LOBSI associated with gram-positive microorganisms in general [16]. Analysis of diagnostic properties of individual markers revealed excellent characteristics of IL-6 followed by CRP and PCT. In the context of LOBSI, CRP is reported to have varying sensitivity, good specificity, and high NPV [4, 6]. The most frequently stated cutoff values are 4-10 mg/L [17, 18]. Our findings are consistent with these data. CRP level alone is considered insufficient for early diagnosis of LOBSI development [19]. According to published data, PCT was expected to perform better than CRP in comparison to individual markers [8, 20]. The sensitivity and specificity of PCT found in our study were very close to the pooled sensitivity and specificity in a large meta-analysis (81% and 79%), which included patients with early and LOS [8]. In the recent prospective study, similar limits to the validity of PCT were described [10]. Our proposed cutoff value for PCT (1.2 µg/L) was near to the threshold of the referred study in neonatal patients with BSI (1.5 µg/L), but remarkably lower than the cutoff value recommended in the large multicentre study in very low birthweight infants (2.4 µg/L) [10, 20]. This further promotes caution when using this individual marker for LOBSI diagnostics. Reported diagnostic properties of IL-6 as an individual marker of late-onset infection vary, although very good characteristics were already published [21–23]. Sonawane et al. presented sensitivity, specificity, PPV, and NPV for identical cutoff values of IL-6, as we propose 96%, 88%, 92%, and 93%, respectively [24]. The main methodological difference from our study is their inclusion of patients with clinical sepsis in the group of patients with LOS. In contrast, our study compared LOBSI group and control uninfected group either with or without clinical sepsis. Involvement of cases with clinical sepsis to the analysis led to increase in false positivity. Thus, specificity and PPV (but not sensitivity and NPV) are diminished. In general, CNSLS patients suffered from an inflammatory event of unknown origin and an undetermined number of blood culture-negative infants were possibly not infected [24]. Therefore, differences in the published data can be partially explained by the inconsistent definition of neonatal sepsis. Patients with CNSLS seem to represent a heterogeneous group [25]. Some of these patients may potentially suffer from LOBSI, in case the blood culture was collected incorrectly, mainly in terms of insufficient volume [25]. Others may have a viral infection [26]. Many very preterm neonates experience clinical impairment due to noninfectious reasons occasionally accompanied by a systemic inflammatory response [25]. This presumed heterogeneity appears to be reflected in the serum levels of biochemical markers of inflammation in such neonates in our dataset. Our logistic regression model seems to confirm that the combination of CRP and IL-6 has great potential in routine rapid diagnostics of LOBSI as proposed previously [9, 10].
The summary of patients with other causes of clinical impairment suggests a significant number of other possible triggers of the late systemic inflammatory response among very preterm infants. Most of these can be distinguished from LOBSI by clinical and/or radiological examination, microbiological tests, and subsequent clinical development with the exception of clinical sepsis.
The main limitation of our study is the retrospective case-control design, which may artificially increase the diagnostic accuracy of the markers. We counteracted this by detailed analysis (primary and complementary) taking into account real clinical situation when the cause of patient’s deterioration is unknown. The results of patients excluded from the analysis are also summarized. It is important to note that the blood samples were certainly obtained at different time points after clinical onset of any symptoms, which has not been specifically documented and could possibly affect the measured values.
In conclusion, elevated serum IL-6 level in very preterm neonates seems to be a very reliable marker of late-onset systemic infection/inflammatory response. Although there are various causes of the systemic inflammatory response in very preterm neonates, there appears to be almost no LOBSI without the elevation of IL-6 > 100 ng/L and CRP > 10 mg/L at the time of clinical onset. High NPV of all tested markers could further encourage early discontinuation of antibiotic treatment.
Notes
Financial support. This work was supported by the Czech Health Research Council [NV 17-31403A].
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.



