-
PDF
- Split View
-
Views
-
Cite
Cite
Yun Wu, Xu-Yu Duan, Guang-Li Liu, Yong Xiang, Bo Shu, Qing-Jun Li, Vegetation context modifies selection on flowering start and plant height in an orchid perennial herb, Journal of Plant Ecology, Volume 14, Issue 5, October 2021, Pages 934–944, https://doi.org/10.1093/jpe/rtab048
Close - Share Icon Share
Abstract
Identifying the potential role of vegetation context (defined as the density, species identity/diversity and height of co-occurring plants) in modifying selection on floral traits is a critical step for clarifying and predicting the floral evolutionary trajectory in complex co-flowering species competition environments. It is also helpful to understand the variation in pollinator-mediated selection.
We experimentally reduced vegetation height around individual plants of Spiranthes sinensis (a bumblebee-pollinated perennial orchid herb) and estimated how vegetation context modified selection on four floral traits (flowering start, plant height, corolla size and number of flowers) through female function and pollen removal over two continuous years. We randomly selected independent plants in each year.
We demonstrated that vegetation context modified selection for earlier flowering start and shorter plant height of S. sinensis. The strength of selection differed between years. In addition, selection was stronger through female function than through pollen removal. Our findings indicate the potential role of vegetation context in shaping the differentiation and diversification of flowers in angiosperms.
摘要
摘要:厘清背景环境因素(本研究定义为目标植株周围物种的数量、密度以及植株高度)对植物花性状获得表型选择压力的影响,是认识和预测同域开花物种-种间竞争环境下植物花性状进化模式和进化轨迹的关键环节;同时,对认识和理解传粉者施加的表型选择在时空上的变化具有重要作用。本研究以兰科多年生草本植物绶草(Spiranthes sinensis)为材料,通过人为降低绶草周围植株高度,连续两年定量衡量背 景环境因素分别通过结实数和花粉输出途径对绶草4个花性状(开花时间、株高、花冠大小和花数目)获得表型选择的影响。研究结果表明,降低周围植株高度导致的背景环境改变对开花时间和株高等表型性状具有选择作用:改变了的背景环境选择开花时间更早、株高更矮的绶草植株个体,且两类性状获得的表型选择强度于年际间存在差异。进一步研究表明,这种背景环境的改变通过雌性适合度产生的影响强于通过雄性适合度。本研究证实了背景环境因素在驱动被子植物花性状分化和塑造花多样性过程中的潜在作用。
INTRODUCTION
Identifying selection mediated by biotic agents is critical to predict the evolutionary trajectory of floral traits in angiosperms (Sletvold et al. 2015). Indeed, experimental studies have demonstrated pollinator-, herbivore-, seed-predator- and nectar-robber-mediated selection on a number of floral traits in wild populations, such as floral visual display, traits influencing pollination efficiency and plant chemical defence traits (Chapurlat et al. 2015; Irwin and Brody 2011; Maron et al. 2019; Phillips et al. 2017; Wu et al. 2018). Despite a large number of existing selection estimates on floral traits, little is known regarding the importance of biotic agents other than pollinators, such as the vegetation context (Caruso 2001; Caruso et al. 2019; Kolb and Ehrlén 2010; Sletvold et al. 2013). Studies that manipulate environmental factors will be critical to improve our understanding of natural selection and accurately predict the evolutionary trajectory of floral traits with complex selection regimes (MacColl 2011).
Vegetation context (defined as the density, species identity/diversity and height of co-occurring plants) may affect selection on floral traits by directly and indirectly influencing plant reproductive success in natural systems (Thomann et al. 2018). First, high density and height of the surrounding vegetation will increase interspecific competition and reduce resource acquisition (such as sunlight) of the focal plant (Ågren et al. 2006). This mechanism is expected to influence the development of floral traits and plant reproductive success. Second, vegetation context may influence the service of pollinators, and this impact depends on specific traits, such as flowering start (Totland 2001), plant height (Reynolds et al. 2010), number of flowers (Wu and Li 2017), flower size (Parachnowitsch and Kessler 2010) and flower colour (Caruso et al. 2010), which are expected to influence the attractiveness of flowers to pollinators. Some experimental studies have supported this hypothesis. For example, variation in the surrounding vegetation results in spatial variation in selection on the inflorescence height of Cypripedium acaule because of its effect on pollinators (O’Connell and Johnston 1998). In natural systems, the environmental vegetation context may simultaneously influence the direction and strength of selection on floral traits (Giménez-Benavides et al. 2011; Sletvold et al. 2013; Valdés and Ehrlén 2018). Variation in vegetation height and/or density may incur variation in interspecific competition for resources and pollinators among co-flowering species. This influences the plant–biotic interaction strength, thus resulting in variation in the direction and/or strength of selection on floral traits. For example, selection for an earlier or later flowering start date and/or taller or shorter plant height may incur avoidance of interspecific competition for pollinators under tall surrounding vegetation height. However, there are still few examples that have experimentally estimated how vegetation context influences spatiotemporal variation in the direction and strength of selection on floral traits (Ågren et al. 2006; Sletvold et al. 2013; Thomann et al. 2018).
In hermaphroditic flowers, floral traits may be subject to selective pressure to simultaneously increase pollen removal (male function) and receipt (female function) (La Rosa and Conner 2017). Ovule number influences the upper limits of plant female reproductive success; in contrast, the fate of pollen is influenced by many factors (e.g. pollen number, pollen transfer efficiency, pollen competition and stigma-pollen compatibility). Indeed, outcrossing opportunity and variance in fitness are expected to be higher for male function than for female function (Arnold and Wade 1984), thus resulting in differential selection pressures on floral traits. Previous studies have indicated that the strength of selection on floral traits through male function is stronger than that through female function (Cuartas-Domínguez and Medel 2010; Hodgins and Barrett 2008). The effect of vegetation context on pollinator behaviours is expected to influence both plant pollen export and receipt. Tall surrounding vegetation results in a negative effect on pollinator visitation of the focal plant due to the reduction in flower attractiveness (Ågren et al. 2006), thus resulting in the reduction of mean population male and female fitness. Mean population fitness is predicted to be inversely related to the opportunity for selection because relative fitness is obtained by dividing the absolute fitness of each plant by the mean population fitness (variance in relative fitness represents the opportunity for selection) (Benkman 2013; Emel et al. 2017). This represents an opportunity to influence the strength of selection. To comprehensively and accurately understand and predict the potential role of vegetation context in driving floral evolution, quantifying selection through both female and male functions is necessary.
Experimental manipulation of vegetation height is a powerful approach to identify its importance as an agent of selection on floral traits (Sletvold et al. 2013). In the present study, we used this approach (tall and short vegetation) to quantify how vegetation context modified selection on floral traits through male and female functions in an orchid species. Spiranthes sinensis is a bumblebee-pollinated orchid herb with multiple flowers in our studied population. According to our own observations, the density of co-flowering species is high (including Prunella vulgaris, Geranium pylzowianum, Gentiana tibetica, Halenia elliptica and some perennial herbs), and the surrounding vegetation height is generally taller than that of S. sinensis. Bumblebees (including Bombus richardsi and B. convexus) are the dominant pollinators for these co-flowering species. During the flowering season, these species often share pollinators. This is expected to cause interspecific competition for resources and pollination services. Floral display influences inter- and intraplant pollinator movements in S. sinensis (Iwata et al. 2012). Tall surrounding vegetation may reduce the floral visual display of this orchid species to pollinators, thus resulting in a reduction in the mean population male and female fitness. Low mean population fitness is generally associated with stronger variation in relative fitness and higher opportunity for selection. Flowering phenology influences the duration of plant-pollinator interactions, thus influencing plant reproductive success. Tall surrounding vegetation from co-flowering species may incur the fitness disadvantage of this orchid species. Selection pressures for an earlier or later flowering start date may be expected in tall environments to avoid interspecific competition for pollinators.
In the present study, we experimentally quantified how vegetation context modified selection on four floral traits (flowering start, plant height, corolla size and number of flowers) of S. sinensis over two continuous years. We estimated directional selection through female fitness and pollen removal. Here, we specifically examine (i) whether vegetation context modifies selection on floral traits and, if so, (ii) whether the direction and/or strength of selection differs between sexual functions and/or years.
MATERIALS AND METHODS
Study species and sites
Spiranthes sinensis is a perennial herb that is widely distributed in Asia, and typical specimens are collected in China. This herb produces multiple flowers and typically has a spiral inflorescence in a single raceme (Fig. 1). Each flower produces nectar and two white pollinia. The flowering period is from July to August, and the fruiting period is from August to September at our study site.

Illustration of phenotypic traits measured in this study on Spiranthes sinensis. (a) Plant height and (b) corolla length and width.
We conducted our experiments in one wild population at the South-East Tibetan Plateau Station for integrated observation and research of the alpine environment (SETS), Chinese Academy of Sciences, China (94°44′19.240″ E, 29°45′58.672″ N, 3335 m a.s.l.). We conducted the experiments in 2018 and 2019. According to our own observations, bumblebees (Bombus richardsi) are the main pollinators for this orchid species. Bumblebees often fed on nectar and removed the pollinia during visitation.
Field experiments and trait measurements
During July and August, we randomly marked 230 and 275 separate individuals in 2018 and 2019 (plants were completely independent in 2 years) and randomly assigned these individuals to one of two treatments: natural vegetation (C) and short vegetation (E). We separately assigned 120 and 110 individuals for the C and E treatments in 2018 and 140 and 135 individuals for the C and E treatments in 2019, respectively. For the E treatment, we cut all vegetation within 20 cm of the focal plant to approximately 2 cm above the ground (Fig. 2). The cutting treatment was performed prior to the start of the flowering period of the focal species and repeated weekly during the flowering period. There were some co-occurring species already flowering at that time.

Short vegetation treatment for Spiranthes sinensis plants at the study site.
We recorded the flowering start (Julian day, the day of the year) for each individual as the day when the first flower opened. At the onset of flowering, we measured the plant height of each individual in the experiment (distance from the ground to the topmost flowers to the nearest 0.1 cm, Fig. 1a). For the first three open flowers of each individual, we measured the corolla length (Fig. 1b) and width (Fig. 1b) to the nearest 0.01 mm using digital callipers. We used the product of the mean corolla length and width to quantify the corolla size for each individual in the present study. We recorded the number of flowers for each individual at the end of the flowering period. Although flower number at the end of the flowering season may have the potential to be interpreted as a fitness measure rather than a pollinator attraction trait in some cases, this type of flower number metric would represent the total attractiveness of plants to pollinators during the flowering season.
To quantify female reproductive success, we recorded the number of fruits at maturation. Fruits were dried at room temperature for at least 1 month, and the dry mass of the three lowest fruits (from low to high in position, the lowest fruits were those that developed earliest from the earliest open flowers) was determined to the nearest 0.1 mg. We estimated the mean weight of the three fruits and used the product of the mean weight per fruit and the number of fruits to determine the female fitness for each individual.
To quantify pollen removal, we recorded the number of flowers that had remaining pollinia (no. of remaining flowers) at the end of the flowering period. Each flower of our studied orchid species produced only two pollinia, and pollinators always simultaneously removed the two pollinia during visitation because pollinia closely contacted each other. We used the following formula to estimate pollen removal for each individual: pollen removal = (no. of total flowers − no. of remaining flowers) × 2.
Statistical analysis
Multiple two-way ANOVAs were used to test the effects of year (2018 vs. 2019) and treatment (C vs. E) and their interaction on floral traits (flowering start, plant height, corolla length, corolla width, corolla size and number of flowers), components of female reproductive success (fruit production, mean weight per fruit and female fitness) and pollen removal. To improve the normal distribution of the data, all the data were log10 transformed prior to ANOVA.
Following the methods of Lande and Arnold (1983), we used multiple linear regression models to estimate directional selection gradients (βi). In the regression models, we used relative female fitness or relative pollen removal (individual value divided by population mean) and the four standardized floral traits (flowering start, plant height, corolla size and number of flowers; with a mean of 0 and a variance of 1) as the response variable and explanatory variables, respectively. To test for multicollinearity in these regression models, we calculated variance inflation factors (VIFs) for the linear terms. All VIFs were <2.4, indicating no serious multicollinearity (Quinn and Keough 2002). We estimated directional selection gradients separately through female fitness and pollen removal in a separate linear regression model. We estimated relative female fitness and pollen removal and standardized the floral traits separately for each year and each treatment. We estimated the opportunity for selection as the variance in relative fitness separately for female fitness and pollen removal.
To test whether net directional selection differed between the 2 years, we used the data from plants in the C treatment in the 2 years in an ANCOVA. The model included the relative female fitness or relative pollen removal as the response variable and the four standardized traits and year and trait × year interactions as the explanatory variables. We tested it separately for female fitness and pollen removal.
To test whether vegetation context-modified selection differed between the 2 years, we used the data from plants in both the C and E treatments in the 2 years in an ANCOVA. The model included the relative female fitness or relative pollen removal as the response variable and the four standardized traits, year, treatment, trait × year, trait × treatment, year × treatment and trait × year × treatment interactions as the explanatory variables. A significant trait × year × treatment interaction term indicated that vegetation context-modified selection differed between years. We tested it separately for female fitness and pollen removal. We further tested the effect of vegetation treatment (C vs. E) on selection gradients separately for each year to determine whether there was significant vegetation context-modified selection. To quantify the vegetation context-modified selection, we subtracted the estimated selection gradients of each trait for plants that received a short vegetation treatment (β E) from the estimate obtained for plants under a natural vegetation treatment (βC) (Δβ = βC − βE). All analyses were performed using R 3.6.1 (R Core Team 2019). We used Excel (2019) (Microsoft, LA, USA) to generate the graphs.
RESULTS
Floral traits and reproductive success
All floral traits did not differ (P > 0.05) between the C and E treatments in any year. However, all floral traits differed between years (Table 1). Earlier flowering start, taller plant height, larger corolla size and greater numbers of flowers were observed in 2018.
Values of floral traits and reproductive success (mean ± SD) for Spiranthes sinensis plants and the effect of years and vegetation treatments on these variables were analysed using two-way ANOVA
| . | 2018 . | 2019 . | Year . | Treatment . | Year × treatment . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Traits and reproductive success . | C (n = 115) . | E (n = 107) . | C (n = 108) . | E (n = 112) . | F1,439 . | P . | F1,439 . | P . | F1,439 . | P . |
| Flowering start | 204.8 ± 3.7 | 205.0 ± 2.6 | 209.4 ± 2.4 | 209.3 ± 3.4 | 228.91 | <0.001 | 0.064 | 0.800 | 0.457 | 0.499 |
| Plant height (cm) | 17.9 ± 3.5 | 17.2 ± 2.8 | 16.7 ± 3.6 | 15.7 ± 2.6 | 21.062 | <0.001 | 5.08 | 0.025 | 0.152 | 0.697 |
| Corolla length (mm) | 4.49 ± 0.53 | 4.58 ± 0.53 | 4.08 ± 0.48 | 4.08 ± 0.61 | 76.87 | <0.001 | 0.394 | 0.531 | 0.849 | 0.357 |
| Corolla width (mm) | 3.23 ± 0.39 | 3.15 ± 0.34 | 3.12 ± 0.43 | 3.04 ± 0.45 | 9.884 | 0.002 | 4.326 | 0.038 | 0.004 | 0.952 |
| Corolla size (mm2) | 14.65 ± 3.25 | 14.52 ± 2.96 | 12.82 ± 2.76 | 12.53 ± 3.01 | 45.732 | <0.001 | 0.712 | 0.399 | 0.308 | 0.579 |
| Number of flowers | 17.0 ± 5.0 | 16.2 ± 4.7 | 15.3 ± 4.9 | 14.6 ± 5.1 | 14.221 | <0.001 | 2.473 | 0.117 | 0.014 | 0.905 |
| Fruit production | 10.2 ± 3.7 | 12.6 ± 4.9 | 11.7 ± 4.8 | 10.7 ± 4.6 | 0.874 | 0.35 | 1.948 | 0.164 | 11.454 | <0.001 |
| Mean weight per fruit (mg) | 2.8 ± 1.0 | 2.5 ± 1.1 | 2.2 ± 0.8 | 2.3 ± 1.1 | 22.119 | <0.001 | 1.232 | 0.268 | 3.154 | 0.076 |
| Female fitness (mg) | 29.2 ± 16.4 | 35.1 ± 26.5 | 28.0 ± 19.4 | 27.6 ± 23.9 | 9.625 | 0.002 | 0.069 | 0.793 | 1.272 | 0.260 |
| Pollen removal | 26.3 ± 9.0 | 27.3 ± 9.1 | 26.2 ± 9.3 | 28.2 ± 10.0 | 0.685 | 0.408 | 4.342 | 0.038 | 0.498 | 0.481 |
| . | 2018 . | 2019 . | Year . | Treatment . | Year × treatment . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Traits and reproductive success . | C (n = 115) . | E (n = 107) . | C (n = 108) . | E (n = 112) . | F1,439 . | P . | F1,439 . | P . | F1,439 . | P . |
| Flowering start | 204.8 ± 3.7 | 205.0 ± 2.6 | 209.4 ± 2.4 | 209.3 ± 3.4 | 228.91 | <0.001 | 0.064 | 0.800 | 0.457 | 0.499 |
| Plant height (cm) | 17.9 ± 3.5 | 17.2 ± 2.8 | 16.7 ± 3.6 | 15.7 ± 2.6 | 21.062 | <0.001 | 5.08 | 0.025 | 0.152 | 0.697 |
| Corolla length (mm) | 4.49 ± 0.53 | 4.58 ± 0.53 | 4.08 ± 0.48 | 4.08 ± 0.61 | 76.87 | <0.001 | 0.394 | 0.531 | 0.849 | 0.357 |
| Corolla width (mm) | 3.23 ± 0.39 | 3.15 ± 0.34 | 3.12 ± 0.43 | 3.04 ± 0.45 | 9.884 | 0.002 | 4.326 | 0.038 | 0.004 | 0.952 |
| Corolla size (mm2) | 14.65 ± 3.25 | 14.52 ± 2.96 | 12.82 ± 2.76 | 12.53 ± 3.01 | 45.732 | <0.001 | 0.712 | 0.399 | 0.308 | 0.579 |
| Number of flowers | 17.0 ± 5.0 | 16.2 ± 4.7 | 15.3 ± 4.9 | 14.6 ± 5.1 | 14.221 | <0.001 | 2.473 | 0.117 | 0.014 | 0.905 |
| Fruit production | 10.2 ± 3.7 | 12.6 ± 4.9 | 11.7 ± 4.8 | 10.7 ± 4.6 | 0.874 | 0.35 | 1.948 | 0.164 | 11.454 | <0.001 |
| Mean weight per fruit (mg) | 2.8 ± 1.0 | 2.5 ± 1.1 | 2.2 ± 0.8 | 2.3 ± 1.1 | 22.119 | <0.001 | 1.232 | 0.268 | 3.154 | 0.076 |
| Female fitness (mg) | 29.2 ± 16.4 | 35.1 ± 26.5 | 28.0 ± 19.4 | 27.6 ± 23.9 | 9.625 | 0.002 | 0.069 | 0.793 | 1.272 | 0.260 |
| Pollen removal | 26.3 ± 9.0 | 27.3 ± 9.1 | 26.2 ± 9.3 | 28.2 ± 10.0 | 0.685 | 0.408 | 4.342 | 0.038 | 0.498 | 0.481 |
C, natural vegetation; E, short vegetation treatment; n is the sample size. Significant estimates and their P-values are indicated in bold.
Values of floral traits and reproductive success (mean ± SD) for Spiranthes sinensis plants and the effect of years and vegetation treatments on these variables were analysed using two-way ANOVA
| . | 2018 . | 2019 . | Year . | Treatment . | Year × treatment . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Traits and reproductive success . | C (n = 115) . | E (n = 107) . | C (n = 108) . | E (n = 112) . | F1,439 . | P . | F1,439 . | P . | F1,439 . | P . |
| Flowering start | 204.8 ± 3.7 | 205.0 ± 2.6 | 209.4 ± 2.4 | 209.3 ± 3.4 | 228.91 | <0.001 | 0.064 | 0.800 | 0.457 | 0.499 |
| Plant height (cm) | 17.9 ± 3.5 | 17.2 ± 2.8 | 16.7 ± 3.6 | 15.7 ± 2.6 | 21.062 | <0.001 | 5.08 | 0.025 | 0.152 | 0.697 |
| Corolla length (mm) | 4.49 ± 0.53 | 4.58 ± 0.53 | 4.08 ± 0.48 | 4.08 ± 0.61 | 76.87 | <0.001 | 0.394 | 0.531 | 0.849 | 0.357 |
| Corolla width (mm) | 3.23 ± 0.39 | 3.15 ± 0.34 | 3.12 ± 0.43 | 3.04 ± 0.45 | 9.884 | 0.002 | 4.326 | 0.038 | 0.004 | 0.952 |
| Corolla size (mm2) | 14.65 ± 3.25 | 14.52 ± 2.96 | 12.82 ± 2.76 | 12.53 ± 3.01 | 45.732 | <0.001 | 0.712 | 0.399 | 0.308 | 0.579 |
| Number of flowers | 17.0 ± 5.0 | 16.2 ± 4.7 | 15.3 ± 4.9 | 14.6 ± 5.1 | 14.221 | <0.001 | 2.473 | 0.117 | 0.014 | 0.905 |
| Fruit production | 10.2 ± 3.7 | 12.6 ± 4.9 | 11.7 ± 4.8 | 10.7 ± 4.6 | 0.874 | 0.35 | 1.948 | 0.164 | 11.454 | <0.001 |
| Mean weight per fruit (mg) | 2.8 ± 1.0 | 2.5 ± 1.1 | 2.2 ± 0.8 | 2.3 ± 1.1 | 22.119 | <0.001 | 1.232 | 0.268 | 3.154 | 0.076 |
| Female fitness (mg) | 29.2 ± 16.4 | 35.1 ± 26.5 | 28.0 ± 19.4 | 27.6 ± 23.9 | 9.625 | 0.002 | 0.069 | 0.793 | 1.272 | 0.260 |
| Pollen removal | 26.3 ± 9.0 | 27.3 ± 9.1 | 26.2 ± 9.3 | 28.2 ± 10.0 | 0.685 | 0.408 | 4.342 | 0.038 | 0.498 | 0.481 |
| . | 2018 . | 2019 . | Year . | Treatment . | Year × treatment . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Traits and reproductive success . | C (n = 115) . | E (n = 107) . | C (n = 108) . | E (n = 112) . | F1,439 . | P . | F1,439 . | P . | F1,439 . | P . |
| Flowering start | 204.8 ± 3.7 | 205.0 ± 2.6 | 209.4 ± 2.4 | 209.3 ± 3.4 | 228.91 | <0.001 | 0.064 | 0.800 | 0.457 | 0.499 |
| Plant height (cm) | 17.9 ± 3.5 | 17.2 ± 2.8 | 16.7 ± 3.6 | 15.7 ± 2.6 | 21.062 | <0.001 | 5.08 | 0.025 | 0.152 | 0.697 |
| Corolla length (mm) | 4.49 ± 0.53 | 4.58 ± 0.53 | 4.08 ± 0.48 | 4.08 ± 0.61 | 76.87 | <0.001 | 0.394 | 0.531 | 0.849 | 0.357 |
| Corolla width (mm) | 3.23 ± 0.39 | 3.15 ± 0.34 | 3.12 ± 0.43 | 3.04 ± 0.45 | 9.884 | 0.002 | 4.326 | 0.038 | 0.004 | 0.952 |
| Corolla size (mm2) | 14.65 ± 3.25 | 14.52 ± 2.96 | 12.82 ± 2.76 | 12.53 ± 3.01 | 45.732 | <0.001 | 0.712 | 0.399 | 0.308 | 0.579 |
| Number of flowers | 17.0 ± 5.0 | 16.2 ± 4.7 | 15.3 ± 4.9 | 14.6 ± 5.1 | 14.221 | <0.001 | 2.473 | 0.117 | 0.014 | 0.905 |
| Fruit production | 10.2 ± 3.7 | 12.6 ± 4.9 | 11.7 ± 4.8 | 10.7 ± 4.6 | 0.874 | 0.35 | 1.948 | 0.164 | 11.454 | <0.001 |
| Mean weight per fruit (mg) | 2.8 ± 1.0 | 2.5 ± 1.1 | 2.2 ± 0.8 | 2.3 ± 1.1 | 22.119 | <0.001 | 1.232 | 0.268 | 3.154 | 0.076 |
| Female fitness (mg) | 29.2 ± 16.4 | 35.1 ± 26.5 | 28.0 ± 19.4 | 27.6 ± 23.9 | 9.625 | 0.002 | 0.069 | 0.793 | 1.272 | 0.260 |
| Pollen removal | 26.3 ± 9.0 | 27.3 ± 9.1 | 26.2 ± 9.3 | 28.2 ± 10.0 | 0.685 | 0.408 | 4.342 | 0.038 | 0.498 | 0.481 |
C, natural vegetation; E, short vegetation treatment; n is the sample size. Significant estimates and their P-values are indicated in bold.
The significant interaction between treatment and year on fruit production was due to higher fruit production in E than in C in 2018, while the opposite was seen in 2019 (Table 1). The mean weight per fruit and female fitness of S. sinensis differed between years, and pollen removal differed between treatments. Plants had higher mean weight per fruit and female fitness in 2018 than in 2019. In both years, plants had higher pollen removal in the E treatment than in the C treatment (Table 1).
Opportunity for selection, net directional selection and vegetation context modification selection
The opportunity for selection through female fitness was 0.3154 and 0.5711 for the C and E treatments in 2018 and 0.4793 and 0.745 for the C and E treatments in 2019, respectively. In contrast, the opportunity for selection through pollen removal was 0.117 and 0.1104 for the C and E treatments in 2018 and 0.1247 and 0.1213 for the C and E treatments in 2019, respectively.
There was net selection for earlier flowering start through female fitness in 2018 (βC = −0.080 ± 0.039, P = 0.043) and through pollen removal in 2019 (βC = −0.042 ± 0.016, P = 0.007, Table 2; Fig. 3a and b). Significant net selection for a greater number of flowers was observed through female fitness and pollen removal in both 2018 and 2019 (Table 2; Fig. 3a and b). Net selection for shorter plant height (βC = −0.050 ± 0.016, P = 0.002) was observed only through pollen removal in 2018. Net selection for a larger corolla size (βC = 0.074 ± 0.039, P = 0.065, marginally significant) was observed through female fitness in 2018. Net directional selection on flowering start and number of flowers differed between years, as indicated by the significant year × flowering start (F1,222 = 7.119, P = 0.008) and year × number of flowers (F1,222 = 10.082, P = 0.002) interactions obtained using ANCOVA through the female fitness function (Supplementary Table S1). The strength of net directional selection on flowering start through female fitness was stronger in 2018 than in 2019. In contrast, the strength of selection on the number of flowers was stronger in 2019 than in 2018.
Directional selection gradients and associated P values among natural vegetation (C) and short vegetation (E) treatments in the Spiranthes sinensis population in 2018 and 2019
| . | . | C . | E . | Vegetation context-modified selection . | |||
|---|---|---|---|---|---|---|---|
| Terms . | Floral traits . | βi ± SE . | P . | βi ± SE . | P . | Δβ . | P . |
| Female fitness | 2018 | ||||||
| Flowering start | −0.080 ± 0.039 | 0.043 | 0.072 ± 0.047 | 0.129 | −0.152 | 0.026 | |
| Plant height | 0.050 ± 0.048 | 0.3 | 0.172 ± 0.070 | 0.015 | −0.122 | 0.002 | |
| Corolla size | 0.074 ± 0.039 | 0.065 | 0.073 ± 0.052 | 0.157 | 0.001 | 0.882 | |
| Number of flowers | 0.332 ± 0.046 | <0.001 | 0.440 ± 0.065 | <0.001 | −0.108 | 0.167 | |
| 2019 | |||||||
| Flowering start | 0.061 ± 0.040 | 0.13 | −0.012 ± 0.049 | 0.807 | 0.073 | 0.345 | |
| Plant height | 0.011 ± 0.043 | 0.788 | 0.245 ± 0.060 | <0.001 | −0.234 | <0.001 | |
| Corolla size | 0.068 ± 0.041 | 0.103 | 0.024 ± 0.054 | 0.658 | 0.044 | 0.518 | |
| Number of flowers | 0.531 ± 0.043 | <0.001 | 0.533 ± 0.060 | <0.001 | −0.002 | 0.973 | |
| Pollen removal | 2018 | ||||||
| Flowering start | −0.020 ± 0.013 | 0.131 | 0.038 ± 0.010 | <0.001 | −0.058 | 0.001 | |
| Plant height | −0.050 ± 0.016 | 0.002 | 0.001 ± 0.015 | 0.95 | −0.051 | 0.068 | |
| Corolla size | 0.010 ± 0.013 | 0.464 | −0.025 ± 0.011 | 0.023 | 0.035 | 0.054 | |
| Number of flowers | 0.336 ± 0.015 | <0.001 | 0.322 ± 0.014 | <0.001 | 0.014 | 0.52 | |
| 2019 | |||||||
| Flowering start | −0.042 ± 0.016 | 0.007 | 0.003 ± 0.004 | 0.488 | −0.045 | 0.002 | |
| Plant height | −0.010 ± 0.016 | 0.525 | 0.005 ± 0.005 | 0.279 | −0.015 | 0.262 | |
| Corolla size | 0.018 ± 0.016 | 0.262 | −0.005 ± 0.004 | 0.218 | 0.023 | 0.222 | |
| Number of flowers | 0.324 ± 0.016 | <0.001 | 0.345 ± 0.005 | <0.001 | −0.021 | 0.25 | |
| . | . | C . | E . | Vegetation context-modified selection . | |||
|---|---|---|---|---|---|---|---|
| Terms . | Floral traits . | βi ± SE . | P . | βi ± SE . | P . | Δβ . | P . |
| Female fitness | 2018 | ||||||
| Flowering start | −0.080 ± 0.039 | 0.043 | 0.072 ± 0.047 | 0.129 | −0.152 | 0.026 | |
| Plant height | 0.050 ± 0.048 | 0.3 | 0.172 ± 0.070 | 0.015 | −0.122 | 0.002 | |
| Corolla size | 0.074 ± 0.039 | 0.065 | 0.073 ± 0.052 | 0.157 | 0.001 | 0.882 | |
| Number of flowers | 0.332 ± 0.046 | <0.001 | 0.440 ± 0.065 | <0.001 | −0.108 | 0.167 | |
| 2019 | |||||||
| Flowering start | 0.061 ± 0.040 | 0.13 | −0.012 ± 0.049 | 0.807 | 0.073 | 0.345 | |
| Plant height | 0.011 ± 0.043 | 0.788 | 0.245 ± 0.060 | <0.001 | −0.234 | <0.001 | |
| Corolla size | 0.068 ± 0.041 | 0.103 | 0.024 ± 0.054 | 0.658 | 0.044 | 0.518 | |
| Number of flowers | 0.531 ± 0.043 | <0.001 | 0.533 ± 0.060 | <0.001 | −0.002 | 0.973 | |
| Pollen removal | 2018 | ||||||
| Flowering start | −0.020 ± 0.013 | 0.131 | 0.038 ± 0.010 | <0.001 | −0.058 | 0.001 | |
| Plant height | −0.050 ± 0.016 | 0.002 | 0.001 ± 0.015 | 0.95 | −0.051 | 0.068 | |
| Corolla size | 0.010 ± 0.013 | 0.464 | −0.025 ± 0.011 | 0.023 | 0.035 | 0.054 | |
| Number of flowers | 0.336 ± 0.015 | <0.001 | 0.322 ± 0.014 | <0.001 | 0.014 | 0.52 | |
| 2019 | |||||||
| Flowering start | −0.042 ± 0.016 | 0.007 | 0.003 ± 0.004 | 0.488 | −0.045 | 0.002 | |
| Plant height | −0.010 ± 0.016 | 0.525 | 0.005 ± 0.005 | 0.279 | −0.015 | 0.262 | |
| Corolla size | 0.018 ± 0.016 | 0.262 | −0.005 ± 0.004 | 0.218 | 0.023 | 0.222 | |
| Number of flowers | 0.324 ± 0.016 | <0.001 | 0.345 ± 0.005 | <0.001 | −0.021 | 0.25 | |
Vegetation context-modified selection gradients (Δβ = βC – βE) and P values associated with the treatment × trait interactions in ANCOVAs conducted separately for each year are also given. Significant selection estimates and their P-values are indicated in bold.
Directional selection gradients and associated P values among natural vegetation (C) and short vegetation (E) treatments in the Spiranthes sinensis population in 2018 and 2019
| . | . | C . | E . | Vegetation context-modified selection . | |||
|---|---|---|---|---|---|---|---|
| Terms . | Floral traits . | βi ± SE . | P . | βi ± SE . | P . | Δβ . | P . |
| Female fitness | 2018 | ||||||
| Flowering start | −0.080 ± 0.039 | 0.043 | 0.072 ± 0.047 | 0.129 | −0.152 | 0.026 | |
| Plant height | 0.050 ± 0.048 | 0.3 | 0.172 ± 0.070 | 0.015 | −0.122 | 0.002 | |
| Corolla size | 0.074 ± 0.039 | 0.065 | 0.073 ± 0.052 | 0.157 | 0.001 | 0.882 | |
| Number of flowers | 0.332 ± 0.046 | <0.001 | 0.440 ± 0.065 | <0.001 | −0.108 | 0.167 | |
| 2019 | |||||||
| Flowering start | 0.061 ± 0.040 | 0.13 | −0.012 ± 0.049 | 0.807 | 0.073 | 0.345 | |
| Plant height | 0.011 ± 0.043 | 0.788 | 0.245 ± 0.060 | <0.001 | −0.234 | <0.001 | |
| Corolla size | 0.068 ± 0.041 | 0.103 | 0.024 ± 0.054 | 0.658 | 0.044 | 0.518 | |
| Number of flowers | 0.531 ± 0.043 | <0.001 | 0.533 ± 0.060 | <0.001 | −0.002 | 0.973 | |
| Pollen removal | 2018 | ||||||
| Flowering start | −0.020 ± 0.013 | 0.131 | 0.038 ± 0.010 | <0.001 | −0.058 | 0.001 | |
| Plant height | −0.050 ± 0.016 | 0.002 | 0.001 ± 0.015 | 0.95 | −0.051 | 0.068 | |
| Corolla size | 0.010 ± 0.013 | 0.464 | −0.025 ± 0.011 | 0.023 | 0.035 | 0.054 | |
| Number of flowers | 0.336 ± 0.015 | <0.001 | 0.322 ± 0.014 | <0.001 | 0.014 | 0.52 | |
| 2019 | |||||||
| Flowering start | −0.042 ± 0.016 | 0.007 | 0.003 ± 0.004 | 0.488 | −0.045 | 0.002 | |
| Plant height | −0.010 ± 0.016 | 0.525 | 0.005 ± 0.005 | 0.279 | −0.015 | 0.262 | |
| Corolla size | 0.018 ± 0.016 | 0.262 | −0.005 ± 0.004 | 0.218 | 0.023 | 0.222 | |
| Number of flowers | 0.324 ± 0.016 | <0.001 | 0.345 ± 0.005 | <0.001 | −0.021 | 0.25 | |
| . | . | C . | E . | Vegetation context-modified selection . | |||
|---|---|---|---|---|---|---|---|
| Terms . | Floral traits . | βi ± SE . | P . | βi ± SE . | P . | Δβ . | P . |
| Female fitness | 2018 | ||||||
| Flowering start | −0.080 ± 0.039 | 0.043 | 0.072 ± 0.047 | 0.129 | −0.152 | 0.026 | |
| Plant height | 0.050 ± 0.048 | 0.3 | 0.172 ± 0.070 | 0.015 | −0.122 | 0.002 | |
| Corolla size | 0.074 ± 0.039 | 0.065 | 0.073 ± 0.052 | 0.157 | 0.001 | 0.882 | |
| Number of flowers | 0.332 ± 0.046 | <0.001 | 0.440 ± 0.065 | <0.001 | −0.108 | 0.167 | |
| 2019 | |||||||
| Flowering start | 0.061 ± 0.040 | 0.13 | −0.012 ± 0.049 | 0.807 | 0.073 | 0.345 | |
| Plant height | 0.011 ± 0.043 | 0.788 | 0.245 ± 0.060 | <0.001 | −0.234 | <0.001 | |
| Corolla size | 0.068 ± 0.041 | 0.103 | 0.024 ± 0.054 | 0.658 | 0.044 | 0.518 | |
| Number of flowers | 0.531 ± 0.043 | <0.001 | 0.533 ± 0.060 | <0.001 | −0.002 | 0.973 | |
| Pollen removal | 2018 | ||||||
| Flowering start | −0.020 ± 0.013 | 0.131 | 0.038 ± 0.010 | <0.001 | −0.058 | 0.001 | |
| Plant height | −0.050 ± 0.016 | 0.002 | 0.001 ± 0.015 | 0.95 | −0.051 | 0.068 | |
| Corolla size | 0.010 ± 0.013 | 0.464 | −0.025 ± 0.011 | 0.023 | 0.035 | 0.054 | |
| Number of flowers | 0.336 ± 0.015 | <0.001 | 0.322 ± 0.014 | <0.001 | 0.014 | 0.52 | |
| 2019 | |||||||
| Flowering start | −0.042 ± 0.016 | 0.007 | 0.003 ± 0.004 | 0.488 | −0.045 | 0.002 | |
| Plant height | −0.010 ± 0.016 | 0.525 | 0.005 ± 0.005 | 0.279 | −0.015 | 0.262 | |
| Corolla size | 0.018 ± 0.016 | 0.262 | −0.005 ± 0.004 | 0.218 | 0.023 | 0.222 | |
| Number of flowers | 0.324 ± 0.016 | <0.001 | 0.345 ± 0.005 | <0.001 | −0.021 | 0.25 | |
Vegetation context-modified selection gradients (Δβ = βC – βE) and P values associated with the treatment × trait interactions in ANCOVAs conducted separately for each year are also given. Significant selection estimates and their P-values are indicated in bold.
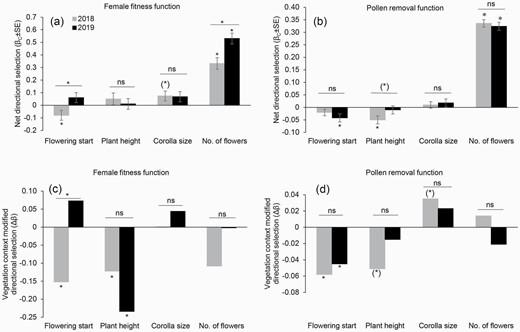
Net directional selection gradients (βC ± SE) and vegetation context-modified selection gradients (Δβ) on flowering start, plant height, corolla size and number of flowers (no. of flowers) of S. sinensis over 2 years. (a) and (c) are estimated through female fitness, and (b) and (d) are estimated through pollen removal. Symbols above the individual bars indicate the level of significance of selection gradients. Symbols above the lines indicate whether selection differs between years. *P < 0.05; (*) P < 0.1; ns, P > 0.1.
Vegetation context-modified selection for earlier flowering start was detected through both female fitness (Δβ = −0.152, P = 0.026) and pollen removal (Δβ = −0.058, P = 0.001) in 2018 and through pollen removal in 2019 (Δβ = −0.045, P = 0.002, Table 2; Fig. 3c and d; Supplementary Fig. S1a, d and g). Selection for shorter plant height through female fitness was detected in both 2018 (Δβ = −0.122, P = 0.002) and 2019 (Δβ = −0.234, P < 0.001, Table 2; Fig. 3c; Supplementary Fig. S1b and c). In addition, vegetation context-modified selection for a shorter plant height through pollen removal was also detected in 2018 (Δβ = −0.051, P = 0.068, marginally significant, Supplementary Fig. S1e). Selection for larger corolla size was detected only through pollen removal in 2018 (Δβ = 0.035, P = 0.054, marginally significant, Fig. 3d; Supplementary Fig. S1f). Vegetation context-modified selection on flowering start differed between years, as indicated by the significant year × treatment × flowering start (F1,441 = 6.814, P = 0.009) interaction obtained using ANCOVA through the female fitness function (Supplementary Table S1). The strength of selection on this trait was stronger in 2018 than in 2019.
DISCUSSION
In the present study, we demonstrated directional selection on flowering start and plant height. Vegetation context affected selection on these floral traits. Temporal variation in the selection on floral traits was also detected. The strength of selection differed between female fitness and pollen removal functions. Our results highlight the role of vegetation context in driving the evolution of floral traits in this orchid species.
Our results showed evidence for vegetation context-modified selection for an earlier flowering start date and shorter plant height through both female fitness and pollen removal. For insect-pollinated plants, an earlier or later flowering phenology is predicted to fit the activity rhythm of pollinators, thus increasing plant reproductive success (Munguía-Rosas et al. 2011; Sandring and Ågren 2009). At our study site, bumblebees (including B. richardsi and B. convexus) are the most important pollinators for these co-flowering species, and their bloom occurs mainly from July to August. Vegetation context-modified selection for an earlier flowering start date is expected to weaken the competition for pollinators between co-flowering species and S. sinensis, resulting in fitness benefits for the focal orchid species. Indeed, our results showed that an earlier flowering start date was linked with higher reproductive success. In addition, our results showed evidence that the selection on flowering start varied between treatments and years. Variation in the direction of selection between treatments suggests the potential effect of vegetation context on driving the evolution of this trait. Our results have demonstrated the significant effect of treatment on pollen removal of S. sinensis. This implies that the vegetation context influences the pollination environment of this orchid species, thus resulting in a shift in the direction of selection. Furthermore, this effect of vegetation context will also be expected to influence pollinator-mediated selection (Sletvold et al. 2013). Variation in the direction and strength of selection on flowering start between years suggests that other agents may influence the process of floral evolution. The precipitation, environmental vegetation density and pollinator abundance differed between the studied years. These biotic and abiotic factors may influence interspecific competition for resources and pollinators, thus resulting in temporal variation in selection on floral traits. To clarify which factor drives the temporal variation in selection on flowering start, more manipulative experiments are needed.
Vegetation context-modified selection for a shorter plant height of S. sinensis suggested that there was some adaptive advantage of being shorter in an environment with tall natural vegetation. This may be attributed to the competition mechanism for resources and pollination services among co-flowering species. A shorter plant height may increase the capability of S. sinensis to obtain soil resources (e.g. soil water and nutrition), thus resulting in an increase in plant reproductive success. Spiranthes sinensis produced a spiral inflorescence with multiple purple flowers, and a shorter plant height may be more striking for pollinators with the surrounding green vegetation context. A shorter plant height will be expected to reduce floral visual displays to attract pollinators. However, it may motivate plants to invest more resources in other floral traits, such as floral scent and nectar, which maintain and/or increase the loyalty of pollinators. This result suggests the competitive strategy of S. sinensis against surrounding co-flowering species at our study site. Previous studies indicate that pollinators always generate selection for a taller plant height (Ågren et al. 2006; Reynolds et al. 2010). Indeed, taller plant height was selected through female fitness in both years in the E treatment. These facts suggest that the selection of plant height via vegetation context and pollinators may be in conflict. Vegetation context modifies selection for shorter plant height, and pollinators mediate selection for taller plant height; as a consequence, the interactive effect of these two biotic agents will be expected to result in stabilizing selective pressure on this trait.
Our results indicated that only vegetation context modified marginally significant selection for a larger corolla size through pollen removal in 2018. Significant selection on corolla size through female fitness was not detected in either year. This suggests the variable effect of vegetation context on the evolution of this trait. There was significant selection for greater flower production in the C and E treatments through both female fitness and pollen removal functions. This suggests that selection on this trait is not modified by vegetation context. Previous studies mainly suggest pollinator-mediated selection for larger corolla size and greater flower production (Benitez-Vieyra et al. 2006; Sánchez-Lafuente and Parra 2009). In this orchid species, pollinators may also be the agent that drives the evolution of these floral traits. However, experimental studies are necessary to confirm this prediction. In the present study, we estimated the total number of flowers at the end of the flowering period as one of the floral attractive traits of S. sinensis. Flower number at the end of the flowering season may be more of a fitness measure than a pollinator attraction trait in some cases and thus may influence our results. To accurately understand phenotypic selection on floral display, the number of open flowers at a given point in the season (e.g. a daily display size measure) will be quantified in future research.
Basically, the vegetation context modifies selection on floral traits by directly influencing plant reproductive success (e.g. resource limitation) or indirectly influencing selective pressures from pollinators (Thomann et al. 2018). In the present study, seed production was roughly equivalent in both treatments, which suggested that plants in the experimental treatment were not released from resource limitation due to the lack of light competition. Significant vegetation context modifies selection on floral traits of S. sinensis, which may imply its potential role in influencing pollinator-mediated selection (Sletvold et al. 2013).
In hermaphroditic flowers, variation in floral traits simultaneously influences pollen export and pollen receipt of plants. Consequently, selection on floral traits may occur through either sexual function and even stand in contrast between sexes (Ashman and Morgan 2004). Conflicting selection on floral traits through male and female function is rare in wild species (Campbell 1989). Most previous studies provide evidence that the strength of selection is stronger through male function than through female function (Benitez-Vieyra et al. 2006; Hodgins and Barrett 2008; La Rosa and Conner 2017). However, our results provided evidence that the strength of selection on floral traits through female fitness was stronger than that through pollen removal. Opportunity for selection positively determines the strength of selection (Benkman 2013). Indeed, the opportunity for selection through pollen removal was lower than that through female fitness at our study site. Each flower of our studied orchid species produces only two pollinia, and pollinators always simultaneously remove the two pollinia during visitation because pollinia closely contact each other, thus resulting in a low variance in relative pollen removal among individuals. Despite the strength of selection on floral traits through pollen removal being weaker than selection through female function, our results showed that more single floral traits (e.g. corolla size) were selected through pollen removal than through female fitness function in this orchid species. This result is consistent with predictions from previous studies (Benitez-Vieyra et al. 2006; Kulbaba and Worley 2012; Stanton et al. 1986). The fate of pollen is deeply linked with floral traits in angiosperms. Variation in these traits influences the quantity and quality of pollen removal. In contrast, plant female reproductive success is simultaneously influenced by interactions with pollinators and resources. These mechanisms suggest the stronger effect of vegetation context on driving the evolution of floral traits through male function. Our results are consistent with the hypothesis from Caruso et al. (2019), which predicts the effect of nonpollinator agents in shaping the floral evolutionary trajectory. To fully explore the variation in vegetation context that modifies selection on floral traits between sexual functions, more male fitness metrics (e.g. pollen competition, the distance of pollen removal and outcrossing rate) and floral traits (e.g. pollination efficiency traits and floral scent) are needed for quantification.
The study of Sletvold et al. (2013) only highlights the effect of vegetation context on pollinator-mediated selection through the female fitness function in a single year. In addition to pollinators, our results have demonstrated that the vegetation context can also generate selective pressures on floral traits. Vegetation context modifies selection on flowering start and plant height in an orchid species. The vegetation context can generate extensive and profound effects in shaping floral phenotypes through both male and female fitness functions. However, the strength of vegetation context-modified selection varies between sexual functions and years. Many studies have demonstrated the key role of pollinators in driving floral evolution, and our results highlight the potential strength of some specific biotic agents (other than pollinators) in this process. To accurately understand and clarify the floral evolutionary trajectory in complex biotic environments, the main and interactive effects of biotic factors on natural selection through both sexual functions should not be neglected.
Supplementary Material
Supplementary material is available at Journal of Plant Ecology online.
Table S1: Variations in directional selection between years via ANCOVA testing for S. sinensis.
Figure S1: Standardized linear phenotypic selection gradients for flowering start, plant height and corolla size in natural vegetation plants (C, open circles, dashed line) and short vegetation plants (E, closed circles, solid line) in 2018 (a, b and d–f) and 2019 (c and g).
Funding
This research was supported by the Funds of the Science and Technology Department of Sichuan Province (2019YJ0393, 2020YFS0309) and Joint Funds of the National Natural Science Foundation of China and Yunnan Provincial Government (U1602263).
Acknowledgements
We thank the South-East Tibetan Plateau Station for integrated observation and research on the alpine environment and the Chinese Academy of Sciences for logistical support.
Conflict of interest statement. The authors declare that they have no conflict of interest.




