-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel García-Pérez, Mariano Ruiz-Ortiz, Irene Panero, Carla Eiriz, Luis Miguel Moreno, Ana García-Reyne, Alfredo García, Patricia Martín-Medina, Elena Salvador-Álvarez, Aurelio Hernández-Lain, Antonio Serrano, Francisco Javier Gil-Etayo, Ana María Castaño-León, Igor Paredes, Ángel Pérez-Núñez, Snorting the Brain Away: Cerebral Damage as an Extension of Cocaine-Induced Midline Destructive Lesions, Journal of Neuropathology & Experimental Neurology, Volume 79, Issue 12, December 2020, Pages 1365–1369, https://doi.org/10.1093/jnen/nlaa097
Close - Share Icon Share
Abstract
Cocaine consumption is associated with a variety of clinical manifestations. Though cocaine intranasal inhalation always determines nasal mucosal damages, extensive septum perforations, and midline destructions—known as cocaine-induced midline destructive lesions (CIMDL)—affect only a limited fraction of patients. CIMDL is viewed as a cocaine-associated autoimmune phenomenon in which the presence of atypical anti-neutrophil cytoplasmic antibody (ANCA) promotes and/or defines the disease phenotype. A 51-year-old man presented with an intracranial tumor-like lesion by its space-occupying effect. CT also revealed the destruction of the nasal septum and skull base. A diagnosis of CIMDL was made in light of the patient’s history as well as findings of the physical and endoscopic examinations, imaging studies, and laboratory testing. There was no evidence of other pathologies. Histopathological results from cerebral biopsy led us to consider the intracranial pathology as an extension of the CIMDL. CIMDL is the result of a necrotizing inflammatory tissue response triggered by cocaine abuse in a subset of predisposed patients. The reported case is the first CIMDL consistent with brain extension mimicking a tumor-like lesion. While the presence of atypical ANCA seems to promote and/or define the disease phenotype, the specific role of these and other circulating autoantibodies needs further investigation.
INTRODUCTION
Cocaine consumption is associated with a variety of local and systemic clinical manifestations, including serious neurological complications. In fact, cocaine has been related with cerebral infarction, intracerebral and subarachnoid hemorrhage, transient ischemic attacks, migraines, and seizures (1). One of these side-effects of cocaine is vasculitis. Two distinct anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitic syndromes due to cocaine intranasal inhalation or “snorting” have been described. One vasculitic syndrome is attributed to levamisole (which is frequently added to cocaine), and manifests characteristically with cutaneous findings, arthralgias, and otolaryngologic involvement. Levamisole-induced vasculitis is known to involve the central nervous system in the form of multifocal leukoencephalopathy (2). On the other hand, cocaine itself leads to cocaine-induced midline destructive lesions (CIMDL), which is characterized by ischemic necrosis of the septal cartilage causing perforation of the nasal septum and vasculitic lesions similar to granulomatosis with polyangiitis (Wegener's; [GPA]) (3). While cocaine-induced perforation of the palate has also been widely recognized, erosion of the anterior cranial fossa resulting in encephalocele from cocaine abuse is very rare (4). Similarly, brain damage due to the propagation of these vasculitic lesions from the nose is exceptional (5). To the best of our knowledge, our patient is the first documented case of CIMDL causing a tumor-like lesion by its intracranial space-occupying effect.
CASE REPORT
A 51-year-old man with type 1 diabetes was admitted to the intensive care unit for a status epilepticus in the context of a hyperglycemic decompensation induced by insulin therapy cessation. Initial computed tomography (CT) scan of the brain revealed cortico-subcortical frontal hypodensity of both frontal lobes with mass effect on brain parenchyma. After contrast administration, diffuse leptomeningeal enhancement was detected. Destruction of the nasal septum was overlooked (Fig. 1A–E).
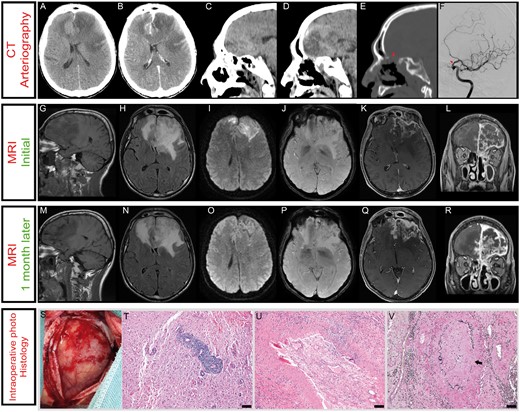
(A) CT showed cortico-subcortical hypodensity of the left frontal lobe which crossed the midline and affected the right frontal lobe. The lesion exerted mass effect with 6 mm midline shift to the right and collapse of the frontal and temporal horns of the left lateral ventricle. (B) Diffuse leptomeningeal enhancement was observed after contrast administration. (C–E) Severe bony erosion in the intranasal cavity and anterior skull base was visualized on the bone windows of the same CT scan. Sagittal CT images showed cribriform plate destruction, which resulted in left nasal cavity and anterior cranial fossa connection (E). We also detected the occupation of several sinuses in relation to chronic sinupathy. (F) Cerebral angiogram demonstrated a focal narrowing at the supraclinoid internal carotid artery (oblique view). (G–L) Initial MRI. (G) The lesion was hypointense on T1-weighted images (T1WI). (H) T2-weighted images (T2WI) hyperintensity involved both frontal lobes, the left insular cortex and left hippocampus. The hyperintensity affected cortical and subcortical parenchyma, exerted mass effect of the ventricular system, shifting the midline 13 mm to the right. (I) Diffusion-weighted imaging (DWI) revealed the presence of signal-intensity abnormality mostly on cortical areas. (J) Hypointense lesions on susceptibility-weighted imaging (SWI) were also observed, suggesting hemorrhagic events. (K–L) Gadolinium-based contrast-enhanced images depicted mild left fronto-basal parenchyma enhancement and a strong diffuse leptomeningeal enhancement, in contact with the cribriform plate. (M–R) A second MRI was performed one month later. (M) T1WI showed augmented necrotic/cystic brain areas. (N) T2WI hyperintensity was slightly diminished on the left hemisphere while it was augmented on the right frontal lobe. (O) Signal intensity on DWI was markedly reduced compared with the previous study. (P) Hypointense lesions on SWI were stable one month later. (Q–R) The new imaging study revealed a marked increase in parenchymal and meningeal contrast enhancement. (S) Intraoperative photograph. A left frontal craniotomy was performed. The dura mater was opened in an X-shaped incision exposing brain. Brain parenchyma was firmer than normal, and necrotic areas were observed. (T–V) Histopathologic features of brain biopsy. (T) Dense polymorphic perivascular and transmural infiltrate, surrounded by reactive astrogliosis (H&E). (U) The lumen of an artery is occluded by an organized thrombus, which is partially recanalized (H&E). (V) Black arrow points to disruption of the internal elastic lamina of the vessel wall (Van Gieson stain). Scale bar: 100 µm.
The patient was transferred to the neurosurgery ward after clinical stabilization. Neurological examination only revealed mild frontal lobe syndrome. A magnetic resonance imaging (MRI) study disclosed the origin of the intracranial lesion from the extension of rinosinusal destruction. MRI also revealed extensive edema of the frontal lobes and extensive meningeal and frontal lobe contrast enhancement, thus suggesting an inflammatory or infectious disease (Fig. 1G–L). Given his medical history, rhino-cerebral mucormycosis was suspected, which prompted rinosinusal examination. Nasal endoscopy revealed extensive destruction of the nasal septum and left ethmoid bone, but no lesions suggestive of rhino-cerebral mucormycosis were observed. When interrogated, the patient admitted nasal cocaine consumption that he had not revealed before, and thus cocaine-induced vasculitis was suggested by the ENT surgeon. Intranasal biopsies revealed tissular necrosis and chronic inflammation, whereas bacterial and fungal cultures were only positive for methicillin susceptible Staphylococcus aureus. The patient started with levofloxacin to avoid CNS bacterial dissemination. Cerebral angiography demonstrated narrowing of the supraclinoid internal carotid artery (Fig. 1F). Laboratory testing and immunofluorescence analysis revealed a positive serum test for atypical ANCA (Fig. 2). ELISA test was positive for lactoferrin antibodies, whereas negative results were obtained for elastase, cathepsin G, or bactericidal permeability increasing protein. We proposed to perform a cerebral biopsy for histological diagnosis but the patient refused and he was discharged upon request.
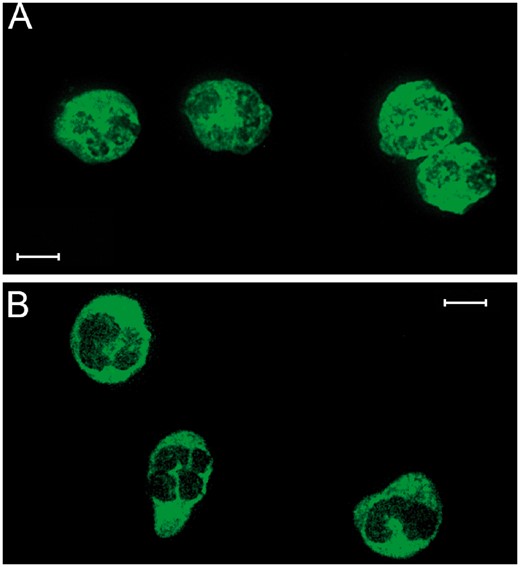
Indirect immunofluorescence for anti-neutrophil cytoplasmic antibodies (ANCA). (A) Ethanol-fixed neutrophils. Atypical ANCA pattern with a granular cytoplasmic staining and perinuclear reinforcement. (B) Formalin-fixed neutrophils. Homogeneous cytoplasmic staining, similar to typical cytoplasmic ANCA pattern. Titration, 1:40. Scale bar: 10 µm. The sample was diluted in phosphate-buffered saline (1:40). Then, the diluted sample was incubated with the fixed neutrophils in ethanol and formalin for 30 minutes at room temperature. After that, the sample was washed 3 times with PBS for 5 minutes. Once the slice was dried, it was incubated with the secondary antibody (antihuman IgG-FITC, Aesku Diagnostics, Wendelsheim, Germany). The slide was then washed as mentioned before.
One month later, he returned to the emergency department due to an intense and progressive headache. He denied cocaine consumption over that period of time. A new MRI showed increased meningeal and parenchymal contrast enhancement and augmented necrotic/cystic brain areas (Fig. 1M–R). The patient underwent a left frontal craniotomy and a partial anterior frontal lobectomy was achieved. Intraoperatively, we found thickened dura mater and arachnoid, with the brain highly fixed to the meninges. These adhesions were far more pronounced as the frontal skull base was approached. Thrombosed veins were also detected. Brain parenchyma was firmer than normal and necrotic areas were also observed (Fig. 1S). The postoperative course was uneventful.
Brain tissue histopathological examination revealed an extensive lymphoplasmocytic infiltrate with perivascular distribution, surrounded by reactive astrogliosis with foci of necrosis (Fig. 1T–V). Active vasculitic lesions were present, which were characterized by transmural inflammation of the blood vessel, fibrinoid necrosis and fragmentation of the endothelium and the internal elastic lamina. In addition, chronic vasculitic lesions, characterized by fibrosis of media and adventitial and intraluminal thrombosis with recanalization, were also detected. Some isolated multinucleated giant cells were detected in the vessel wall, without granuloma formation. Lymphoid cells were not atypical and they were mostly reactive T lymphocytes (CD3-positive). IgG/IgG4 ratio was within the normal limits. No fungus structures or other microorganisms were identified. Our results are consistent with an intracranial extension of CIMDL.
DISCUSSION
With the widespread illicit use of cocaine, a broad spectrum of clinical pathologies related to this drug is emerging. One of the less known effects of cocaine use is its ability to induce several types of vasculitis, especially those that mimic ANCA-associated vasculitis (3).
The most frequently used administration method of cocaine is intranasal inhalation. Habitual nasal insufflations cause mucosal lesions and, if cocaine use becomes chronic, progressive damage of the mucosa and perichondrium can lead to ischemic necrosis and perforation of the septal cartilage. Occasionally, cocaine-induced lesions cause extensive destruction of the osteocartilaginous structures of the nose, sinuses, and palate, known as CIMDL, which mimic the clinical picture of other necrotizing midfacial diseases (4). Destruction of the neighboring skull base or craniovertebral junction is rare but has been described before (4). Importantly, our case is the second documented CIMDL extending through the cribriform plate into the brain (5), and the first report presenting as a tumor-like lesion by its space-occupying effect. As a consequence, this is the first time that a detailed brain histopathological analysis has been performed.
As previously stated, clinical differentiation between CIMDL and other diseases, such as infections, neoplasms, sarcoidosis, or GPA, may be very difficult. For instance, CIMDL can mimic invasive fungal rhinosinusitis. Invasive fungal diseases often occur in the context of immunosuppression or immunodeficiency, and the diagnosis is confirmed by specific histopathology, which shows fungal hyphae infiltrating mucosa or blood vessels (6). Given that our patient suffered from type 1 diabetes, an important step was to exclude rhino-cerebral mucormycosis. Multifocal demyelinating leukoencephalopathy induced by levamisole was also included in the differential diagnosis. The extensive meningeal and cortical involvement of the lesion, and the absence of white matter lesions separated from the frontal nucleus of the pathology made this diagnosis unlikely, in addition to the absence of many other features well described for levamisole-induced lesions (2, 7). CIMDL and limited GPA to the upper respiratory tract lesions may be clinically indistinguishable. The degrees of local destruction are usually much more significant in CIMDL compared with nasal involvement of GPA (4). Nonetheless, our patient presented with limited osteocartilaginous destruction compared with the extensive intracranial disease.
Serologic abnormalities can help to differentiate between CIMDL and GPA. Patients with GPA most often have ANCAs that generate a cytoplasmic ANCA staining pattern that react with proteinase 3. In our patient, ANCA tests resulted in positive cytoplasmic and perinuclear staining pattern for atypical antigens, a result highly specific for CIMDL (8, 9). However, such serologic findings are characteristic of CIMDL but not specific to this entity, since similar serologic profiles can be encountered in other drug-induced ANCA-associated vasculitides. For instance, levamisole-associated autoimmune disease shows atypical perinuclear ANCA-associated antigens within the neutrophil granules, such as human neutrophil elastase, lactoferrin, and cathepsin G (10).
To the best of our knowledge, there exist no reports of the histological analysis of cerebral CIMDL. This fact is probably due to the greater accessibility and lower morbidity that entails biopsying the upper airway and the scarceness of CIMDL cases with brain extension (5). In our case, the pseudotumoral presentation made it advisable to obtain brain biopsy to rule out other processes. We analyzed the histopathological alterations in our patient against those described in CIMDL series with intranasal biopsies and that of brain biopsies from cocaine-induced vasculitis (regardless of CIMDL spectrum). In both groups, despite being nonspecific, the findings were similar to our case: A perivascular inflammatory infiltrate and sporadically disruption of the vascular wall with fibrinoid necrosis, signs of small vessel vasculitis (1, 4, 5). A relevant difference with GPA (and others like sarcoidosis, reasonably included in the differential diagnosis of CIMDL) seems to be the absence of granuloma formation (4). The histopathological findings in our patient differ from other brain diseases related to cocaine consumption. For instance, cocaine-associated leukoencephalopathy is characterized by vacuolar degeneration of the deep white matter with relative sparing of the subcortical U fibers, whereas levamisole‐induced leukoencephalopathy is associated with multifocal demyelinating lesions (2, 11).
The pathogenesis of CIMDL remains poorly understood. Cocaine can cause the destruction of midline facial structures secondary to several mechanisms, including ischemic necrosis, cocaine-induced apoptosis, direct trauma during its administration, irritation by chemical adulterants, and/or recurrent nasal infections (4). However, given the widespread cocaine abuse on the one hand and the relative scarcity of CIMDL on the other hand, additional individual host factors must be key elements to develop CIMDL.
Thus, CIMDL has been proposed as cocaine-associated autoimmune phenomena, where the presence of ANCA distinguishes patients with CIMDL from individuals with similar cocaine use patterns without CIMDL (8). Specifically, the presence of ANCA may significantly enhance the local inflammatory response to injury. Bacterial superinfection (which has been documented in essentially all patients with CIMDL [4]) with superantigen-producing organisms may lead to ANCA production and long‐term persistence in predisposed individuals (12). Given the nature of the disease process, the augmented hypointensity on follow-up MRI, suggesting increased necrosis and cystic brain areas despite cocaine cessation, might be attributable to the persistence of circulating ANCA. Interestingly, autoantibodies are strongly associated with the development of type 1 diabetes (13). The presence of circulating autoantibodies related with diabetes might have been associated with an increased risk of CIMDL development, maintenance and/or brain tropism.
Cerebral ischemia from excessive cocaine use might have also been a major element in the development of extensive intracranial CIMDL in our patient. Brain imaging studies performed at initial presentation showed intensity changes in diffusion-weighted imaging (DWI) in the frontal left lobe together with a focal narrowing at the supraclinoid internal left carotid artery. These findings were consistent with acute infarction. In contrast, follow-up MRI findings one month later showed interval resolution of DWI hyperintensity. In agreement, it has been described that cocaine-induced cerebral vasculitis is a condition belonging to the group of reversible cerebral vasoconstriction syndromes (14), so reversibility of vascular abnormalities within days to weeks can be observed if cocaine consumption is stopped. Interestingly, it has been hypothesized that prolonged vasoconstriction could progress to secondary angiitis (15). Thus, the histological picture seen in our patient might be, at least in part, attributable to a combination of both the vasoconstrictive and proinflammatory effects of cocaine at the CNS.
In summary, CIMDL is the result of a necrotizing inflammatory tissue response triggered by cocaine abuse in a subset of predisposed patients. The reported case is the first CIMDL consistent with brain extension mimicking a tumor-like lesion. While the presence of atypical ANCAs seem to promote and/or define the disease phenotype, the specific role of these and other circulating autoantibodies needs further investigation.
The authors received no financial support for the research, authorship, and/or publication of this articleThe authors have no duality or conflicts of interest to declare.



