-
PDF
- Split View
-
Views
-
Cite
Cite
Inna Y Gong, Matthew C Cheung, Kelvin K W Chan, Sumedha Arya, Neil Faught, Andrew Calzavara, Ning Liu, Oreofe O Odejide, Gregory Abel, Paul Kurdyak, Michael J Raphael, Thomas Kuczmarski, Anca Prica, Lee Mozessohn, Mortality among patients with diffuse large B-cell lymphoma and mental disorders: a population-based study, JNCI: Journal of the National Cancer Institute, Volume 115, Issue 10, October 2023, Pages 1194–1203, https://doi.org/10.1093/jnci/djad149
Close - Share Icon Share
Abstract
Mental disorders have been reported in patients with diffuse large B-cell lymphoma (DLBCL), but studies examining their association with mortality are lacking.
We conducted a population-based study using linked administrative health-care databases from Ontario, Canada. All patients with DLBCL 18 years of age or older treated with rituximab-based therapy between January 1, 2005, and December 31, 2017, were identified and followed until March 1, 2020. Mental disorders were defined as either preexisting or postdiagnosis (after lymphoma treatment initiation). Cox proportional hazards models were used to estimate the adjusted hazard ratio (HR) between mental disorders and 1-year and all-cause mortality while controlling for covariates.
We identified 10 299 patients with DLBCL. The median age of the cohort was 67 years; 46% of patients were female, and 28% had a preexisting mental disorder. At 1-year follow-up, 892 (9%) had a postdiagnosis mental disorder, and a total of 2008 (20%) patients died. Preexisting mental disorders were not associated with 1-year mortality (adjusted HR = 1.06, 95% confidence interval [CI] = 0.96 to 1.17, P = .25), but postdiagnosis disorders were (adjusted HR = 1.51, 95% CI = 1.26 to 1.82, P = .0001). During a median follow-up of 5.2 years, 2111 (22%) patients had a postdiagnosis mental disorder, and 4084 (40%) patients died. Both preexisting and postdiagnosis mental disorders were associated with worse all-cause mortality (preexisting adjusted HR = 1.12, 95% CI = 1.04 to 1.20, P = .0024; postdiagnosis adjusted HR = 1.63, 95% CI = 1.49 to 1.79, P < .0001).
Patients with DLBCL and mental disorders had worse short-term and long-term mortality, particularly those with postdiagnosis mental disorders. Further studies are needed to examine mental health service utilization and factors mediating the relationship between mental disorders and inferior mortality.
The prevalence of mental disorders such as depression and anxiety in patients with cancer is high, with increased rates during the months before and after a cancer diagnosis (1-3). Similar to the well-established association between mental disorders and mortality in the primary psychiatric literature (4-9), several studies have shown this association in the solid cancer population (10-17). Whether mental disorders are associated with inferior survival, however, has been less well studied in patients with hematologic malignancies.
Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma (18,19). As survival outcomes of DLBCL improve over time, the management of physical and psychological well-being during treatment and survivorship has become increasingly important (20,21). Recent studies indicate a high prevalence of mental disorders in patients with DLBCL (22-24), which may be related to the psychological burden of an aggressive lymphoma diagnosis, uncertainties toward the cancer and its treatments, and fear of relapse and uncertain prognosis (25,26). Although studies have reported on the prevalence of mental disorders in patients with lymphoma (22-24,27-31), the literature on its association with mortality—most of which is studies with small sample sizes—is limited (30,32-35). Because of the limitations of the existing literature, we set out to examine the relationship between mental disorders and mortality risk in patients with newly diagnosed DLBCL.
Methods
Study design
We conducted a population-based, retrospective cohort study in which patients were linked to administrative databases using unique encoded identifiers from health card numbers. Data were analyzed at ICES, an independent nonprofit research institute whose legal status under Ontario’s health information policy law allows it to collect and analyze health-care and demographic data, without consent, for health system evaluation and improvement. Use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act and does not require review by a research ethics board.
Cohort creation
The study cohort consisted of Ontario residents 18 years of age or older with DLBCL (including transformed follicular lymphoma) who had received first-line curative-intent treatment with rituximab-containing chemoimmunotherapy, whereby therapy was identified through the New Drug Funding Program between January 1, 2005, and December 31, 2017. The cohort included only patients with confirmed histology showing aggressive B-cell lymphoma, identified through the Ontario Cancer Registry, using International Classification of Diseases for Oncology, Third Revision codes. The Ontario Cancer Registry is a provincewide cancer registry that has captured more than 95% of cancer diagnoses and all cancer deaths in Ontario residents since 1964 (36,37). The following exclusion criteria were applied: central nervous system DLBCL, HIV-associated lymphoma, invalid health card number, and a competing cancer diagnosis in the year before cohort entry. Date of cohort entry (ie, the index date) was defined as the date of the first dose of rituximab.
Exposure
The exposure was a mental disorder, defined as either an emergency department visit or hospitalization with a responsible discharge diagnosis for any of the mental disorders shown in Supplementary Table 1 (available online) or an outpatient visit to a primary care physician or psychiatry specialist for a mental disorder. Discharge diagnoses for emergency department visits or hospitalizations were identified using International Statistical Classification of Diseases, Tenth Revision codes (Supplementary Table 1, available online) and the following databases: Canadian Institute for Health Information Hospital Discharge Abstract Database, which contains data on all inpatient hospitalizations; the National Ambulatory Care Reporting System database, which contains information about hospital and community-based ambulatory care; and the Ontario Mental Health Reporting System, which contains data on patients in adult inpatient mental health beds. Mental health–related outpatient visits were identified using physician billing claims to the Ontario Health Insurance Plan database with a high sensitivity (81%) and specificity (97%), as previously validated (Supplementary Table 2, available online) (38).
Mental disorders were categorized as either preexisting (occurring within 2 years before the index date) or postdiagnosis (occurring after the index date in those without a prior history). A 2-year look-back period was chosen, as it is generally accepted for identification of preexisting cases (39). Subtypes of mental disorders were divided as follows: mood (including nonpsychotic disorders identified through physician billing codes), anxiety, substance use, or psychotic. For exploratory analyses, we examined the temporal relationship between mental disorders and all-cause mortality and defined the time frame of mental disorder occurrence relative to treatment as follows: preexisting, during treatment (start of treatment to 90 days after the last cycle), and after completion of treatment (>90 days since the last cycle of therapy).
Covariates
Demographic variables, including age, sex, and vital status, were identified from the Registered Persons Database. Patients were categorized as living in a rural location (<10 000 residents) based on postal code. Income quintile was assigned from Canadian Census data at the neighborhood level based on postal code, whereby the lowest quintile represents the lowest household income. Comorbidity burden was measured using the Johns Hopkins ACG System, version 10 (39), whereby patients were assigned to up to 32 Aggregated Diagnosis Groups to characterize their comorbidity burden in the 2 years before the index date (cancer Aggregated Diagnosis Groups were excluded).
Mortality outcome
The outcomes were 1-year mortality, all-cause mortality, and lymphoma-specific mortality. Patients were followed from the index date until death or the end of the study period (March 1, 2020), whichever occurred first. March 1, 2020, was chosen as the end of follow-up as the COVID-19 pandemic had widespread effects on health-care utilization in Ontario after this date.
Statistical analysis
Descriptive statistics of baseline characteristics are presented using mean (SD), medians (interquartile range), or frequency with percentage, where appropriate.
We used univariate and multivariable Cox hazards models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of mental disorders on mortality. A multivariable cause-specific model was used for lymphoma-specific mortality. Mental disorders were modeled as preexisting or postdiagnosis using a 2-level time-varying covariate. Preexisting mental disorders were coded as yes for the entire follow-up, whereas mental disorders diagnosed after the index date were coded as yes at the time of diagnosis. The hazard ratio was reported for both levels to evaluate their relative association with outcomes. Multivariable models adjusted for comorbidity burden using Aggregated Diagnosis Groups, age modeled in 10-year interval increments, rural residence status, and income quintile. All-cause mortality models were repeated for each subtype of mental disorder.
A sensitivity analysis was conducted excluding patients with transformed follicular lymphoma, given that prior lines of therapy and duration of disease may introduce heterogeneity and bias. In addition, a sensitivity analysis was conducted with preexisting mental disorders defined as within 5 years before the index date.
For exploratory analyses, univariate and multivariable Cox proportional hazards models, adjusting for treatment delay and number of treatment cycles, were carried out. Treatment delay was defined as a delay of more than 24 days between treatments to allow for variation in the standard 21-day schedule used in the setting of statutory holidays. Number of cycles was defined as a dichotomous variable with fewer than 3 vs 3 or more cycles of chemoimmunotherapy, with the former representing failure to complete planned treatment considering that 3 cycles may be a standard for patients with limited-stage disease.
To further delineate the temporal association of mental disorders and all-cause mortality, time-covariate interactions with restricted cubic splines were used to model the hazard ratios as functions of time (40).
For all analyses, a 2-tailed P < .05 was considered statistically significant. Analyses were conducted using SAS, version 9.4, statistical software (SAS Institute Inc, Cary, NC).
Results
Study population
The study cohort comprised 10 299 patients diagnosed with DLBCL between January 1, 2005, to December 31, 2017 (Table 1 summarizes baseline characteristics). Of these patients, the majority (95%) had de novo DLBCL, whereas 5% had transformed follicular lymphoma. Median (interquartile range) age was 67 (56-76) years, and 46% of the patients were women.
| Characteristic . | Patients with DLBCL (N = 10 299) . | No prior mental disorders (n = 7405) . | Prior mental disorders (n = 2894) . |
|---|---|---|---|
| Age, mean (SD) | 64 (15) | 65 (15) | 63 (15) |
| Female sex, No. (%) | 4727 (46) | 3208 (43) | 1519 (53) |
| Rural residence, No. (%) | 1365 (13) | 1059 (14) | 306 (11) |
| Income quintile, No. (%) | |||
| 1 | 1724 (17) | 1234 (17) | 490 (17) |
| 2 | 2046 (20) | 1491 (20) | 555 (19) |
| 3 | 2059 (20) | 1463 (20) | 596 (21) |
| 4 | 2160 (21) | 1532 (21) | 628 (22) |
| 5 | 2284 (22) | 1666 (23) | 618 (21) |
| Lymphoma subtype, No. (%) | |||
| DLBCL | 9756 (95) | 7035 (95) | 2721 (94) |
| Transformed follicular lymphoma | 543 (5) | 370 (5) | 173 (6) |
| Aggregated Diagnosis Groups, No. (%) | |||
| Low (0-5) | 1633 (16) | 1305 (18) | 328 (11) |
| Moderate (6-9) | 4635 (45) | 3446 (47) | 1189 (41) |
| High (≥10) | 4031 (39) | 2654 (36) | 1377 (48) |
| Aggregated Diagnosis Group quartile, No. (%) | |||
| 1 | 2620 (25) | 2044 (28) | 576 (20) |
| 2 | 3648 (35) | 2707 (37) | 941 (33) |
| 3 | 2004 (20) | 1371 (19) | 633 (22) |
| 4 | 2027 (20) | 1283 (17) | 744 (26) |
| Characteristic . | Patients with DLBCL (N = 10 299) . | No prior mental disorders (n = 7405) . | Prior mental disorders (n = 2894) . |
|---|---|---|---|
| Age, mean (SD) | 64 (15) | 65 (15) | 63 (15) |
| Female sex, No. (%) | 4727 (46) | 3208 (43) | 1519 (53) |
| Rural residence, No. (%) | 1365 (13) | 1059 (14) | 306 (11) |
| Income quintile, No. (%) | |||
| 1 | 1724 (17) | 1234 (17) | 490 (17) |
| 2 | 2046 (20) | 1491 (20) | 555 (19) |
| 3 | 2059 (20) | 1463 (20) | 596 (21) |
| 4 | 2160 (21) | 1532 (21) | 628 (22) |
| 5 | 2284 (22) | 1666 (23) | 618 (21) |
| Lymphoma subtype, No. (%) | |||
| DLBCL | 9756 (95) | 7035 (95) | 2721 (94) |
| Transformed follicular lymphoma | 543 (5) | 370 (5) | 173 (6) |
| Aggregated Diagnosis Groups, No. (%) | |||
| Low (0-5) | 1633 (16) | 1305 (18) | 328 (11) |
| Moderate (6-9) | 4635 (45) | 3446 (47) | 1189 (41) |
| High (≥10) | 4031 (39) | 2654 (36) | 1377 (48) |
| Aggregated Diagnosis Group quartile, No. (%) | |||
| 1 | 2620 (25) | 2044 (28) | 576 (20) |
| 2 | 3648 (35) | 2707 (37) | 941 (33) |
| 3 | 2004 (20) | 1371 (19) | 633 (22) |
| 4 | 2027 (20) | 1283 (17) | 744 (26) |
DLBCL = diffuse large B-cell lymphoma.
| Characteristic . | Patients with DLBCL (N = 10 299) . | No prior mental disorders (n = 7405) . | Prior mental disorders (n = 2894) . |
|---|---|---|---|
| Age, mean (SD) | 64 (15) | 65 (15) | 63 (15) |
| Female sex, No. (%) | 4727 (46) | 3208 (43) | 1519 (53) |
| Rural residence, No. (%) | 1365 (13) | 1059 (14) | 306 (11) |
| Income quintile, No. (%) | |||
| 1 | 1724 (17) | 1234 (17) | 490 (17) |
| 2 | 2046 (20) | 1491 (20) | 555 (19) |
| 3 | 2059 (20) | 1463 (20) | 596 (21) |
| 4 | 2160 (21) | 1532 (21) | 628 (22) |
| 5 | 2284 (22) | 1666 (23) | 618 (21) |
| Lymphoma subtype, No. (%) | |||
| DLBCL | 9756 (95) | 7035 (95) | 2721 (94) |
| Transformed follicular lymphoma | 543 (5) | 370 (5) | 173 (6) |
| Aggregated Diagnosis Groups, No. (%) | |||
| Low (0-5) | 1633 (16) | 1305 (18) | 328 (11) |
| Moderate (6-9) | 4635 (45) | 3446 (47) | 1189 (41) |
| High (≥10) | 4031 (39) | 2654 (36) | 1377 (48) |
| Aggregated Diagnosis Group quartile, No. (%) | |||
| 1 | 2620 (25) | 2044 (28) | 576 (20) |
| 2 | 3648 (35) | 2707 (37) | 941 (33) |
| 3 | 2004 (20) | 1371 (19) | 633 (22) |
| 4 | 2027 (20) | 1283 (17) | 744 (26) |
| Characteristic . | Patients with DLBCL (N = 10 299) . | No prior mental disorders (n = 7405) . | Prior mental disorders (n = 2894) . |
|---|---|---|---|
| Age, mean (SD) | 64 (15) | 65 (15) | 63 (15) |
| Female sex, No. (%) | 4727 (46) | 3208 (43) | 1519 (53) |
| Rural residence, No. (%) | 1365 (13) | 1059 (14) | 306 (11) |
| Income quintile, No. (%) | |||
| 1 | 1724 (17) | 1234 (17) | 490 (17) |
| 2 | 2046 (20) | 1491 (20) | 555 (19) |
| 3 | 2059 (20) | 1463 (20) | 596 (21) |
| 4 | 2160 (21) | 1532 (21) | 628 (22) |
| 5 | 2284 (22) | 1666 (23) | 618 (21) |
| Lymphoma subtype, No. (%) | |||
| DLBCL | 9756 (95) | 7035 (95) | 2721 (94) |
| Transformed follicular lymphoma | 543 (5) | 370 (5) | 173 (6) |
| Aggregated Diagnosis Groups, No. (%) | |||
| Low (0-5) | 1633 (16) | 1305 (18) | 328 (11) |
| Moderate (6-9) | 4635 (45) | 3446 (47) | 1189 (41) |
| High (≥10) | 4031 (39) | 2654 (36) | 1377 (48) |
| Aggregated Diagnosis Group quartile, No. (%) | |||
| 1 | 2620 (25) | 2044 (28) | 576 (20) |
| 2 | 3648 (35) | 2707 (37) | 941 (33) |
| 3 | 2004 (20) | 1371 (19) | 633 (22) |
| 4 | 2027 (20) | 1283 (17) | 744 (26) |
DLBCL = diffuse large B-cell lymphoma.
Mental disorders
In our cohort, 2887 patients (28%) had a preexisting mental disorder, of which 677 (7%) were mood disorders, 2384 (23%) were anxiety disorders, 123 (1%) were substance user disorders, and 154 (2%) were psychotic disorders. During follow-up, 2211 (22%) patients had a postdiagnosis mental disorder, of which 955 (10%) were mood disorders, 1939 (19%) were anxiety disorders, 153 (2%) were substance user disorders, and 218 (2%) were psychotic disorders.
Mortality outcome
During the first year of treatment, 2008 patients died. Of these, 570 (28.4%) had a preexisting mental disorder, and 128 (6.4%) had a postdiagnosis mental disorder. In the univariate analysis for 1-year mortality, preexisting mental disorders were not statistically significantly associated with survival (HR = 1.05, 95% CI = 0.95 to 1.15, P = .37), whereas postdiagnosis mental disorders were statistically significantly associated with inferior survival (HR = 1.51, 95% CI = 1.26 to 1.81, P < .0001). In multivariable modeling, controlling for age, sex, comorbidity burden, and income quintile, preexisting mental disorders were similarly not statistically significantly associated with 1-year mortality (adjusted HR = 1.06, 95% CI = 0.96 to 1.17, P = .3), but postdiagnosis mental disorders were (adjusted HR = 1.51, 95% CI = 1.26 to 1.81, P < .0001) (Table 2). Older age, greater comorbidity burden, and male sex were also associated with greater 1-year mortality (Supplementary Table 3, available online).
Multivariable models examining the association between mental disorders and mortality
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| 1-y mortalitya | |||
| Mental health | Preexisting vs none | 1.06 (0.96 to 1.17) | .3 |
| Postdiagnosis vs none | 1.51 (1.26 to 1.82) | <.0001 | |
| All-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.12 (1.04 to 1.20) | .002 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Model, adjusting for treatment delay in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.04 to 1.20) | .004 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Treatment delay | 1.24 (1.16 to 1.32) | <.0001 | |
| Model, adjusting for No. of cycles in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.03 to 1.19) | .005 |
| Postdiagnosis vs none | 1.57 (1.44 to 1.73) | <.0001 | |
| No. of cycles | ≥3 vs <3 | 0.40 (0.36 to 0.44) | <.0001 |
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| 1-y mortalitya | |||
| Mental health | Preexisting vs none | 1.06 (0.96 to 1.17) | .3 |
| Postdiagnosis vs none | 1.51 (1.26 to 1.82) | <.0001 | |
| All-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.12 (1.04 to 1.20) | .002 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Model, adjusting for treatment delay in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.04 to 1.20) | .004 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Treatment delay | 1.24 (1.16 to 1.32) | <.0001 | |
| Model, adjusting for No. of cycles in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.03 to 1.19) | .005 |
| Postdiagnosis vs none | 1.57 (1.44 to 1.73) | <.0001 | |
| No. of cycles | ≥3 vs <3 | 0.40 (0.36 to 0.44) | <.0001 |
Models adjusted for age, sex, rural residence, Aggregated Diagnosis Group, and income quintile. CI = confidence interval; HR = hazard ratio.
Multivariable models examining the association between mental disorders and mortality
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| 1-y mortalitya | |||
| Mental health | Preexisting vs none | 1.06 (0.96 to 1.17) | .3 |
| Postdiagnosis vs none | 1.51 (1.26 to 1.82) | <.0001 | |
| All-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.12 (1.04 to 1.20) | .002 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Model, adjusting for treatment delay in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.04 to 1.20) | .004 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Treatment delay | 1.24 (1.16 to 1.32) | <.0001 | |
| Model, adjusting for No. of cycles in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.03 to 1.19) | .005 |
| Postdiagnosis vs none | 1.57 (1.44 to 1.73) | <.0001 | |
| No. of cycles | ≥3 vs <3 | 0.40 (0.36 to 0.44) | <.0001 |
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| 1-y mortalitya | |||
| Mental health | Preexisting vs none | 1.06 (0.96 to 1.17) | .3 |
| Postdiagnosis vs none | 1.51 (1.26 to 1.82) | <.0001 | |
| All-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.12 (1.04 to 1.20) | .002 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Model, adjusting for treatment delay in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.04 to 1.20) | .004 |
| Postdiagnosis vs none | 1.63 (1.49 to 1.79) | <.0001 | |
| Treatment delay | 1.24 (1.16 to 1.32) | <.0001 | |
| Model, adjusting for No. of cycles in all-cause mortalitya | |||
| Mental health | Preexisting vs none | 1.11 (1.03 to 1.19) | .005 |
| Postdiagnosis vs none | 1.57 (1.44 to 1.73) | <.0001 | |
| No. of cycles | ≥3 vs <3 | 0.40 (0.36 to 0.44) | <.0001 |
Models adjusted for age, sex, rural residence, Aggregated Diagnosis Group, and income quintile. CI = confidence interval; HR = hazard ratio.
During a median (interquartile range) follow-up of 5.2 (1-8) years, 4093 (40%) patients died. Of these, 1153 (28.2%) had a preexisting mental disorder, and 656 (16.0%) had a postdiagnosis mental disorder. On univariate analysis, preexisting mental disorders were not statistically significantly associated with all-cause mortality (HR = 1.06, 95% CI = 0.99 to 1.14, P = .09), but postdiagnosis mental disorders were (HR = 1.58, 95% CI = 1.44 to 1.74, P < .0001). On multivariable analysis, both preexisting and postdiagnosis mental disorders were independently associated with all-cause mortality (preexisting adjusted HR = 1.12, 95% CI = 1.04 to 1.20, P = .002; postdiagnosis adjusted HR = 1.63, 95% CI = 1.49 to 1.79, P < .0001) (Table 2). Similar to 1-year mortality, covariates associated with worse all-cause mortality included older age, higher comorbidity burden, lower income quintile, and male sex (Supplementary Table 4, available online). The statistically significant association between postdiagnosis mental disorders were similarly observed for lymphoma-specific mortality (adjusted HR = 1.52, 95% CI = 1.36 to 1.70, P < .0001). Sensitivity analysis, excluding patients with transformed follicular lymphoma, did not change these findings (Supplementary Tables 5 and 6, available online). Similarly, a sensitivity analysis using a 5-year look-back period for defining preexisting mental disorders did not alter these results (Supplementary Table 7, available online).
Subtypes of mental disorders and all-cause mortality
Table 3 summarizes the association between subtypes of mental disorders and all-cause mortality. Postdiagnosis anxiety disorders were statistically significantly associated with inferior all-cause mortality (adjusted HR = 1.38, 95% CI = 1.25 to 1.52, P = .0001), but preexisting anxiety disorders were not (adjusted HR = 1.04, 95% CI = 0.97 to 1.13, P = .27). Similarly, postdiagnosis mood disorders were statistically significantly associated with inferior all-cause mortality (adjusted HR = 1.46, 95% CI = 1.28 to 1.67, P = .0001), but preexisting disorders were not (adjusted HR = 1.06, 95% CI = 0.93 to 1.19, P = .4). For psychotic disorders, both preexisting (adjusted HR = 1.39, 95% CI = 1.09 to 1.78, P = .008), and postdiagnosis mental disorders were statistically significantly associated with inferior all-cause mortality (adjusted HR = 1.75, 95% CI = 1.37 to 2.24, P = .0001). In contrast, for substance user disorders, preexisting disorders were statistically significantly associated with inferior all-cause mortality (adjusted HR = 1.49, 95% CI = 1.14 to 1.94, P = .003), but postdiagnosis disorders were not (adjusted HR = 0.99, 95% CI = 0.67 to 1.47, P = .97).
Multivariable models examining the association between subtypes of mental disorders and mortality, adjusting for clinical characteristics
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| Overall survivala | |||
| Anxiety | Preexisting vs none | 1.04 (0.97 to 1.13) | .3 |
| Postdiagnosis vs none | 1.38 (1.25 to 1.52) | <.0001 | |
| Mood | Preexisting vs none | 1.06 (0.93 to 1.19) | .4 |
| Postdiagnosis vs none | 1.46 (1.28 to 1.67) | <.0001 | |
| Substance use | Preexisting vs none | 1.49 (1.14 to 1.94) | .003 |
| Postdiagnosis vs none | 0.99 (0.67 to 1.47) | .97 | |
| Psychotic | Preexisting vs none | 1.39 (1.09 to 1.78) | .008 |
| Postdiagnosis vs none | 1.75 (1.37 to 2.24) | <.0001 | |
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| Overall survivala | |||
| Anxiety | Preexisting vs none | 1.04 (0.97 to 1.13) | .3 |
| Postdiagnosis vs none | 1.38 (1.25 to 1.52) | <.0001 | |
| Mood | Preexisting vs none | 1.06 (0.93 to 1.19) | .4 |
| Postdiagnosis vs none | 1.46 (1.28 to 1.67) | <.0001 | |
| Substance use | Preexisting vs none | 1.49 (1.14 to 1.94) | .003 |
| Postdiagnosis vs none | 0.99 (0.67 to 1.47) | .97 | |
| Psychotic | Preexisting vs none | 1.39 (1.09 to 1.78) | .008 |
| Postdiagnosis vs none | 1.75 (1.37 to 2.24) | <.0001 | |
Models adjusted for age, sex, rural residence, Aggregated Diagnosis Group, and income quintile. CI = confidence interval; HR = hazard ratio.
Multivariable models examining the association between subtypes of mental disorders and mortality, adjusting for clinical characteristics
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| Overall survivala | |||
| Anxiety | Preexisting vs none | 1.04 (0.97 to 1.13) | .3 |
| Postdiagnosis vs none | 1.38 (1.25 to 1.52) | <.0001 | |
| Mood | Preexisting vs none | 1.06 (0.93 to 1.19) | .4 |
| Postdiagnosis vs none | 1.46 (1.28 to 1.67) | <.0001 | |
| Substance use | Preexisting vs none | 1.49 (1.14 to 1.94) | .003 |
| Postdiagnosis vs none | 0.99 (0.67 to 1.47) | .97 | |
| Psychotic | Preexisting vs none | 1.39 (1.09 to 1.78) | .008 |
| Postdiagnosis vs none | 1.75 (1.37 to 2.24) | <.0001 | |
| Variable . | Level . | Multivariable HR (95% CI) . | P . |
|---|---|---|---|
| Overall survivala | |||
| Anxiety | Preexisting vs none | 1.04 (0.97 to 1.13) | .3 |
| Postdiagnosis vs none | 1.38 (1.25 to 1.52) | <.0001 | |
| Mood | Preexisting vs none | 1.06 (0.93 to 1.19) | .4 |
| Postdiagnosis vs none | 1.46 (1.28 to 1.67) | <.0001 | |
| Substance use | Preexisting vs none | 1.49 (1.14 to 1.94) | .003 |
| Postdiagnosis vs none | 0.99 (0.67 to 1.47) | .97 | |
| Psychotic | Preexisting vs none | 1.39 (1.09 to 1.78) | .008 |
| Postdiagnosis vs none | 1.75 (1.37 to 2.24) | <.0001 | |
Models adjusted for age, sex, rural residence, Aggregated Diagnosis Group, and income quintile. CI = confidence interval; HR = hazard ratio.
Exploratory analyses
To examine whether the inferior all-cause mortality in patients with mental disorders may be attributed to treatment delays or failure to complete primary treatment, models adjusting for these variables were separately conducted. In the multivariable model, adjusting for treatment delay, mental disorders remained independently statistically significantly associated with inferior all-cause mortality (postdiagnosis mental disorders adjusted HR = 1.63, 95% CI = 1.49 to 1.79, P < .0001; preexisting mental disorders adjusted HR = 1.11, 95% CI = 1.04 to 1.20, P = .004). Similarly, in the model adjusting for fewer than 3 vs 3 or more cycles, mental disorders remained independently statistically significantly associated with inferior all-cause mortality (postdiagnosis mental disorders adjusted HR = 1.57, 95% CI = 1.44 to 1.73, P < .0001; preexisting mental disorders adjusted HR = 1.11, 95% CI = 1.03 to 1.19, P = .005). No association was found between mental disorders and treatment delay or time to completion of third chemoimmunotherapy cycle (data not shown).
Figure 1 shows the estimated cumulative incidence of all-cause mortality after each year of follow-up in patients without a mental disorder and those with a mental disorder diagnosed at various time points. Patients with a preexisting mental disorder and those with a mental disorder diagnosed during therapy had a similar association with all-cause mortality, and incidence rates approach those without mental disorders, despite a greater proportion of patients without a mental disorder, suggesting excess mortality. Interestingly, patients diagnosed with a mental disorder after completion of treatment had the highest relative mortality (adjusted HR = 1.94, 95% CI = 1.73 to 2.16, P < .0001) (Figure 1). Mortality was lower for patients diagnosed before treatment initiation (adjusted HR = 1.12, 95% CI = 1.04 to 1.21, P = .002) and during treatment (adjusted HR = 1.25, 95% CI = 1.09 to 1.45, P = .002).
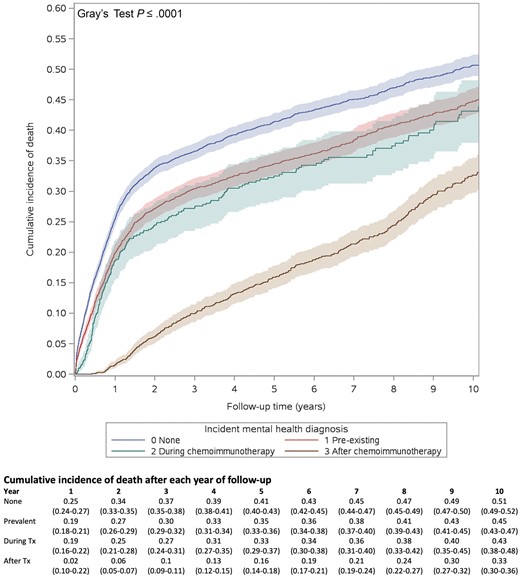
Cumulative incidence of death in patients with diffuse large B-cell lymphoma by time frame of mental disorder occurrence relative to treatment (no preexisting or postdiagnosis mental disorder, preexisting mental disorder, mental disorder occurring during or after chemoimmunotherapy). Shaded areas indicate 95% confidence intervals. Tx = treatment.
Figure 2 depicts the hazard ratio for the association between mental disorders and all-cause mortality as a function of time, modeled using restricted cubic splines, to examine the temporal dynamics of the hazard ratio over time (41). For those with a preexisting mental disorder or a postdiagnosis mental disorder during treatment, we observed that the hazard ratio for survival was less than 1 between 0 and 3 years after the index date and increased slightly over time at 4 years and beyond (the 10-year hazard ratios for preexisting mental disorders and postdiagnosis mental disorders during treatment were 1.14 [95% CI = 0.89 to 1.46] and 1.22 [95% CI = 0.78 to 1.89]) (Figure 2). For patients with a postdiagnosis mental disorder after completion of treatment, we observed a substantial increase in risk at 6 years of follow-up (HR = 1.53, 95% CI = 1.28 to 1.83) that persisted up to 10 years, with HR = 1.66 (95% CI = 1.30 to 2.11) (Figure 2).
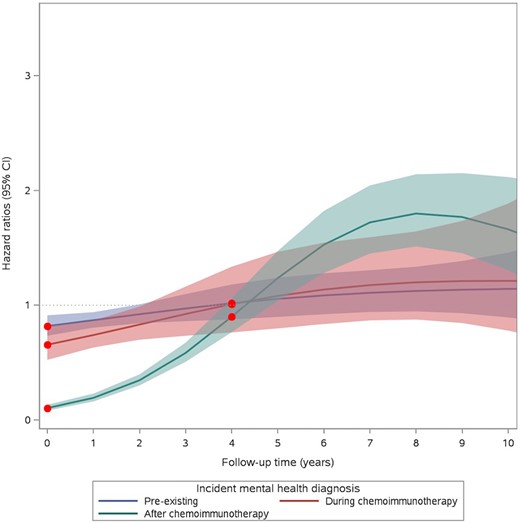
Estimated hazard ratios as a function of time, modeled using restricted cubic splines during follow-up for the association between mental disorders and all-cause mortality in patients with diffuse large B-cell lymphoma. Shaded areas indicate 95% confidence intervals (CIs).
Discussion
In this large population-based study of more than 10 000 patients with newly diagnosed DLBCL, more than one-quarter of patients had a preexisting mental disorder, and 1 in 5 had a postdiagnosis mental disorder, indicating a high burden of mental disorders in this patient population. We found that although postdiagnosis mental disorders were associated with worse short-term (1-year) and long-term mortality, preexisting mental disorders were associated with all-cause mortality only. Furthermore, our results suggest that mental disorders diagnosed after therapy completion had the highest relative mortality. Our study adds to the limited literature in its evaluation of both preexisting and postdiagnosis mental disorders and their temporal association with mortality.
The high prevalence of mental disorders reported here is congruent with prior reports, which demonstrate a greater prevalence of mental disorders in patients with lymphoma than in the general population (42-44) and even compared with other cancer survivors (45). In the primary psychiatric literature, numerous studies have reported on the association between mental disorders and mortality (4-9), including specific mental health disorders such as schizophrenia (8), depression (46), and bipolar disorder (47). The association has also been observed in the cancer population with greater all-cause and cancer-related mortality (48-51), despite similar incidence of cancer in patients with mental disorders compared with the general population (48).
In patients with hematologic malignancies, only a few existing studies have examined the association between mental disorders and mortality. An association with inferior survival has been demonstrated in patients with Hodgkin lymphoma or leukemia and in stem cell transplant populations (30,32-35). The only study examining the association between mental disorders and outcomes in patients with DLBCL, however, is a recently published study by Kuczmarski et al. that showed that preexisting depression and anxiety predicted poor overall survival and lymphoma-specific survival (52). Although this study was a large population-based study similar to ours, their study was limited to patients 67 years of age or older and examined only preexisting mental disorders, whereas ours had no age restriction and examined the relative association of preexisting and postdiagnosis mental disorders with mortality, which makes our study a unique contribution to the existing literature. We found that postdiagnosis mental disorders had a higher relative risk of all-cause mortality than preexisting mental disorders, a finding that warrants further exploration of potential mechanisms and their impact on management. Here, whether treatment delays and an inability to complete primary treatment mediated the association of mental disorders with mortality was explored. Despite adjusting for these factors, postdiagnosis mental disorders were independently associated with worse all-cause mortality, suggesting that the poor outcomes were not related to undertreatment or poor treatment compliance.
We examined the temporal dynamics of the association between mental disorders and mortality. Interestingly, patients with mental disorders diagnosed during treatment had lower mortality, which we postulate may be a result of earlier recognition and intervention for the mental disorders. Patients who had a mental disorder after end of treatment had the highest relative association with mortality, which remained high up to 10 years after diagnosis, whereas the number of mental disorders during treatment was relatively small. This finding suggests that the association between mental disorders and inferior survival cannot be explained by an inability to complete therapy. Given that the relative risk was only slightly attenuated for lymphoma-specific death, 1 possible explanation is that the higher mortality in patients with mental disorders after treatment completion may be related to lower adherence to follow-up recommendations for DLBCL relapse, which requires further study. Nevertheless, given the limitations of our dataset, we cannot rule out the possibility of reverse causation—that is, a new diagnosis of a mental disorder may be correlated with higher-risk disease or poorer response to treatment.
Although the precise reason for inferior survival could not be delineated from our study, we can postulate several explanations. Cancer survival is dependent on early diagnosis, timely access to effective therapies, and adherence to surveillance recommendations. It may be that a lower likelihood of participation in regular health screening leads to more advanced disease at presentation in patients with mental disorders (53-55). Additional reasons include inequitable access to health-care services, which may be in part the result of lower socioeconomic status, lower education, rural residency, and lower health literacy (56). Moreover, lifestyle and behavioral differences may contribute, as individuals with mental disorders may partake in less physical inactivity and have higher rates of malnutrition (57), and they may potentially be less adherent to follow-up (58), all of which could increase the morbidity and mortality associated with cancer. Finally, from a biologic perspective, mental disorders may lead to an abnormal activation of the hypothalamic-pituitary-adrenal axis, resulting in high levels of cortisol and consequent suppression of immune responses against cancer (59). Mental disorders such as depression and anxiety have also been associated with inflammatory markers such as C-reactive protein and interleukin 6, which may contribute to tumorigenesis and poorer prognosis (60,61).
Irrespective of the mechanism for inferior survival, our study supports existing guidelines for integrating psychological assessment and management into cancer care and highlights the need for vigilance of mental disorders at the time of cancer diagnosis during treatment and follow-up (62,63). Strategies to reduce excess mortality in patients with cancer and mental disorders should be identified and implemented. These measures may include increased screening and prevention, expanded access to care, and the fostering of healthier behavior and safer living conditions. In the oncology clinic, important strategies may include increasing physician awareness for recognizing mental disorders, integrated care between psychiatrists/psychologists and oncologists to diagnose and implement mental health–directed interventions and treatments, and prompt screening and management of chronic medical conditions (64,65).
Our study has several limitations. An important limitation is that the definition of mental disorders herein may underestimate the true burden of mental disorders. For instance, we could not identify care from nonphysicians practitioners, such as social workers and psychologists. Moreover, outpatient visits are ascertained through physician billing claims, which may underestimate the rate of outpatient visits if the claim is not filed for mild psychological concerns or if the visit was focused on another medical concern. Furthermore, patients with mental health–related concerns may not have adequate access to mental health services. The crude percentage of patients with a preexisting mental disorder was higher (28%) than the percentage of patients with a postdiagnosis mental disorder (22%). It is important to note that patient behavior and follow-up care are different before and after DLBCL diagnosis, and the circumstances whereby a mental disorder comes to medical attention is also likely to differ between these 2 periods. Although the reason for the lower percentage of mental disorders during the postdiagnosis period is not evident, we caution interpretation of these percentages because they are crude calculations. We were unable to account for ethnicity; education level; and lifestyle factors, such as diet, smoking, alcohol use, and type of substance use. As such, we were unable to study their association with mortality nor adjust for these in the models, which may be sources of residual confounding.
This study is also limited by lack of granularity of clinical data. There may also be residual confounding from unrecognized and unavailable factors, such as stage/disease burden at diagnosis; prognostic scores, such as the International Prognostic Index score; time from diagnosis to treatment initiation; treatment response; and relapse/refractory disease during follow-up, all of which are known to be associated with survival in patients with DLBCL. Our study could not delineate the exact mechanism for inferior survival among those with mental disorders in patients with DLBCL, and importantly, we could not determine whether the relationship is causal. To this end, future studies are warranted to examine potential factors that may mediate the relationship between mental disorders and survival. Our study included patients who initiated curative-intent treatment, and we were not able to examine the association between mental disorders on likelihood of therapy initiation. We could not examine the adherence to care and specialist visits, which may have also affected outcomes. Although prior studies have defined mental health events by including those who were receiving psychotropic medications (23,66), because of data availability (limited to individuals ≥65 years old), we were unable to ascertain all patients receiving psychotropic medications. As such, we could not adjust for psychotropic medication use in our models or examine whether such use could mitigate and reduce the associated risk between mental disorders and inferior outcomes.
Despite these limitations, our study supports the association between mental disorders and worse outcomes in patients with DLBCL; it also highlights the need for future studies to delineate the causal pathway between mental disorders and mortality. Moreover, a better understanding of mental health service utilization and health-care utilization in these patients compared with the general population is required. Characterization of utilization patterns may provide strategies on improving mental health and influences of mental health on adverse health outcomes.
In this large population-based study of more than 10 000 patients with DLBCL receiving curative-intent therapy, patients with mental disorders were associated with all-cause and lymphoma-specific mortality. This study highlights the importance of regular mental disorder assessments at diagnosis and during treatment. Future studies are required to delineate health-care utilization, access to care, and frequency of monitoring and surveillance in patients with DLBCL and mental disorders.
Data availability
The dataset from this study is held securely in coded form at ICES. Although legal data sharing agreements between ICES and providers (eg, health-care organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Author contributions
Inna Ying Gong, MD, PhD (Investigation; Methodology; Writing—original draft; Writing—review & editing), Matthew C. Cheung, MD, MSc (Conceptualization; Funding acquisition; Methodology; Resources; Supervision; Writing—original draft; Writing—review & editing), Kelvin K. W. Chan, MD, MSc, PhD (Conceptualization; Writing—original draft; Writing—review & editing), Sumedha Arya, MD (Writing—original draft; Writing—review & editing), Neil Faught, MSc (Formal analysis; Writing—review & editing), Andrew Calzavara, MSc (Formal analysis; Writing—review & editing), Ning Liu, PhD (Methodology; Writing—review & editing), Oreofe O. Odejide, MD, MPH (Writing—review & editing), Gregory Abel, MD, MPH (Writing—review & editing), Paul Kurdyak, MD, PhD (Writing—review & editing), Michael J. Raphael, MD, MSc (Writing—review & editing), Thomas Kuczmarski, MD (Writing—review & editing), Anca Prica, MD (Writing—review & editing), Lee Mozessohn, MD, MPH (Conceptualization; Methodology; Resources; Supervision; Writing—original draft; Writing—review & editing).
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Dr Cheung is supported by philanthropy funding from Roy and Marjorie Linden.
Conflicts of interest
The authors have nothing to disclose.
Acknowledgements
We thank Dr Christopher M. Booth for his expertise and contribution to manuscript review.
This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information, Ontario Health, the Ontario Ministry of Health, and the Ontario Registrar General information on deaths, the original source of which is ServiceOntario. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources, including the Ministry of Public and Business Service Delivery; no endorsement is intended or should be inferred. We thank IQVIA Solutions Canada Inc for use of their Drug Information File.
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; or the writing of the manuscript and decision to submit it for publication.


