-
PDF
- Split View
-
Views
-
Cite
Cite
N. W. Beebe, A. F. van den Hurk, H. F. Chapman, S. P. Frances, C. R. Williams, R. D. Cooper, Development and Evaluation of a Species Diagnostic Polymerase Chain Reaction-Restriction Fragment-Length Polymorphism Procedure for Cryptic Members of the Culex sitiens (Diptera: Culicidae) Subgroup in Australia and the Southwest Pacific , Journal of Medical Entomology, Volume 39, Issue 2, 1 March 2002, Pages 362–369, https://doi.org/10.1603/0022-2585-39.2.362
Close - Share Icon Share
Abstract
Members of the Culex sitiens subgroup are important vectors of arboviruses, including Japanese encephalitis virus, Murray Valley encephalitis virus and Ross River virus. Of the eight described species, Cx. annulirostris Skuse, Cx. sitiens Wiedemann, and Cx. palpalis Taylor appear to be the most abundant and widespread throughout northern Australia and Papua New Guinea (PNG). Recent investigations using allozymes have shown this subgroup to contain cryptic species that possess overlapping adult morphology. We report the development of a polymerase chain reaction-restriction fragment-length polymorphism (PCR-RFLP) procedure that reliably separates these three species. This procedure utilizes the sequence variation in the ribosomal DNA ITS1 and demonstrates species-specific PCR-RFLP profiles from both colony and field collected material. Assessment of the consistency of this procedure was undertaken on mosquitoes sampled from a wide geographic area including Australia, PNG, and the Solomon Islands. Overlapping adult morphology was observed for Cx. annulirostris and Cx. palpalis in both northern Queensland and PNG and for all three species at one site in northwest Queensland.
The evolution of mosquito populations into separate species can be subtle and may not be accompanied by overt changes in external morphology, creating cryptic species groups. It is now known that many important vector taxa consist of closely related cryptic species which, when examined under a microscope, are morphologically identical (sibling or isomorphic species) or are polymorphic for characters that were previously thought to be diagnostic. The presence of cryptic species within medically important groups of mosquitoes confounds previous studies of behavior and biology and may compromise vector control efforts. Consequently, the elucidation of species status, natural mating barriers, and the identification of intraspecific populations is vital to any study of vector borne disease.
In the Australasian zoogeographical region, the Culex sitiens subgroup includes several important vectors of arboviruses. This subgroup contains eight species that are currently described using morphological characters (Lee et al. 1989). The most abundant, widespread and medically important species within this subgroup are Culex annulirostris Skuse, Culex sitiens Wiedemann, and Culex palpalis Taylor. These three species also appear to be the most morphologically ambiguous (Marks 1982a, Chapman et al. 2000). However, no study on the genetic and morphology status of the other five less common members has been carried out. In Australia, Cx. annulirostris is the major vector of arboviruses, including Ross River (RR), Murray Valley encephalitis (MVE), and Kunjin viruses (Mackenzie et al. 1998, Russell 1998). More recently, this species has been identified as the primary vector of the Japanese encephalitis (JE) virus during outbreaks in north Queensland in 1995 and 1998 (Ritchie et al. 1997, Johansen et al. 2001). In Australia, Cx. sitiens is a vector of RR, MVE, and other arboviruses (Russell 1998), whereas in Southeast Asia it has been implicated as a vector of JE virus (Vythilingham et al. 1994). With Cx. palpalis, MVE virus has been isolated from this species collected in Western Australia (Mackenzie et al. 1994). During investigations of the source of the JE virus incursions into northern Queensland, an isolate of JE virus was obtained from Cx. sitiens subgroup mosquitoes collected from the Western Province of Papua New Guinea (PNG) (Johansen et al. 2000). Allozyme analysis of a sample of these mosquitoes demonstrated that 88% of the collection was Cx. palpalis and 12% Cx. annulirostris (Chapman et al. 2000); therefore, it is possible that Cx. palpalis may also be involved in JE transmission.
The separation of Cx. annulirostris, Cx. sitiens, and Cx. palpalis adults using morphological characteristics has always been difficult (Marks 1982a). Chapman et al. (2000) designed an electrophoretic method for identifying these species and verified that a certain degree of polymorphism existed in the morphological characters used to separate them. Chapman et al. (2000) also identified the possible existence of separate populations within these species based on allozyme frequencies but could not resolve the issue any further. Additionally, allozyme analysis requires mosquito material to be stored at below -50°C; this is logistically difficult when working in remote regions. The importance of these species in the transmission of arboviruses requires that a method be available to accurately identify the different species.
DNA-based diagnostic methods are now required to identify species from this subgroup from field collections with emphasis on the procedure being robust, simple, and consistent over the large geographic range of these species. The ribosomal DNA (rDNA) region of the mosquito genome has received particular attention with regard to mosquito taxonomy and systematics as the polymorphic nature of rDNA spacers can allow evaluation of population structures and in turn, the identification of species (Collins and Paskewitz 1996, Beebe and Cooper 2000). The rDNA consists of an array of tandemly repeated transcribed units composed of structural genes (28S, 5.8S, and 18S) separated by an intergenetic spacer (IGS), external transcribed spacer (ETS) and two internal transcribed spacer sequences (ITS1 and ITS2). Thus, within this gene family there are several regions of varying conservation and complexity which may allow interspecific and intraspecific evaluation of mosquito populations.
Mosquitoes in the Culex vishnui subgroup are vectors of JE virus in Asia and are closely related to the Cx. sitiens subgroup (Lee et al. 1989). An allele-specific polymerase chain reaction (PCR) diagnostic procedure, based on DNA sequence variation in the rDNA ITS1, has been developed to separate the cryptic species within this subgroup (Toma et al. 2000). However, this procedure has not yet been tested on field-collected material over the geographical range of the species involved. Problems may subsequently arise as the internal transcribed spacers of Culex mosquitoes appear to be relatively polymorphic (Crabtree et al. 1995, Miller et al. 1996, Toma et al. 2000), which may reduce the effectiveness of allele-specific primers that are designed to these polymorphic regions.
In this article the rDNA ITS1 spacer is examined as a molecular marker to develop a species-diagnostic PCR-restriction fragment length polymorphism (PCR-RFLP) procedure to separate the important arbovirus vector species in the Cx. sitiens subgroup: Cx. annulirostris, Cx. palpalis, and Cx. sitiens. The subsequent application of this technique for identifying field collected Cx. sitiens subgroup members throughout Australia, PNG and the southwest Pacific is then assessed.
Materials and Methods
Mosquito Material.
Members of the Cx. sitiens subgroup were collected from 17 sites located in Australia, PNG, and the Solomon Islands (Fig. 1; Table 1). Adult mosquitoes were collected using CO2-baited encephalitis virus surveillance (EVS) traps or Centers for Disease Control (CDC) light traps baited with CO2 + 1-octen-3-ol (octenol). Specimens were morphologically identified using the keys of Marks (1982b) and Lee et al. (1989). After identification, mosquitoes were stored in liquid nitrogen, dry ice, on silica gel, or in 70% ethanol.
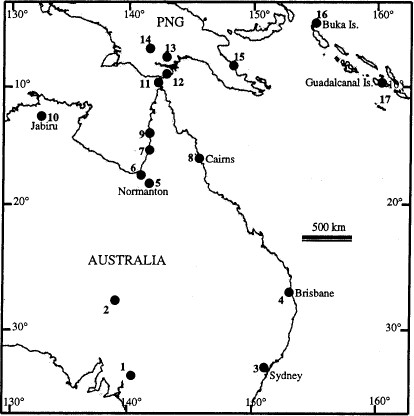
Southwest Pacific region showing the collection sites for mosquitoes of the Culex sitiens subgroup. Specific localities are summarized in Table 1.

To develop molecular tools for the identification of species members in the Cx. sitiens subgroup, voucher specimens of each species, identified using both morphology and allozymes, were required. Culex annulirostris and Cx. sitiens were obtained from Brisbane (Table 1; Fig. 1, site 4) and maintained as colonies by the Australian Army Malaria Institute (AMI). Voucher specimens of Cx. palpalis were obtained from field collections at Jabiru in the Northern Territory, Australia, in 1998 (Table 1; Fig. 1, site 10), and were all identified electrophoretically by Chapman et al. (2000).
DNA Extraction.
Mosquitoes (either partial or whole) were thoroughly ground in a 1.5-ml microfuge tube containing 50 μl of lysis buffer (1.0 M NaCl, 0.2 M sucrose, 0.1 M Tris-HCl, pH 9.0), 0.5 M EDTA, and 0.5% sodium dodecyl sulfate (SDS) using a 1.5-ml plastic grinding pestle (Kontes Glass, Vineland, NJ). Tubes were pulse centrifuged to concentrate the homogenate at the bottom of the tube before incubation at 65°C for 30 min. To each tube, 7 μl of 8.0 M KAc was added and mixed, the tubes were placed on ice for 15–30 min, and microfuged for 15 min at 12,000 × g. Supernatants were placed in a new tube, to which 100 μl of 100% EtOH was added and immediately centrifuged at 12,000 × g for 15 min. Supernatants were removed, 100 μl of 70% EtOH was added and tubes were centrifuged again at 12,000 × g for 5 min. Supernatants were again removed, tubes were air dried and resuspended in 50 μl TE containing RNase (5 μg/ml).
PCR-RFLP Analysis.
All polymerase chain reactions were carried out in a 48-well 0.2-ml PCR microtiter plate in a 25 μl volume on a MJ-PTC200 Thermo-cycler (MJ Research, Maltham, MA). The final PCR mixture contained 1–10 ng of template DNA, 20 pM of each primer, 1.25 mM MgCl2, 1.5 mM of each dNTP, 1 × Taq reaction buffer and 1 U of Taq DNA polymerase. Primers used for amplification of the ITS1 were those designed by Beebe et al. (2000). The ITS1A forward primer is the flanking segment of the 18S gene (5′-CCTTTGTACACACCGCCCGTCG). The ITS1B primer is the reverse complement of the ITS2A primer on the 5.8S gene (5′-ATGTGTCCTGCAGTTCACA). Cycling involved an initial denaturation at 94°C for 4 min, followed by 30 cycles of 94°C for 30 s, 51°C for 40 s, and 72°C for 30 s.
Restriction endonuclease digestion was carried out in a 0.5-ml microfuge tube containing 3 μl of PCR product, to which 3 μl of 2 × RsaI buffer (premade stock) containing 1 U RsaI enzyme/reaction (NEB II, New England Biolabs, Beverly, MA). The mixture was incubated at 37°C for 1 h. The digest was then run out on a 3.0% agarose gel at 100 V for 30 min and the gel was stained with ethidium bromide (5 μg/ml) for 15 min and viewed on an UV transilluminator at 312 nm.
Cloning PCR Products.
The ITS1 was cloned from the voucher specimens for each species. Culex annulirostris and Cx. sitiens specimens were from colonies. Culex palpalis mosquitoes were from Jabiru in the Northern Territory of Australia. Amplification products were ligated into a pGem-T vector according to the manufacturer’s recommendation (Promega, Madison, WI). The ligation was transformed into Escherichia coli (DH5α) in accordance with that recommended by Promega. White colonies were selected with a sterile pipette tip, reamplified by dipping the tip into the reaction mixture, and cycled as above for 25 cycles. Amplified ITS1 products were then subjected to restriction enzyme digestion to identify banding profiles.
DNA Sequencing.
PCR products were purified using Qiagen PCR-purification columns (Valencia, CA) following manufacturer’s directions. Purified ITS1 products were then sequenced using a 373 ABI automated sequencer with dye-terminator chemistry as recommended by manufacturer (Perkin Elmer, Wellesley, MA). Sequence analysis was performed using the Australian National Genomic Information Service (ANGIS, http://www.angis.org.au/). One clone sequence from each Cx. annulirostris and Cx. sitiens and two sequenced clones from Cx. palpalis (pal1 and pal2) were subjected to the PILEUP program (GCG 1994) using default settings. For publication the output was then subjected to the PRETTYPLOT program (GCG 1994).
Results
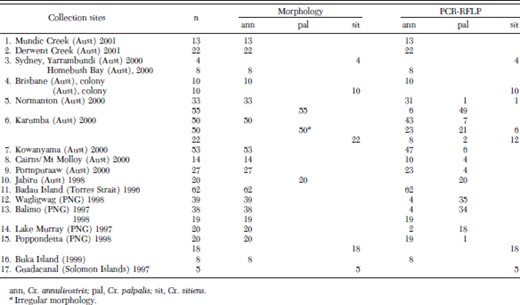
On PCR amplification of the ITS1 region, single products of ≈650 bp were produced for each species. A variety of restriction enzymes and combinations thereof were tested for their ability to produce diagnostic banding profiles. The enzyme RsaI produced serviceable species diagnostic profiles (Fig. 2). Culex annulirostris produced two clear bands at 300 and 180 bp, with less defined bands under 100 bp. Culex sitiens produced a cluster of three bands at ≈210, 180, and 170 bp and a fourth band at ≈130 bp. Culex palpalis showed main bands at 180 and 150 bp, and two less defined bands under 100 bp that appeared similar to those of Cx. annulirostris. Subpopulations of Cx. palpalis showed a 300 bp band similar to that of Cx. annulirostris (Fig. 2, lane 4). This 300 bp band appeared consistently, in the same individuals, at the same intensity, on repeated PCR-RFLP analyses, indicating that it was neither a PCR artifact nor the result of an incomplete restriction digest.
PCR amplified ITS1 region from mosquitoes in the Culex sitiens subgroup digested with RsaI and run in a 3.0% agarose gel. Lane 1, Cx. annulirostris (Brisbane); lane 2, Cx. sitiens (Brisbane), lanes 3 and 4 are the two RFLP profiles shown by Cx. palpalis (Jabiru). Lanes 5 and 6 are the two separate ITS1RFLP profiles derived from clones of the Cx. palpalis genotype in lane 4.
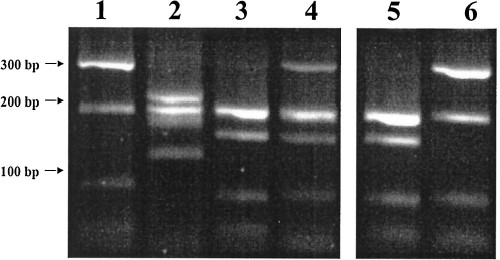
In most regions where Cx. palpalis populations were identified (Jabiru, Balimo, Normanton, and Karumba), a subpopulation of each collection showed a 300 bp band. An example of this can be seen in Fig. 3, where field material collected from Balimo (1997) was subjected to the PCR-RFLP procedure. As with the allozyme identification, most of the material was identified as Cx. palpalis with one Cx. annulirostris (Fig. 3, lane 9). These results appear comparable to the species ratios from allozyme studies by Chapman et al. (2000), which identified ≈95% of this material as Cx. palpalis (Table 2). Thus, mosquitoes from the same traps, though not the same individual mosquitoes, collected at Lake Murray (1997) and Balimo (1997 and 1998) have been identified using both allozyme markers and PCR-RFLP analysis and (see Table 2 for detailed comparisons). The PCR-RFLP procedure appeared robust, and generated consistent banding profiles from material collected from southern Australia, PNG and the Solomon Islands.
ITS1 PCR-RFLP analysis of Culex sitiens subgroup mosquitoes collected from Balimo, PNG, in 1997. Most of the mosquitoes were identified as Cx. palpalis with one Cx. annulirostris (lane 9). Allozyme analysis of material from the same collection pool showed 94.9% Cx. palpalis (see Table 3 for allozyme comparisons). A 300 bp band can be observed in a subpopulation of Cx. palpalis individuals.
Allozyme and PCR identification comparisons of different mosquitoes from the same collection pools in the Western Province of PNG
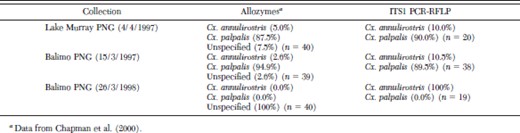
Allozyme and PCR identification comparisons of different mosquitoes from the same collection pools in the Western Province of PNG

Individuals of Cx. annulirostris, Cx. sitiens, and both Cx. palpalis genotype profiles were cloned, single colonies were selected, amplified, and digested to identify the ITS1 sequences. Three colonies from each individual were selected and sequenced. Amplified colonies of Cx. palpalis that showed the 300 bp band produced two separate RFLP profiles upon digestion, one of which appeared identical to the Cx. palpalis RFLP profile with the second showing similarities to the Cx. annulirostris RFLP profile (Fig. 2, lanes 5 and 6). Three clones from amplified colonies of each species were sequenced, including the two different Cx. palpalis profiles and submitted to Genbank (AY035882-AY035885). An alignment of one sequence from Cx. annulirostris and Cx. sitiens and the two RFLP profile sequence clones from Cx. palpalis are shown in Fig. 4. A summary of these sequences and the alignment are shown in Table 3.
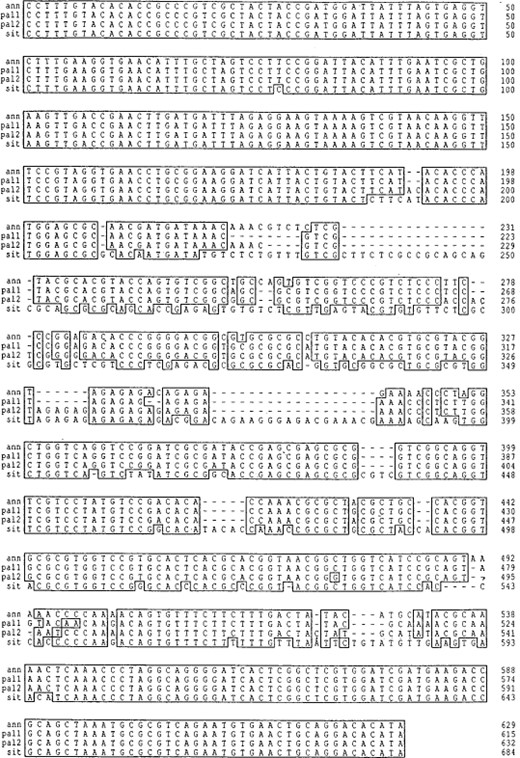
DNA sequence alignment of the rDNA ITS1 from three members of the Cx. sitiens subgroup. Clones that produced the species-specific profiles for Cx. annulirostris (ann), Cx. sitiens (sit) and Cx. palpalis (pal1 and pal2) were sequenced, and then aligned with the regions of sequence similarity highlighted. Two ITS1 sequences were found to be responsible for the two RFLP profile types in Cx. palpalis.
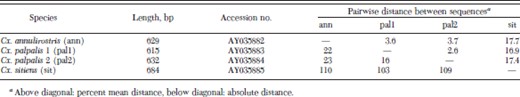

The ITS1 sequence alignment contains conserved and variable regions with 25.3% of the alignment variable; excluding Cx. sitiens, only 7.8% of the alignment was variable. The two different RFLP profile sequences of Cx. palpalis (pal1 and pal2) appear to display more similarity to each other (2.6% variability) than to Cx. annulirostris (pal1/ann 3.6% and pal2/ann 3.7%). Culex sitiens showed ≈17% sequence variation to the other two species. Sequencing of clones from single mosquitoes revealed small amounts of intragenomic di- and tri-nucleotide sequence polymorphisms, all of which occur within the variable regions of the alignment in Fig. 4. Moreover, running the ITS1 PCR products on a 7.0% acrylamide gel showed a large amount of heteroduplex products indicating substantial intragenomic ITS1 polymorphism within individual mosquitoes (data not shown). This will require a separate study to be documented in detail.
When comparing identification techniques (morphology and PCR-RFLP analysis), the occurrence of overlapping or irregular morphological characteristics was demonstrated in specimens collected from north Queensland (sites 5–9) and PNG (sites 11–15). This was particularly apparent with Cx. annulirostris and Cx. palpalis specimens collected from the Western Province (sites 11–14) and all three species collected from Normanton and Karumba in northwest Queensland.
Discussion
In this study we demonstrated that the ITS1 region provides adequate sequence variation between species for the development of a simple PCR-RFLP tool to separate Cx. annulirostris, Cx. sitiens, and Cx. palpalis; the three main species of the Cx. sitiens subgroup that occur in Australia and PNG. By using the rDNA ITS1 as a marker, it was possible to generate consistent species-specific PCR-RFLP profiles from both colony and field-collected material over a large geographic distribution.
Comparisons between the results from the allozyme analysis of Chapman et al. (2000) with those from the PCR-RFLP analysis on mosquitoes from the same collection trap appeared consistent (Table 2). However, Chapman et al. (2000) were unable to identify 6.9% of their field-collected material from PNG and Australia, and in particular, were not able to identify 37% of material from sites in the Western Province of PNG detailed in Table 2. These individuals were placed into four separate genetic groups as they did not agree at all loci with Cx. annulirostris and Cx. palpalis, though had definite similarities. Most of these mosquitoes comprised of individuals that showed homozygous allozyme loci and it was suggested that they may constitute sibling species. However, the PCR-RFLP procedure could identify all mosquitoes from these pools. Moreover, it appeared that the unknown individuals (by allozyme) were Cx. annulirostris.
Allozyme electrophoresis can be sensitive to population changes and shifts (Richardson et al. 1986), and the inability to identify any mosquitoes from Balimo in PNG in 1998 (see Table 2) may be due to severe shifts in mosquito populations driven by a drought which occurred throughout PNG in 1997 and ended in March 1998. Stagnant pools along the shores near Balimo in 1997 resulted in large collections of Cx. palpalis—a permanent and semipermanent water breeder (Chapman et al. 2000). In the following year after the drought had ended, Chapman et al. (2000) could not identify any of their mosquitoes using allozymes, hovever, the PCR-RFLP identified these mosquitoes as Cx. annulirostris. Thus, it appears that the PCR-RFLP procedure is not as sensitive to population (genotype) fluctuations than the allozyme markers and therefore will be more useful as a species-diagnostic tool across their geographic range.
The polymorphic nature of the rDNA ITS1 in this subgroup is interesting. The RFLP profile of the pal1 and pal2 sequences in the Cx. palpalis genotype may initially appear as a hybridization with Cx. annulirostris. However, on sequence analysis, pal2 was more homologous to pal1 (2.6%) than to the Cx. annulirostris sequence (3.7%), suggesting that pal2 is of Cx. palpalis origin and that the similarity between the RFLP profile and Cx. annulirostris is due to mutations in a polymorphic region at ≈495 bp-500 bp rather than any possible introgression with Cx. annulirostris. It is important to note that ITS1 sequences exist within Cx. palpalis, which upon digestion can produce RFLP profiles similar to that of Cx. annulirostris. The pal1 sequence that produces the distinctive and diagnostic 150 bp band identifying Cx. palpalis appears to be the primary ITS1 sequence and the pal2 sequence seems to appear at a variable copy number. If indeed populations of Cx. palpalis exist that contain only the pal2 sequence polymorphism, it may be difficult to separate Cx. palpalis from Cx. annulirostris using this procedure. However, these populations have not yet been identified and may not exist. To overcome this potential problem, allele-specific PCR primers would be required, similar to those of Scott et al. (1993) for the An. gambiae complex and Walton et al. (1999) for the An. dirus complex. Thus, each species sequence could be identified separately and the pal1 and pal2 sequences could also be viewed independently.
The separate identification of pal1 and pal2 may prove useful in understanding why some subpopulations of Cx. palpalis contain, maintain or amplify particular sequences in the genome. Clarification will probably require the use of allele-specific PCR to identify pal1 and pal2 used in conjunction with an independent genetic marker such as one from the maternally inherited mitochondria.
Genbank database searches of the ITS1 sequences revealed sequence highest similarity between Cx. sitiens subgroup members and members of the Culex vishnui subgroup from the Ryukyu Archipelago, Japan (Toma et al. 2000). Interestingly, the Cx. sitiens ITS1 sequence showed greater sequence similarity to the Cx. vishuni subgroup members than to Cx. annulirostris and Cx. palpalis.
Because there are up to eight morphological species in the Cx. sitiens subgroup, the current use of universal primers for the PCR amplification of the ITS1 means that all mosquitoes amplified will produce a PCR product and subsequently permit resolution of anomalies that may appear due to incorrect identification based on morphology or the discovery of new species or genotypes. The presence of intra- and interindividual sequence copy heterogeneity may initially complicate or reduce the effectiveness of an allele-specific PCR diagnostic procedure. It appears from our observations on the Cx. sitiens subgroup and those of Crabtree et al. (1995), Miller et al. (1996) and Toma et al. (2000) on other Culex species that the ITS1 region can be highly polymorphic. Thus, all procedures should be fully tested on field-collected material throughout the geographic range of each species.
Acknowledgements
The authors thank Scott Ritchie, Peter Foley, Cheryl Johansen, and John Mackenzie for assistance with organizing and undertaking field collections. This article is published with the approval of the Director General Defense Health Services (Australia). This work was funded by the National Health and Medical Research Council of Australia (117102) and Queensland Health.
References Cited



