-
PDF
- Split View
-
Views
-
Cite
Cite
Susan R. Dean, Roger W. Meola, Effect of Diet Composition on Weight Gain, Sperm Transfer, and Insemination in the Cat Flea (Siphonaptera: Pulicidae), Journal of Medical Entomology, Volume 39, Issue 2, 1 March 2002, Pages 370–375, https://doi.org/10.1603/0022-2585-39.2.370
Close - Share Icon Share
Abstract
Weight gain by adult cat fleas, Ctenocephalides felis (Bouché), was influenced primarily by the concentrations of protein and sodium chloride in the feeding solution. After 48 h of feeding, fleas fed whole blood weighed almost twice as much as fleas fed plasma or hemolyzed blood and 1.25 times as much as fleas fed 0.15 M sodium chloride. When fleas were fed sodium chloride solutions ranging from 0 to 0.5 M, weight gain was greatest on the 0.15- or 0.2-M solutions. Weight gain decreased significantly when fleas were fed plasma, hemolyzed blood or 0.3 or 0.5 M sodium chloride in place of whole blood, but improved when plasma was diluted 100% and when hemolyzed blood was diluted 10% with distilled water. Adenosine-5′-triphosphate did not appear to stimulate weight gain in cat fleas; weight gain was unchanged in fleas fed hemolyzed blood or 0.15 M sodium chloride to which 0.005 M ATP was added. Insemination did not occur in starved fleas or those fed protein-free diets. When fleas were starved or fed distilled water, sodium chloride, or other salt solutions, sperm was transferred from the testes to the vas deferens in 91–94% of males, but no females were inseminated. In contrast, when fleas were fed whole blood, hemolyzed blood, plasma, or bovine serum albumin (3.5 or 7.0 g/deciliter) dissolved in 0.15 M saline, 80, 80, 10, and 10% of the females were inseminated, respectively.
Obligate blood-feeding insects such as the cat flea, Ctenocephalides felis (Bouché), are able to obtain all of the nutrients required for survival and reproduction from blood. Apparently, the protein-rich red cell fraction of blood is essential for egg development in cat fleas. Wade and Georgi (1988) demonstrated that cat fleas fed only on plasma lay few eggs and survivability increases two-fold when fleas are fed whole cattle blood versus plasma. Moreover, Georgi (2001) recently reported that increasing the concentration of erythrocytes in the blood diet resulted in an increase in the quantity of blood consumed as well as the number of viable offspring produced.
Galun (1966) demonstrated that the amount of feeding by oriental rat fleas, Xenopsylla cheopis (Rothschild), decreased on hemolyzed blood versus whole blood and hypothesized that this may be a result of the breakdown of ATP, a phagostimulant in some insects, during hemolysis. The addition of 0.005 M ATP to plasma or 0.15 M sodium chloride doubled weight gain by X. cheopis to an amount equivalent to that on whole blood. However, it is not known whether ATP acts as a phagostimulant in the cat flea. When cat fleas were fed plasma or whole blood to which 0.005 M ATP had been added, neither survival nor fecundity increased (Wade and Georgi 1988).
Hemolysis results in the release of hemoglobin, sodium, potassium, chloride, phosphate, sulfate, and other ions from red blood cells into plasma with a concurrent increase in tonicity (Langley and Pimley 1973). Galun (1966) demonstrated that the concentration of a salt in solution influenced the amount of feeding by X. cheopis. When she fed fleas sodium chloride solutions ranging from 0.04 to 0.15 M, maximum feeding occurred on a 0.15-M solution. However, when the sodium chloride concentration was increased to 0.3 M, no feeding occurred. In addition, she reported that the amount of feeding by X. cheopis on other sodium salt solutions was influenced by molecular weight; fleas fed abundantly on sodium chloride but little on sodium citrate, which has a larger molecular weight. As a result Galun (1966) speculated that an increase in tonicity and concentration of large molecular weight salts may decrease the amount of feeding by rat fleas by inhibiting diffusion and active transport of ions across midgut epithelial cell membranes.
Blood constituents also appear to indirectly influence insemination in the cat flea. For example, Akin (1984) showed that before insemination can take place, the testicular plug that blocks passage of sperm from the testes to the vas deferens must be dissolved. Testicular plug dissolution, usually associated with bloodfeeding, is completed in cat fleas within 24 h of bloodfeeding on a host (Akin 1984). Potent or inseminated matings have not been reported in unfed male and female fleas housed together for 7–30 d (Zakson-Aiken et al. 1996, Dean and Meola 1997). Moreover, unfed males do not attempt to mate with unfed or blood-fed females (Hsu and Wu 2001), possibly as a result of blockage due to their intact testicular plugs. Bai and Prasad (1979a) demonstrated that 55% of Xenopsylla astia (Rothschild) and 40% of X. cheopis males dissolve their testicular plugs when they feed for 24 h on 0.15 M saline to which 0.0045 M ATP is added, versus 100 and 75% plug dissolution, respectively, when fed blood. Hence, blood constituents other than proteins may prepare fleas for mating and insemination by promoting testicular plug dissolution.
The objective of this study was to identify constituents of blood that stimulate weight gain, sperm transfer and insemination in the cat flea.
Materials and Methods
Laboratory Insects.
Fleas were obtained from a colony originally supplied by Zoecon (Dallas, TX) in 1987 and maintained in culture at Texas A&M University. Adult fleas for the colony were fed and maintained on four domestic cats housed in the Laboratory Animal Resources and Research facility on campus. Eggs were collected daily and brought to the laboratory at the Center for Urban and Structural Entomology where they were placed on 30 g of larval rearing medium made from 6 g dried bovine blood, 23 g finely ground cat chow, and 200 mg powdered brewer’s yeast in 310 g of fine sand in plastic rearing containers. Cocoons were sifted from the rearing medium and stored in plastic containers in an environmental chamber maintained at 25°C, 70–80% RH, and a photoperiod of 14:10 (L:D) h. Adult eclosion began ≈7 d after initial cocoon formation.
Experimental Procedure.
Five experiments were designed to determine the effect of various feeding solutions on weight gain, sperm transfer and insemination in fleas. Newly eclosed fleas of both sexes were collected by a vacuum separator (FleaData, Freeville, NY) and placed in plastic cages in groups of ≈100 each. Five replicate cages of fleas were used in each experimental treatment. Dog hair was added to each cage to act as a resting substrate for fleas. The upper lid of each cage was constructed of a plastic mesh through which fleas could feed. Cages were maintained at 37°C on an artificial membrane system previously described by Pullen and Meola (1995). Each cage was supplied with an individual feeding reservoir which either remained empty (starved fleas) or was sealed with a parafilm membrane and filled with 7-ml feeding solution. Each day, the feeding reservoirs were removed and replaced with fresh feeding solution in clean reservoirs with new membranes. Blood used in these experiments was collected aseptically from a herd of donor cattle maintained without insecticide or antihelminthic treatments. Sodium citrate (0.02 M) was added to whole blood as an anticoagulant.
Measurement of Weight Gain.
Twenty live females were collected from each experimental cage ≈48 h after they began feeding or after ≈48 h of starvation. Each group of 20 fleas was weighed in a microcentrifuge tube and returned to its respective cage. Weight gain was reported at this time because fleas attain their maximum size by expansion of the abdominal segments at ≈48 h after the initiation of bloodfeeding, and mortality increases in saline-fed fleas at 72 h. Only females were used in weight gain measurements because we determined, from our preliminary investigations, that they gain more weight from feeding than males and because significant differences in weight gain on different feeding solutions were only observed with females.
Determination of Percentage Sperm Transfer and Insemination and Observation of Gut Distention.
Twenty females and 20 males were removed from each experimental cage after 6 d to determine percentage insemination (sperm in the spermathecae) and sperm transfer (sperm in the epididymis), respectively. Insects were dissected in 0.15 M saline and viewed under a dissecting microscope. Blood and other feeding solutions were visible within the midgut and the amount of midgut distention was compared with that of control fleas.
Experiment 1: Fleas Fed Sodium Chloride Solutions.
Fleas were starved or fed 0 (distilled water), 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, and 0.5 M sodium chloride to investigate the effect of sodium chloride concentration on weight gain and insemination. Maximum feeding concentration was estimated by comparing weights of fed fleas with those of starved fleas.
Experiment 2: Fleas Fed Dilutions of Cattle Blood.
Fleas were fed blood in which the plasma was replaced by an equal volume of distilled water or 0.15 M saline to determine which constituents of plasma contribute to weight gain and insemination in cat fleas. Plasma was isolated from whole blood by centrifuging blood in test tubes three times at 5,000 rpm for 10 min each, and removing the supernatant after each centrifugation. In addition, whole blood was diluted with an equal volume or twice the volume of plasma (100% and 200% dilutions, respectively) to investigate the effect of increased plasma volume on weight gain and insemination. Fleas fed undiluted blood served as controls.
Experiment 3: Fleas Fed Dilutions of Cattle Blood Plasma.
Plasma that had been removed from cattle blood by centrifugation in the previous experiment was diluted with an equal volume or 1.5 times the volume (100% and 150% dilutions, respectively) of distilled water or 0.15 M saline to investigate the effect of dilution of plasma on weight gain and insemination. Fleas fed undiluted plasma served as controls.
Experiment 4: Fleas Fed Dilutions of Thawed Frozen Cattle Blood.
Blood that was frozen for 30 d at -12°C was thawed for 24 h at 4°C and diluted 3.3, 6.6, 9.9, or 16% with either distilled water or 0.15 M saline to determine the effect of dilution on weight gain and insemination. In addition, fleas were fed thawed frozen blood to which 0.005 M adenosine-5′-triphosphate (Sigma, St. Louis, MO) had been added to determine whether ATP stimulated weight gain. Fleas fed undiluted thawed frozen blood served as controls.
Experiment 5: Fleas Fed Salt Solutions.
To compare weight gain on different salt solutions, the weight of fleas fed 0.15 M sodium chloride, potassium chloride, sodium phosphate (Na2HPO4), and sodium citrate was measured. The weight gain of fleas fed 0.15 M sodium chloride to which 0.02 M sodium citrate was added was measured to investigate the effect of sodium citrate, in the same concentration that it is used as a blood anticoagulant, on flea weight gain. To determine whether adenosine-5′-triphosphate stimulates weight gain, we weighed fleas fed 0.15 M sodium chloride to which 0.005 M ATP was added. Fleas were fed 0.15 M sodium chloride to which 1.5, 3.5, or 7.0 g/deciliter bovine serum albumin (Sigma) was added to determine whether albumin (the major plasma protein) concentrations influence weight gain and insemination.
Statistics.
Means were compared with the control using a two-sample, unpaired t-test (P > 0.05).
Results
Fleas Fed Sodium Chloride Solutions.
Weight gain was significantly increased when fleas were provided with distilled water or any concentration of sodium chloride in comparison with starved fleas (water: t = 6.48, df = 8, P < 0.05; 0.05 M: t = 7.33, df = 8, P < 0.05; 0.10 M: t = 22.8, df = 8, P < 0.05; 0.15 M: t = 26.31, df = 8, P < 0.05; 0.20 M; t = 20.51, df = 8, P < 0.05; 0.25 M: t = 33.94, df = 8, P < 0.05; 0.3 M: t = 14.14, df = 8, P < 0.05; 0.5 M: t = 6.71, df = 8, P < 0.05) (Fig. 1). Maximum weight gain was achieved when fleas were fed 0.15 or 0.20 M sodium chloride. Fleas fed to repletion on 0.15 or 0.2 M sodium chloride as evidenced by their distended midguts and expanded abdomens. In contrast, the midguts of fleas fed 0.3 or 0.5 M sodium chloride were distended but the abdomens remained unexpanded, suggesting that little saline was absorbed from the midgut. Mean percentage sperm transfer in males varied from 91 to 94% and did not differ significantly regardless of whether fleas were starved, or fed distilled water or any of the concentrations of sodium chloride listed above. No females were inseminated regardless of whether they were starved or fed water or any of the concentrations of sodium chloride listed above.
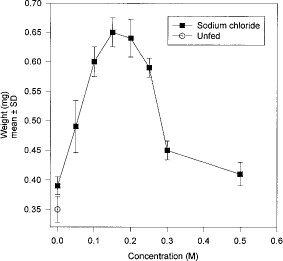
Live weights of female cat fleas fed sodium chloride solutions for 48h.
Fleas Fed Dilutions of Cattle Blood.
Replacement of plasma with an equal volume of saline did not significantly affect weight gain or percentage insemination during the first 3 d of bloodfeeding (Table 1). Fleas fed to repletion on undiluted whole blood or blood in which plasma was replaced by 0.15 M saline as evidenced by their distended midguts and expanded abdomens. After 3 d, however, blood in which plasma was replaced by saline appeared to be hemolyzed and flea weights decreased. Hemolysis also occurred when the plasma that was removed from whole blood was replaced by distilled water, and fleas fed on this solution had significant decreases in both weight gain and percentage insemination in comparison with the control. Likewise, when cattle blood was diluted 200% with plasma, both flea weight gain and percentage insemination were significantly decreased. Sperm transfer was completed by 90–95% of male fleas in all treatments by 6 d.
Percentage insemination of cat fleas fed dilutions of cattle blood on an artificial membrane system at 37°C for 6 d (mean ± SD)
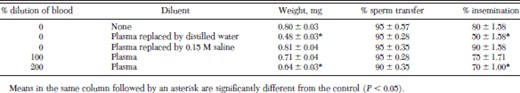
Percentage insemination of cat fleas fed dilutions of cattle blood on an artificial membrane system at 37°C for 6 d (mean ± SD)

Fleas Fed Dilutions of Cattle Blood Plasma.
The weight gain and percentage insemination of fleas fed plasma diluted 100% with distilled water were significantly greater than those of fleas fed undiluted plasma (Table 2). The midguts of fleas fed undiluted plasma or plasma diluted with water or saline were distended but their abdomens did not expand. Sperm transfer was completed by 90–95% of male fleas in all treatments by 6 d.
Percentage insemination of cat fleas fed dilutions of cattle blood plasma on an artificial membrane system at 37°C for 6 d (mean ± SD)
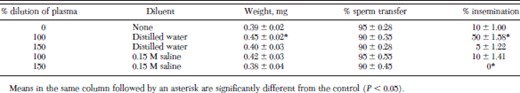
Percentage insemination of cat fleas fed dilutions of cattle blood plasma on an artificial membrane system at 37°C for 6 d (mean ± SD)

Fleas Fed Dilutions of Thawed Frozen Cattle Blood.
The weight gain and percentage insemination of fleas fed thawed frozen blood diluted 9.9% with distilled water were significantly greater than those of fleas fed undiluted thawed frozen blood or blood diluted with 0.15 M saline (Table 3). The addition of 0.005 M ATP to thawed frozen blood did not increase weight gain or percentage insemination. The midguts of fleas fed undiluted thawed frozen blood or blood diluted with water or saline were distended but their abdomens did not expand. Sperm transfer was completed by 90–95% of male fleas in all treatments by 6 d.
Percentage insemination of cat fleas fed dilutions of thawed frozen cattle blood on an artificial membrane system at 37°C for 6 d (mean ± SD)
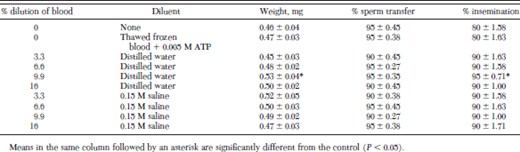
Percentage insemination of cat fleas fed dilutions of thawed frozen cattle blood on an artificial membrane system at 37°C for 6 d (mean ± SD)

Fleas Fed Salt Solutions.
Weight gain was significantly decreased when fleas were fed 0.15 M potassium chloride, sodium phosphate and sodium citrate when compared with the control (Table 4). Fleas fed sodium citrate gained the least weight. The weight of fleas fed 0.15 M sodium chloride to which 0.02 M sodium citrate had been added was significantly decreased in comparison to that of the control. The weight gain of fleas fed 0.15 M sodium chloride to which 0.005 M ATP had been added did not increase significantly. Compared with the control, weight gain increased significantly when fleas were fed 7.0 g/deciliter bovine serum albumin in 0.15 M sodium chloride and percentage insemination was significantly greater when fleas were fed either 3.5 or 7.0 g/deciliter bovine serum albumin solutions. Sperm transfer was completed by 90–95% of male fleas in all treatments by 6 d.
Percentage insemination of cat fleas fed salt solutions on an artificial membrane system at 37°C for 6 d (mean ± SD)
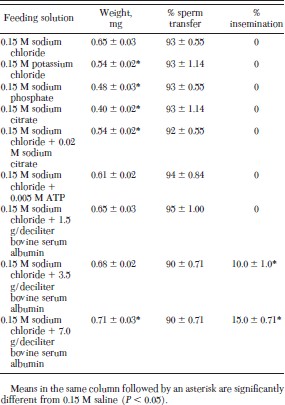
Percentage insemination of cat fleas fed salt solutions on an artificial membrane system at 37°C for 6 d (mean ± SD)

Discussion
In cat fleas, weight gain appeared to be influenced primarily by the concentrations of protein and sodium chloride in the feeding solution. Fleas fed undiluted whole blood weighed almost twice as much as fleas fed hemolyzed blood or plasma and 1.25 times as much as those fed 0.15 M sodium chloride. This finding is in general agreement with those of Georgi (2001) with cat fleas and Galun (1966) with rat fleas. Likewise, Hinkle et al. (1991) reported that cat fleas fed whole blood double their body weights within 24 h. The discrepancy in weights reported by Hinkle et al. (1991) for cat fleas (0.19 mg/unfed flea) and those that we observed was probably due to our selective reporting of female weights. Joseph (1976) reported that cat flea females weigh 2.5 times more than males. The difference in weight gained by blood-fed fleas could not be explained by taking into account the negligible difference in specific gravities of the feeding solutions; undiluted whole blood weighed only 0.8% >0.15 M sodium chloride, the feeding solution with the lowest specific gravity. Hinkle et al. (1991) observed that protein levels of adult fleas more than tripled after feeding on blood, and remained higher than those of unfed fleas even after starvation for 12 or 24 h. Utilization of digested blood proteins by fleas to synthesize vitellogenins and structural proteins such as those found in the cuticle may have accounted for much of the observed weight gain. Although fleas fed 0.15 or 0.2 M sodium chloride fed to repletion, they gained less weight than those fed undiluted whole blood probably because they were fed a protein-free diet and depleted their fat body reserves as an energy source for survival. Plasma proteins, i.e., albumin and globulins, appeared to play a lesser role in weight gain. Weights were unchanged in fleas fed whole blood in which plasma was replaced with 0.15 M saline, and the weight gain of fleas fed bovine serum albumin in concentrations approximating those found in plasma was less than that of whole blood. It may be that this difference in weight gain was due to the relative abundance of hemoglobin in blood, because whole blood contains approximately four times as much hemoglobin as plasma proteins (Ruckebusch et al. 1991). However, the amino acid composition of the protein may also be a factor in determining weight gain. Bai and Prasad (1979b) determined that mortality was higher when the rat fleas X. cheopis and X. astia were fed a diet in which bovine albumin was substituted for bovine hemoglobin.
Weight gains of cat fleas fed hemolyzed blood, plasma and whole blood diluted with plasma were significantly less than on whole blood, but improved when plasma was diluted 100% and hemolyzed blood 10% with water.
Cat fleas, like many blood-feeding insects, use active transport of sodium ions from midgut epithelial cells to the hemolymph to co-transport chloride, water and amino acids (Lehane and Billingsley 1996). Sodium chloride is abundant in most mammalian blood and often accounts for as much as 85% of the plasma tonicity. The sodium concentration in cattle plasma normally ranges between 0.14 and 0.15 M (Ruckebusch et al. 1991). Cat fleas fed 0.15 or 0.2 M sodium chloride gained significantly more weight than those fed higher or lower concentrations of saline. The optimal feeding concentration of sodium chloride for rat fleas and tsetse flies was also 0.15 M (Galun 1966, Langley and Pimley 1973). Because the sodium pumps used in active transport are located on the basolateral membranes of the epithelial cells and these membranes unfold in fleas fed whole blood or solutions isotonic to blood, exposing a large surface area to the hemolymph (Rothschild et al. 1986), sodium pump activity and hence active transport may be modulated by the tonicity of the feeding solution. The epithelial cells of fleas may shrink in response to hypertonic feeding solutions resulting in a refolding of the basolateral membranes with a concurrent loss of sodium pump activity and inhibition of active transport (Langley and Pimley 1973). This could explain why weight gain was decreased when fleas were fed 0.3 or 0.5 M sodium chloride.
When cat fleas were fed 0.15 M sodium chloride, potassium chloride, sodium phosphate or sodium citrate, the amount of weight gain was inversely related to the molecular weight of the salt. Galun (1966) reported that X. cheopis also fed readily on 0.15 M sodium chloride but fed little on equimolar solutions of potassium chloride or sodium citrate. She hypothesized that this result was due to the difficulty that large molecular weight ions versus smaller ones have in diffusing across epithelial cell membranes. Peacock (1986) also reported that smaller anions such as chloride passed across epithelial cell membranes much more readily than larger ones such as phosphate during diuresis in the tsetse fly. Likewise, the addition of sodium citrate to blood, at a concentration of 0.02 M, as an anticoagulant may inhibit weight gain in cat fleas; fleas fed 0.15 M sodium chloride to which 0.02 M sodium citrate was added weighed less than those fed on 0.15 M sodium chloride.
We could not demonstrate that ATP stimulated weight gain in the cat flea. Weight gain did not increase when 0.005 M ATP was added to hemolyzed blood or 0.15 M sodium chloride.
Insemination did not take place when cat fleas were starved or fed protein-free diets. Although 90–95% of male fleas completed sperm transfer by 6 d at 37°C regardless of whether they were starved or fed distilled water, sodium chloride, other salt solutions, whole blood, plasma or thawed frozen blood, none of the starved female fleas or those fed distilled water or saline were inseminated. In contrast, when fleas were fed whole blood, hemolyzed blood, plasma or 3.5 or 7.0 g/deciliter bovine serum albumin solutions, 80, 80, 10, and 10%, respectively, were inseminated. Inseminated matings, as measured by percentage insemination, increased with an increase in the protein concentration of the feeding solution. It may be that starved fleas or those that are fed distilled water or saline deplete their fat body reserves as an energy source for survival and are unable to meet the energy requirements necessary to seek mates or mate successfully. Hsu and Wu (2000) reported that cat flea matings may be lengthy, lasting from 2 to 157 min with an average of ≈60 min, and successive matings may be required because only 44% of first matings result in ovipositon of viable eggs. An alternate explanation for the observed absence of insemination in starved fleas or those fed water or saline was that complete abdominal extension observed in blood-fed fleas and to a lesser extent in plasma and albumin-fed fleas is a requirement for inseminated mating. Hsu and Wu (2001) observed that blood-fed males were unable to mate with unfed 4-d-old females and postulated that this is a result of abdominal shrinkage in starved females.
Acknowledgements
We acknowledge the help of Jay R. Georgi in reviewing the manuscript. This research was funded by Texas Agricultural Experiment Station Project 6526.
In conducting the research described in this report, the investigators adhered to the "Guide for the Care and Use of Laboratory Animals," as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The facilities are fully accredited by the American Association of Laboratory Animal Care.
References Cited



