-
PDF
- Split View
-
Views
-
Cite
Cite
Shaoxue Ling, Zonghao You, Yang Li, Jian Zhang, Shuwu Zhao, Yongzhi He, Xi Chen, The role of γδ T17 cells in cardiovascular disease, Journal of Leukocyte Biology, Volume 112, Issue 6, December 2022, Pages 1649–1661, https://doi.org/10.1002/JLB.3MR0822-761RR
Close - Share Icon Share
Abstract
Due to the ability of γδ T cells to bridge adaptive and innate immunity, γδ T cells can respond to a variety of molecular cues and acquire the ability to induce a variety of cytokines such as IL-17 family, IFN-γ, IL-4, and IL-10. IL-17+ γδ T cells (γδ T17 cells) populations have recently received considerable interest as they are the major early source of IL-17A in many immune response models. However, the exact mechanism of γδ T17 cells is still poorly understood, especially in the context of cardiovascular disease (CVD). CVD is the leading cause of death in the world, and it tends to be younger. Here, we offer a review of the cardiovascular inflammatory and immune functions of γδ T17 cells in order to understand their role in CVD, which may be the key to developing new clinical applications.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide and poses a serious threat to human health.1–3 These diseases involved injury in the heart or blood vessels, including myocardial infarction (MI), atherosclerosis (AS), aortic aneurysm (AA), ischemia-reperfusion injury (IRI), and so on.4,5 At present, it has been recognized that γδ T cells are closely related to CVD.6
γδ T cells are a group of T lymphocytes that are distinct from αβ T cells, whose surface TCR is composed of γ chain and δ chain. γδ T cells only account for 1–5% of T lymphocytes in peripheral blood circulation and lymphatic circulation, and they were mainly distributed in mucosa and subcutaneous tissue.7–9 γδ T cells have been classified in a variety of ways, among which the most common classification is based on composition of TCR chains10 and cytokine secretion.11
γδ T cells as a bridge contact innate and adaptive immunity,12 such as they can act as APC to perform the antigen presentation function,13 and secrete a variety of cytokines, including IL-17 family, IFN-γ, IL-4, IL-10, and so on.14–17 In this group of cytokines, the IL-17 family consists of 6 structurally related cytokines,18 and the research hotspots are focused on IL-17A and IL-17F.
γδ T cells that produce IL-17 family, which are termed γδ T17 cells (or alternatively γδ17 cells, γδT-17 cells, Tγδ17 cells),19,20 and they are the primary early source of IL-17A in immune response.21 Two different types of γδ T17 cells have been found in each human and mice.22 γδ T17 cells can rapidly not only produce large amounts of IL-17A/F, but also IL-22, IL-21, and GM-CSF, which are attributed to their innate feature.23,24 In this review, we introduced the classification, differentiation, and characteristics of γδ T17 cells. Then, we sorted out the role of γδ T17 cells in CVD.
CLASSIFICATION OF γδ T CELLS
Classification based on TCR chains
γδ T cells can be divided into different subsets according to different composition of γ chains and δ chains. Human γδ T cells can be distinguished by δ chain expression, including Vδ1, Vδ2, Vδ3, and Vδ5 subtypes.10,25 However, murine γδ T cells can be distinguished by their γ chain expression.26 According to Heilig and Tonegawa nomenclature,27 there are 7 subtypes of mouse γδ T cells, Vγ1–Vγ728 (Table 1).
| . | Subset . | Paired TCR chains . | Tissue resident . | Reference . |
|---|---|---|---|---|
| Human Murine | Vδ1 Vδ2 Vδ3 Vδ5 Vγ1 Vγ2 Vγ3 Vγ4 Vγ5 Vγ6 Vγ7 | Vγ2/3/4/5/8/9 Vγ9 Vγ2/3/4 Vγ4 Vδ6.3/6.4 Vδ4 Vδ1 Vδ4 Vδ1 Vδ1 Vδ4/5/6 | Skin, liver, spleen mucosal tissues, PB (peripheral blood) Skin, PB Liver, PB PB Skin, lung, colon, liver, PB Skin, lung, colon, liver, PB Skin Skin, lung, colon, liver, PB, joint Skin, liver Genital tract, tongue, lung, colon, skin, adipose tissue Intraepithelial lymphocytes | 29,30,31 32,33,34 35,36 37 38,39 40,41,42,43 44,45 39,46,47,48 49,50 51,52 53 |
| . | Subset . | Paired TCR chains . | Tissue resident . | Reference . |
|---|---|---|---|---|
| Human Murine | Vδ1 Vδ2 Vδ3 Vδ5 Vγ1 Vγ2 Vγ3 Vγ4 Vγ5 Vγ6 Vγ7 | Vγ2/3/4/5/8/9 Vγ9 Vγ2/3/4 Vγ4 Vδ6.3/6.4 Vδ4 Vδ1 Vδ4 Vδ1 Vδ1 Vδ4/5/6 | Skin, liver, spleen mucosal tissues, PB (peripheral blood) Skin, PB Liver, PB PB Skin, lung, colon, liver, PB Skin, lung, colon, liver, PB Skin Skin, lung, colon, liver, PB, joint Skin, liver Genital tract, tongue, lung, colon, skin, adipose tissue Intraepithelial lymphocytes | 29,30,31 32,33,34 35,36 37 38,39 40,41,42,43 44,45 39,46,47,48 49,50 51,52 53 |
| . | Subset . | Paired TCR chains . | Tissue resident . | Reference . |
|---|---|---|---|---|
| Human Murine | Vδ1 Vδ2 Vδ3 Vδ5 Vγ1 Vγ2 Vγ3 Vγ4 Vγ5 Vγ6 Vγ7 | Vγ2/3/4/5/8/9 Vγ9 Vγ2/3/4 Vγ4 Vδ6.3/6.4 Vδ4 Vδ1 Vδ4 Vδ1 Vδ1 Vδ4/5/6 | Skin, liver, spleen mucosal tissues, PB (peripheral blood) Skin, PB Liver, PB PB Skin, lung, colon, liver, PB Skin, lung, colon, liver, PB Skin Skin, lung, colon, liver, PB, joint Skin, liver Genital tract, tongue, lung, colon, skin, adipose tissue Intraepithelial lymphocytes | 29,30,31 32,33,34 35,36 37 38,39 40,41,42,43 44,45 39,46,47,48 49,50 51,52 53 |
| . | Subset . | Paired TCR chains . | Tissue resident . | Reference . |
|---|---|---|---|---|
| Human Murine | Vδ1 Vδ2 Vδ3 Vδ5 Vγ1 Vγ2 Vγ3 Vγ4 Vγ5 Vγ6 Vγ7 | Vγ2/3/4/5/8/9 Vγ9 Vγ2/3/4 Vγ4 Vδ6.3/6.4 Vδ4 Vδ1 Vδ4 Vδ1 Vδ1 Vδ4/5/6 | Skin, liver, spleen mucosal tissues, PB (peripheral blood) Skin, PB Liver, PB PB Skin, lung, colon, liver, PB Skin, lung, colon, liver, PB Skin Skin, lung, colon, liver, PB, joint Skin, liver Genital tract, tongue, lung, colon, skin, adipose tissue Intraepithelial lymphocytes | 29,30,31 32,33,34 35,36 37 38,39 40,41,42,43 44,45 39,46,47,48 49,50 51,52 53 |
Although classification based on TCR chains has been accepted as the acknowledged method, 1 subtype of γδ T cells can secrete a variety of cytokines, making this cell subtype multifunctional. For example, the role of Vδ1 T cells is debatable. Vδ1 T cells have 2 opposite effects, both secreting IFN-γ to produce antitumor effects29,30 and secreting IL-17 to promote tumor growth.54,55 Therefore, classification based on TCR may have some limitation reflecting cell function.
Classification of cytokines secreted
According to the secreted cytokines, γδ T cells can be divided into IL-17+ γδ T cells, IFN-γ+ γδ T cells, IL-4+ γδ T cells, and so on (Table 2). This classification method can avoid the inconsistency in the description of γδ T cells due to different species. Therefore, the classification of cell function is widely used in practical applications.14,61,64
| . | Subset . | Function . | Reference . |
|---|---|---|---|
| Human Murine | IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T IL-17+ γδ T IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T KGF+ γδ T IL-17+ γδ T | Antitumor, anti-infection Anti-inflammatory Immune tolerance Proinflammatory Anti-infection Anti-inflammatory, allergenic Protect liver, anti-infection Protect the integrity of damaged epithelial surfaces Proatherogenic, promote tumor | 56,57 58 17 59 60 15,16 61 62 63 |
| . | Subset . | Function . | Reference . |
|---|---|---|---|
| Human Murine | IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T IL-17+ γδ T IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T KGF+ γδ T IL-17+ γδ T | Antitumor, anti-infection Anti-inflammatory Immune tolerance Proinflammatory Anti-infection Anti-inflammatory, allergenic Protect liver, anti-infection Protect the integrity of damaged epithelial surfaces Proatherogenic, promote tumor | 56,57 58 17 59 60 15,16 61 62 63 |
| . | Subset . | Function . | Reference . |
|---|---|---|---|
| Human Murine | IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T IL-17+ γδ T IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T KGF+ γδ T IL-17+ γδ T | Antitumor, anti-infection Anti-inflammatory Immune tolerance Proinflammatory Anti-infection Anti-inflammatory, allergenic Protect liver, anti-infection Protect the integrity of damaged epithelial surfaces Proatherogenic, promote tumor | 56,57 58 17 59 60 15,16 61 62 63 |
| . | Subset . | Function . | Reference . |
|---|---|---|---|
| Human Murine | IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T IL-17+ γδ T IFN-γ+ γδ T IL-4+ γδ T IL-10+ γδ T KGF+ γδ T IL-17+ γδ T | Antitumor, anti-infection Anti-inflammatory Immune tolerance Proinflammatory Anti-infection Anti-inflammatory, allergenic Protect liver, anti-infection Protect the integrity of damaged epithelial surfaces Proatherogenic, promote tumor | 56,57 58 17 59 60 15,16 61 62 63 |
γδ T cells can secrete a variety of cytokines and chemokines, including proinflammatory cytokines, IFN-γ.57 In addition, IL-4+ γδ T cells infiltrating the spleen and heart are major early producers of IL-4, inhibiting acute cardiac inflammation in a mouse model of human viral myocarditis induced by Coxsackievirus of group B3 (CVB3) infection.15 IL-10+ γδ T cells controlled the expansion of CD8+ T cells and reduced TNF-α secretion by activated CD8+ T cells.61 Certain γδ T cells secrete specific cytokines, such as keratinocyte growth factor (KGF), that play important roles in the control of epithelial integrity, fibrin production, and wound repair.62
γδ T cells produce IL-17A, which promotes CVB3-induced viral pancreatitis.48 In addition, γδ T17 cells induced neutrophil infiltration into meninges by CXCL1 and CXCL2 and aggravated ischemic brain injury.65,66 IL-17A produced by γδ T cells in the aorta of C57BL/6 mice induced proinflammatory chemokines to recruit neutrophils and monocytes to promotes atherogenesis.63
γδ T17 CELLS
Differentiation of γδ T17 cells
The thymic programming of human γδ T cells appears to differ from that described in mice.57 In human, γδ T17 cells production occurs peripherally,57 and unlike murine γδ T cells that “naturally” produce IL-17 during thymus development, human γδ T cells acquire this ability only under inflammatory conditions, similar to Th17 cells. Therefore, γδ T17 cells are absent in the human thymus57 but can accumulate in inflamed tissues.67 Circulating Vγ9Vδ2 T cells from healthy adult donors produce little or no IL-1768, unless under inflammatory stimulation. For example, IL-17+ Vγ9Vδ2 T cells were significantly increased in patients with bacterial meningitis.67 Differentiation of human γδ T17 cells in vitro requires TCR activation69 and maximization with other cytokines, including IL-23, IL-6, TGF-β, and IL-1β.67 Human γδ T17 cells were significantly amplified by IL-7 and TCR agonist68 (Figure 1).
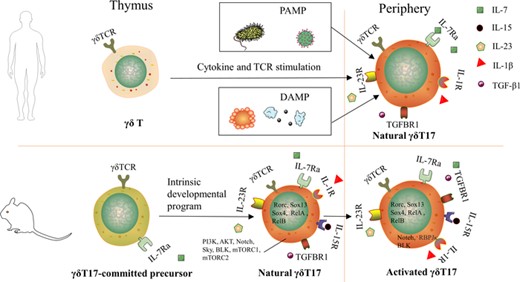
Development of γδ T17 cells in human and mouse. γδ T17 cells do not exist in human thymus, and human γδ T17 cells only appear in peripheral inflammatory conditions, whether pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP). In mice, γδ T17 cells are thought to be uniquely developed in the fetal/perinatal thymus. The precommitted γδ T17 cells differentiated into mature γδ T17 cells through the intrinsic developmental program and were then activated in the peripheral
In mice, γδ T17 cells are identified to be developed in the fetal/perinatal thymus, suggested that physiologic development of γδ T17 cells is terminated before birth and that these cells subsequently persist as a long-lived, self-renewing population.70
Murine γδ T17 cells differentiation requires IL-23, IL-1β, IL-7, and other cytokines. When γδ T17 cells in the thymus, it requires TCR signaling to develop. Subsequently, γδ T17 cells leaved thymus to periphery, in this process, the influence of TCR signaling on γδ T17 cells gradually weakens, the activity of these cells largely guided by innate signaling, such as IL-23 and IL-1β.24,71,72 But there is a research that shows that IL-23 is indispensable for the differentiation of γδ T17 cells in thymus.73,74
IL-7 is an essential cytokine for T cells development and survival, and γδ T cells is no exception.75 γδ T cells in the thymus of IL-7R–/– mice were severely reduced,76 partly due to the effect of IL-7 in V-J recombination of the TCR γ gene.77 γδ T17 cells are dependent on IL-7 for regeneration and survival, because IL-7 connects to up-regulate antiapoptotic molecules Bcl-2 and Bcl-xL.78
The role of IL-6 and IL-15Rα in γδ T17 cells development is controversial. The addition of IL-6 impair induction of γδ T17 cells,72,79 and the lack of IL-15Rα increases γδ T17 cells and their precursors.80 In the thymus, TGF-β1 plays an irreplaceable role in the acquisition of IL-17-producing capacity,73,81 but it inhibited the expansion of γδ T17 cells in vitro and negatively regulated γδ T17 cells expansion or survival.72,82
Transcription factors (TFs) and signaling pathways involved in γδ T17 cells differentiation include RORγt, SRY-related HMG-box (SOX) 4, and SOX13, B-lymphoid tyrosine kinase (Blk), PI3K/Akt, Notch/Hes-1, NF-κB, and mTOR.83 RORγt is the master TF for cytokine IL-17.84 The expression of RORγt was significantly increased in γδ T17 cells from IL-10−/– mice, indicating that RORγt is influenced by IL-10 in γδ T17 cells differentiation.85 SOX4 and SOX13 constitute the central positive regulators of γδ T17 cells differentiation.86,87 SOX4/13 are paramount in the acquisition of the γδ T17 cells effector fate by controlling Rorc (the cardinal TF for γδT17 cells) transcription, enhancing IL-7Rα signaling pathway, and inhibiting Rorc-repressing TFs.87,88 Blk, a B-cell-specific member of the Src family that encodes protein tyrosine kinases, is preferentially expressed in γδ T cells over αβ T cells. BLK affects γδ T17 cells differentiation possibly by initiating its genetic signaling pathway or regulating the γδTCR signaling threshold.89 And, RasGRP1-deficient γδ T cells are damaged and unable to produce IL-17.90
The PI3K/Akt pathway plays an important role in the transcription process of γδ T17 lineage. PI3K inhibition reduced the expression of the TFs RORγt and SOX13 in developing γδ T cells. Syk induces PI3K/Akt pathway activation under γδ TCR stimulation and significantly induces γδ T17 cells development.91,92 Notch/Hes1 signaling pathway plays a crucial part in γδ T17 cells formation. Hes1 expression was associated with IL-17 production, and hes1-deficient mice had almost no γδ T17 cells.93 The expression of IL-7R in γδ T cells can be controlled by the Notch–RBPjκ signaling pathway. Deletion of RBPJκ significantly reduced the size of γδ T17 cells banks in peripheral organs of adult mice.94 γδ T cells require RelA and RelB, members of the NF-κB family, and lymphotoxin β receptors for IL-17 production.95 mTORC1 and mTORC2 signals synergistically promote IL-17 secretion in γδ T17 cells, mTORC1 promotes IL-17 expression by mediating glucose-dependent glycolysis, and mTORC2 enhances γδ T17 differentiation by inhibiting the production of mitoROS.96
Characteristics of γδ T17 cells
Many cell surface markers have been shown to distinguish γδ T17 cells, human and murine γδ T17 cells can be defined as CD3high, IL-7Rαhigh, IL-18Rhigh, and CCR6+ cells; in addition, CD161+ is unique to human γδ T17 cells.64,97–101 γδ T17 cells mainly reside in the heart, blood vessels, lung, skin, vagina, oral barrier sites, and endotheliocyte102,103; these cells are characterized by their rapid production of IL-17A/F23, IL-2224, IL-8, TNF-α, IL-21, and GM-CSF.104 The migration of murine γδ T17 cells is regulated by chemokine receptors CCR2 (during inflammation) and CCR6 (homeostasis).105 An animal study reported that γδ T17 cells appear to directly recognize microbiome-derived lipids provided by nonclassical MHC molecule CD1d.20 CD1d is a typical lipid presenting molecule for NKT cells and can also present lipid antigens to γδ TCR and activate γδ T cells. Hepatocytes express CD1d microbiome lipid antigens rather than pathogen-associated molecular pattern (PAMP) or cytokine signaling to maintain liver-resident γδ T17 cells homeostasis. Low endogenous levels of IL-1β regulated by skin commensals to maintain γδ T17 cells homeostasis in mouse skin, and dysbiosis of the microbiome increases the secretion of IL-1β, leading to γδ T17 cells expansion, which induce dermal inflammation.106 Madecassic acid can inhibit γδ T17 cells activation through PPARγ–pTEN/Akt/GSK3β/NFAT pathway and contributes to the improvement of colitis.107
Both Th17 cells and γδ T17 cells produce IL-17,108 but the 2 cells differ in many ways (Table 3). Th17 cells and γδ T17 cells express different types of TCR, namely αβTCR or γδTCR, which are composed of different groups of somatic rearrangement TCR chains and CD3 subunits.109 The antigen recognition and differentiation requirements of γδ T17 cells are different from those of Th17 cells. Th17 cells development and function depend on αβTCR recognition of antigenic peptides presented by MHC proteins. Upon recognition of the peptide–MHC complex, αβT cells differentiate into effector Th17 cells, producing the cytokine IL-17 that activates innate immune cells or B cells, thereby preventing invasion of pathogens.110 However, TCR γδ can recognize and bind antigen molecules directly without binding MHC molecules or APC.
| . | Th17 . | γδT17 . |
|---|---|---|
| TCR MHC Markers Transcription factors Proximal regulators Effector molecules Location Main targets | TCRαβ+T MHC CD3+, CD4+, IL-23R+, CCR6+ and IL-1R+ RORγt, RORa, STAT3 TGF-β, IL-1β, IL-21, IL-6 and IL-23 IL-17A, IL-17F, IL-21, IL-22, IL-26, TNF-α, CCL20 and GM-CSF Mucosal surface Infection, autoimmune diseases, transplant rejection and tumors | TCRγδ+T Nonclassical MHC CD3+, IL-7Rαhigh, IL-18Rhigh CCR6+ and CD161+ RORγt, STAT3 IL-2, IL-23, IL-1β, IL-7 and TGF-β IL-17A, IL-17F, IL-22, IL-8, IL-21, TNF-α and GM-CSF Mucosa and subcutaneous tissue Infection, autoimmune diseases, tumors, inflammation the central nervous system and psoriasis |
| . | Th17 . | γδT17 . |
|---|---|---|
| TCR MHC Markers Transcription factors Proximal regulators Effector molecules Location Main targets | TCRαβ+T MHC CD3+, CD4+, IL-23R+, CCR6+ and IL-1R+ RORγt, RORa, STAT3 TGF-β, IL-1β, IL-21, IL-6 and IL-23 IL-17A, IL-17F, IL-21, IL-22, IL-26, TNF-α, CCL20 and GM-CSF Mucosal surface Infection, autoimmune diseases, transplant rejection and tumors | TCRγδ+T Nonclassical MHC CD3+, IL-7Rαhigh, IL-18Rhigh CCR6+ and CD161+ RORγt, STAT3 IL-2, IL-23, IL-1β, IL-7 and TGF-β IL-17A, IL-17F, IL-22, IL-8, IL-21, TNF-α and GM-CSF Mucosa and subcutaneous tissue Infection, autoimmune diseases, tumors, inflammation the central nervous system and psoriasis |
| . | Th17 . | γδT17 . |
|---|---|---|
| TCR MHC Markers Transcription factors Proximal regulators Effector molecules Location Main targets | TCRαβ+T MHC CD3+, CD4+, IL-23R+, CCR6+ and IL-1R+ RORγt, RORa, STAT3 TGF-β, IL-1β, IL-21, IL-6 and IL-23 IL-17A, IL-17F, IL-21, IL-22, IL-26, TNF-α, CCL20 and GM-CSF Mucosal surface Infection, autoimmune diseases, transplant rejection and tumors | TCRγδ+T Nonclassical MHC CD3+, IL-7Rαhigh, IL-18Rhigh CCR6+ and CD161+ RORγt, STAT3 IL-2, IL-23, IL-1β, IL-7 and TGF-β IL-17A, IL-17F, IL-22, IL-8, IL-21, TNF-α and GM-CSF Mucosa and subcutaneous tissue Infection, autoimmune diseases, tumors, inflammation the central nervous system and psoriasis |
| . | Th17 . | γδT17 . |
|---|---|---|
| TCR MHC Markers Transcription factors Proximal regulators Effector molecules Location Main targets | TCRαβ+T MHC CD3+, CD4+, IL-23R+, CCR6+ and IL-1R+ RORγt, RORa, STAT3 TGF-β, IL-1β, IL-21, IL-6 and IL-23 IL-17A, IL-17F, IL-21, IL-22, IL-26, TNF-α, CCL20 and GM-CSF Mucosal surface Infection, autoimmune diseases, transplant rejection and tumors | TCRγδ+T Nonclassical MHC CD3+, IL-7Rαhigh, IL-18Rhigh CCR6+ and CD161+ RORγt, STAT3 IL-2, IL-23, IL-1β, IL-7 and TGF-β IL-17A, IL-17F, IL-22, IL-8, IL-21, TNF-α and GM-CSF Mucosa and subcutaneous tissue Infection, autoimmune diseases, tumors, inflammation the central nervous system and psoriasis |
ROLE OF γδ T17 CELLS IN CVD
Myocardial infarction
Myocardial infarction (MI) is a subset of a series of acute coronary syndromes. Plaque or thrombosis results in reduced myocardial perfusion, then occurs MI.111,112 Tissue injury after MI leads to secondary inflammation; there are many immune cells involved, including γδ T cells, macrophages, and neutrophils.113–115 Beyond that, these immune cells also participate in ventricular remodeling.116 It has been reported that almost 90% of the IL-17A-secreting leukocytes were T cells, including approximately 75% γδ T cells and 10% CD4+ T cells on MI, which proved that γδ T cells were the major source of IL-17A in the infarct of the heart.117 By analyzing the clinical blood sample of patients with MI, it was found that compared with normal subjects, restrictive expression of TCR γδ repertoire and alteration expression of IL-17A gene are the important characteristics of γδ T cells in MI patients.118
In mouse, γδ T17 cells has the effects of promoting apoptosis, fibrosis, or inflammation in ventricular remodeling after MI (Figure 2). The apoptotic cardiomyocytes and damaged matrix will release “danger signal” molecules called damage-associated molecular pattern (DAMP),119 such as mitochondrial DNA, F-actin, heat-shock protein, peroxiredoxins, and so on.120–122 TLRs on the surfaces of macrophages, neutrophils, and dendritic cells respond to these DAMP.123–125 These cells were stimulated to release IL-23 and IL-1β. IL-23 is an indispensable upstream regulator of IL-17A production in γδ T cells and can recruit γδ T cells to damaged regions.126 IL-1β be produced by caspase-1 and inflammatory complex, it can work in concert with IL-23 to drive γδ T cells secrete IL-17A. This is IL-23/IL-17 axis.24,99,127,128 Some studies have shown that IL-1 receptor blockers play a protective role in post-MI heart.129,130 Importantly, production of IL-17A by γδ T cells can increase the infarct size and myocardial fibrosis after MI.131 In vitro, IL-17A produced by γδ T17 cells can promote cardiomyocyte apoptosis by regulating caspase-3 activity, pro- to antiapoptotic protein (Bax/Bcl-2) ratio, induce neutrophil migration, and directly activate the p38 MAPK-p53-Bax signaling pathway.117 By contrast, genetic IL-17A deficiency had the opposite effect.117,132

γδ T17 cells participate in the injury process of heart disease. In MI, damaged cardiomyocytes release signaling molecules, which are recognized by TLRs on macrophages and dendritic cells, stimulating the release of IL-23 and active IL-1β, act synergistically to drive IL-17A production in γδ T cells, which also act synergistically with IL-23 in direct response to TLR stimulation. Activated γδ T17 cells can produce cytokines such as IL-17A and IL-22. IL-17RA is expressed on the surface of fibroblasts and macrophages in the heart. On the one hand, it can stabilize the chemotaxis of CXCL1 and other cells in fibroblasts. The expression of the factor, CXCL1, can recruit peripheral neutrophils to the injured myocardium, and neutrophils release reactive oxygen species (ROS) and proteolytic enzymes, thereby causing damage to the cardiomyocytes. On the other hand, IL-17A enhanced M1 macrophage polarization and directly activated macrophages to express TNF-α, IL-6, and IL-1β, further aggravating myocardial injury.
In addition, IL-17A promote the expression of CXCL1 in cardiac fibroblasts, CXCL1 leads to infiltration of neutrophils. These neutrophils release proteolytic enzymes or reactive oxygen species to destroy surrounding muscle cells.102,131,133 And anti-IL-17A antibody can reduce the mouse cardiomyocytes death, which is cultured in low serum and hypoxia.102 IL-17A can also enhance polarization of macrophages toward M1 and directly activated the expression of TNF-α, IL-6, IL-1β, and matrix metalloproteinases (MMPs), which further aggravated myocardial injury.134–136 So, the myocardial interstitial fibrosis and increased production of MMP associated with persistent inflammation contribute to subsequent adverse remodeling.135
Besides, IL-17A increased the proliferation of mouse cardiac fibroblasts in a dose-dependent manner in vitro. One week after MI, fibroblasts selected from IL-17A-KO mice expressed lower levels of fibrosis-related genes such as collagen 1, collagen 3, periostin, and TGF-β than those of wild-type (WT) mice. The survival rate of TCR γδ-KO mice in the later stage of MI was significantly higher than that of WT mice, the lack of γδ T cells limited infarct expansion in myocardium.117,131And the number of neutrophils, macrophages, and T cells in the infarcted heart of TCR γδ-KO mice rather than IL-17A-KO mice decreased.131 The result implicates the functional significance of mediating damage by cardiac γδ T cells after MI.
In terms of γδ T cell migration, except IL-23, the sphingosine-1-phosphate receptor helps to mediate γδ T cells egress from lymph nodes,137,138 and CCL20 acts as a chemokine can recruit γδ T cells to the sites of damaged myocardium.131
Atherosclerosis
Atherosclerosis (AS) is a chronic inflammatory disease of large and medium-sized arterial walls, caused by elevated levels of low-density lipoprotein and cholesterol in the blood.139 AS is a complex disease in which blood vessels, metabolism, and immune system are involved. One of the pathologic manifestation of AS is the formation of plaque. Arterial plaques are characterized by lipid accumulation in the arterial wall and infiltration of immune cells140–143 (Figure 3).
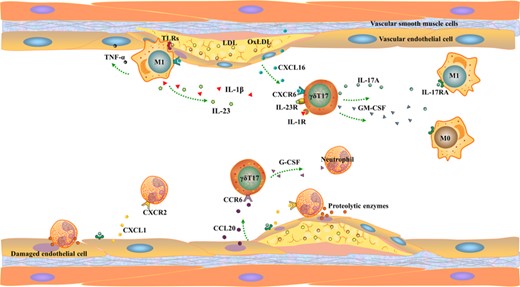
γδ T17 cells participate in vascular disease injury process. γδ T17 cells in arteries are mainly recruited to the damaged site by CXCL16 and CCL20 released by damaged vascular endothelial cells (ECs). Second, the ligand CXCR6 of CXCL16 is also expressed on the surface of macrophages. Activated γδ T17 cells recruit macrophages and neutrophils in peripheral blood by releasing IL-17A, GM-CSF, and G-CSF and aggravate vascular damage. Third, endothelial cells stabilize recruited immune cells by releasing ICAM-1
T cells proliferation is closely related to the regulation of intracellular cholesterol level and cholesterol metabolism-related genes.144,145 Lipid rafts and raft-related proteins can activate TCR signaling pathway and enhance ERK1/2 phosphorylation, which leads to T cells activation and proliferation; this regulatory function is also applicable to γδ T cells.146,147 In apolipoprotein E-deficient (ApoE KO) mice, the number of T cells expressing IL-17A was significantly increased; γδ T17 cells are the majority of these cells. Through the mouse model of human familial hypercholesterolemia, IL-17+ cells were found in the root of the model mice aorta.148 Inhibition of IL-17 expression or signal transduction will decrease the formation of aortic lesions and leukocyte accumulation149,150; it is suggested that γδ T cells can regulate AS through the production of IL-17A.24
In the early stage of AS, γδ T17 cells were the main T cell subsets of aortic root and aortic arch lesions in ApoE KO mice and were mainly distributed in the aortic adventitia.151 ApoE/γδ T Double KO mice showed that γδ T cell deficiency reduced aortic root and arch lesion size in Western diet-fed mice. Similar to the role of IL-23/IL-17 axis in MI, IL-23R+ γδ T cells can locally promote the formation of early atherosclerotic lesions, plaque fall off, and inflammation in aortic roots.152 In fact, after γδ T17 cells are activated,153 the spread of inflammatory response depends on the effect of the IL-17 produced by γδ T17 cells on other immune cells and epithelial cells. IL-17 produced by γδ T cells induces the secretion of proinflammatory cytokines (such as IL-6, TNF-α, and G-CSF) and chemokine (CXCL1, CCL2, CXCL8, and CXCL2) through multiple pathways.18,154,155 For example, IL-17A supports the production of IL-6 and IL-8 and the chemokines CXCL1, CCL2, CCL5, CXCL12, and CXCL10 in several cell types, including endothelial, vascular smooth muscle cells (VSMCs),156,157 fibroblasts, and epithelial cells.154,158 Among them, CXCL1 can promote the recruitment of monocytes to the arterial wall during AS, resulting in a pro-∖inflammatory effect.159 And CXCR4 and its ligand CXCL12 are involved in neutrophil excretion from the bone marrow and neutrophil recruitment to atherosclerotic lesions.160
In addition, IL-17A regulates levels of G-CSF, which affects the number of neutrophils in the blood. The neutrophil infiltration was positively correlated with the degree of early aortic lesion progression in the aortic root of ApoE KO mice, and neutrophil depletion reduced lesion progression.151 And the adenovirus-mediated blockade of IL-17A resulted in decreased levels of IL-6 and G-CSF plasma and reduced the recruitment of neutrophils and macrophages in the aorta, that alleviates the damage. Therefore, γδ T cells can promote the progression of new lesions and increase neutrophils in the process of early AS.160,161
Aortic aneurysm
Aortic aneurysm (AA) is a vascular disease, which can occur anywhere in the aorta. Among them, abdominal aortic aneurysm (AAA) is more common than other aneurysms.162 AAA is characterized by hypoxia and inflammation caused by apoptosis of VSMCs.163,164 However, in addition to VSMCs, other cells are also involved in the occurrence and development of AAA, including γδ T cells165 and other immune cells.166–169 And some studies show that, CXCR5+ γδ T has more effect functions in AAs increased.170,171
The current study found that γδ T17 cells are the main source of IL-17A in AAA patients.171 Analysis of the tumor tissue of AAA patients showed that γδ T cells were involved in the inflammatory response, and IL-17A could induce the production of inflammatory cytokines, chemokines, and nitric oxide.172 Related inflammatory cytokines can further activate MMP, such as MMP-1,173 MMP-2,174 MMP-9,175 MMP-12,176 and cathepsin production.177 These factors can promote inflammation and apoptosis in the aortic wall and can further damage the smooth muscle cells leading to aneurysm expansion and rupture.
A previous study found that dilatation and rupture of AAs are closely related to hypertension, while IL-17A derived from γδ T17 cells mediates hypertension and hypertensive heart injury.178 This also indirectly provides a link between γδ T cells and AA development. In addition, γδ T cells can increase apoptosis of cells that the aortic wall by inhibiting genes involved in phosphoinositide 3-kinase/AKT signaling pathway and the proliferation phase (Sos1, Mtor, Myc), whereas apoptosis-related genes (Pten, Bcl1, Bad) were up-regulated.179 But the inflammatory cytokines including IL-1β, Mcp-1, and TNF-α were down-regulated in the aneurysm tissues of γδ T KO mice.179 And the expression of inflammation-related genes such as IL19, Nlrp3, Spp1, and IL3 down-regulates and then affects the production of related inflammatory cytokines.180–184
Ischemia–reperfusion injury
Ischemia–reperfusion injury (IRI) mostly occurs in clinical, such as organ transplantation, resection, trauma, and septic, or hemorrhagic shock and leads to significant mortality.185,186 Immune cells in the ischemia–reperfusion area release various cytokines, thereby triggering a violent inflammatory response and ultimately aggravating tissue damage. The latest research proves the γδ T17 cells play an important role in the initial stage of immune response in IRI.187
γδ T17 cells are considered to be the main source of IL-17A in myocardial ischemia–reperfusion.188 IL-17A induce cardiomyocyte apoptosis, neutrophil infiltration, and macrophage migration in myocardial injury.102,189 The apoptotic cells cause damage by releasing oxygen-free radicals, cytokines, and recruiting granulocytes in IRI.190–192 The exogenous long pentraxin-3, a member of a phylogenetically conserved group of acute-phase reactants that are involved in inflammation and innate immunity, can improve cardiomyocyte apoptosis and infiltration of neutrophils and macrophages by inhibiting the expansion of γδ T cells and reducing the expression of IL-23 and IL-17A. It plays a protective role in the IRI of cardiomyocytes.193 NKG2D, are activating or coactivating receptor on γδ T cells, stimulating γδ T cells to secrete cytokines. And the blockade of NKG2D will also reduce the damage caused by reperfusion.188 The above indirectly proves the damage effect of γδ T17 cells in myocardial ischemia–reperfusion.
Importantly, recent studies in other IRI models also show that γδ T17 cells have a vital contribution to IRI.65,66,194–196 For example, γδ T cells can be considered as mediators promoting the inflammatory response in the acute stage of intestinal IRI. Selective depletion of γδ T cells after IRI significantly reduced proinflammatory cytokine levels and neutrophil infiltration in the intestinal tract compared with the control group.196 In addition, γδ T KO delayed inflammatory reaction in early renal IRI, but with the extension of reperfusion time, the survival rate of knockout mice decreased.194,197,198
Other CVDs
In addition to the 4 types mentioned above, γδ T17 cells also promote inflammation in other types of CVD, such as stroke, hypertensive heart disease, and viral myocarditis. Stroke is an acute CVD. γδ T17 cells are rapid inflammatory effector cells involved in the delayed period of cerebral ischemia (day 1–4).199,200 IL-17 from γδ T cells acts on macrophages and brain cells directly and promotes the expression of inflammatory mediators that enhance apoptotic neuronal cell death and blood–brain barrier breakdown.201 γδ T17 cells mediate cardiac damage and fibrosis in hypertensive patients. γδ T17 cells secrete IL-17A and accelerate the differentiation of myofibroblasts by promoting cardiac fibroblasts to produce IL-6178. Early activation of γδ T17 cells has positive effect in viral myocarditis by promoting neutrophil infiltration and Th17 induction.48
DISCUSSION ABOUT THE ROLE OF γδ T17 CELLS IN OTHER DISEASES
Not limited to CVD, γδ T17 cells function applies to the whole body. γδ T17 cells have been shown to be involved in a variety of acute injuries, chronic inflammations, and tumors. γδ T17 cells mainly plays a protective role in infection202,203 and plays a pathogenic factor in inflammatory diseases, including arthritis,204,205 colitis,107 diabetes,206 and psoriasis,106,207–209 which has been reviewed and reported. In a variety of murine models, γδ T17 cells have been shown to be critical in fungal,210 bacterial,211 and viral infections,212 primarily through their ability to produce IL-17 and activate epithelial cells and recruit innate cells at the site of infection. In humans, although direct evidence is lacking, studies of loss of function mutations suggest that γδ T17 cells may be important for clearing fungal213,214 and extracellular bacterial infections.215 Psoriasis is a chronic inflammatory skin disease characterized by abnormal proliferation and differentiation of keratinocytes and an influx of inflammatory cells.216 Dysregulation of the microbiome up-regulated IL-1β, resulting in the amplification of γδ T17 cells and skin inflammation. IL-1β can directly activate γδ T17 cells in mice and also stimulate keratinocytes to secrete chemokines, thus attracting more IL-17-producing cells from the periphery, thus establishing an amplified inflammatory response, leading to skin inflammation, which is the pathogenesis of psoriasis.106
However, γδ T17 cells play a dual role in tumor growth. On the one hand, the tumor-promoting function of γδ T17 cells has been demonstrated in various cancer clinical studies and animal models, such as oral cancer,217 human colorectal cancer,104 and Helicobacter felis-infected mouse models of gastric MALT lymphoma.218 Activated γδ T17 cells secrete a large number of cytokines, including IL-17, IL-8, and GM-CSF, which may mobilize and recruit polymorphonuclear myeloid suppressor cells in tumors to establish an effective immunosuppressive environment that promotes tumor progression and immune avoidance.104,219 In multiple tumor models, tumor-infiltrating γδ T17 cells promote tumor growth by supporting angiogenesis.220–223 In addition to promoting angiogenesis, γδ T17 cells also promote tumor growth by inhibiting cytotoxic T cells.224,225 On the other hand, the antitumor function of γδ T17 cells has also been reported. Aging induces γδ T cells to produce IL-17A, which plays a key role in the development of resistance to lung melanoma in elderly mice.226 Infiltration of γδ T17 cells was observed in epithelial tumors after chemotherapy. γδ T17 cells enhanced recruitment of IFN-γ-producing CD8+ T cells, which mediated antitumor function.227 In addition, γδ T17 cells in human malignant pleural effusion secrete more IL-17A than peripheral blood, thereby improved patient survival, suggesting an antitumor role of γδ T17 cells in human cancer.85 Because of the plasticity of γδ T17 cells and the complexity of the tumor microenvironment, the role of γδ T17 cells in tumors is controversial and needs further study.
CLOSING REMARKS
CVD is a general term for vascular disease and heart disease. In recent years, researchers have paid attention to the important role of γδ T cells in disease progression. We summed up that γδ T17 cells predominate in CVD, such as MI, AS, AA, and IRI. In these diseases, γδ T17 cells recruit other immune cells, such as neutrophils or macrophages, by secreting cytokines and chemokines that mediate heart or blood vessel damage.
In previous studies, immunotherapy about γδ T17 cells in CVD treatment was not found. However, there are many types of immunotherapies for CVD, such as therapeutic strategies targeting cytokines, chemokines and their receptors, and immune checkpoints. Anti-IL-1β antibody treatment is currently the only immunotherapy available to treat CVD. Anti-IL-1β therapy improved primary and secondary CVD outcomes.228 Blocking of CCR2 with MLN1202, a highly specific humanized mAb that inhibits CCL2 binding, reduced serum C-reactive protein levels in patients with risk factors for CVD in a phase 2 trial.229 The CD80/86-CD28 and CD80/86-CTLA4 immune checkpoint proteins are pivotal regulators during AS. Inhibition of B7-1 (CD80) by RhuDex® reduces LPS-mediated inflammation in human atherosclerotic lesions.230
By summarizing the pathogenesis of γδ T17 cells in CVD, it may provide some enlightenment for immunotherapy of CVD, including: (1) mAbs inhibit γδ T17 cell-associated chemokines and their receptors; (2) inhibitor blocking checkpoints in γδ T17 cells; (3) drugs modulate γδ T17 cell-related signaling pathways to ameliorate CVD; (4) miRNA inhibits the synthesis of cytokines or chemokines, such as IL-17 and CCL20.
In conclusion, this review provides a more comprehensive understanding of the biology of γδ T17 cells and their role in CVD, which may be the key to effective immunotherapy.
AUTHORSHIP
S. L. and Z. Y. wrote manuscript and designed figures. Y. L. completed the writing of the classification of γδ T cells. J. Z. and S. Z. completed the writing of the differentiation of γδ T17 cells. X. C. and Y. H. completed the writing of introduction and summary. All authors contributed to the article and approved the submitted version. S. L. and Z. Y. contributed equally to this work.
ACKNOWLEDGMENTS
This work was supported by The National Natural Science Foundation of China (No. 81873100).
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
Abbreviations
- CVD
ardiovascular disease
- MI
myocardial infarction
- AS
atherosclerosis
- AA
aortic aneurysm
- IRI
ischemia-reperfusion injury
- TCR
T cells receptors
- CVB3
Coxsackievirus of group B3
- KGF
keratinocyte growth factor
- TFs
Transcription factors
- BLK
B-lymphoid tyrosine kinase
- LTβR
lymphotoxin β receptors
- MHC
major histocompatibility complex
- NKT
natural killer T
- PAMP
pathogen-associated molecular pattern
- MA
Madecassic acid
- pMHC
peptide-MHC
- DAMP
damage-associated molecular pattern
- HSP
heat-shock protein
- TLRs
Toll-like receptors
- DCs
dendritic cells
- ROS
reactive oxygen species
- MMPs
matrix metalloproteinases
- WT
wild-type
- LDL
low density lipoprotein
- VSMCs
vascular smooth muscle cells
- AAA
abdominal aortic aneurysm
- MALT
mucosa-associated lymphoid tissue
- PMN-MDSC
polymorphonuclear myeloid suppressor cells
- MPE
malignant pleural effusion



