-
PDF
- Split View
-
Views
-
Cite
Cite
Jonas S Heitmann, Melanie Märklin, Felicia M Truckenmüller, Clemens Hinterleitner, Daniela Dörfel, Michael Haap, Hans-Georg Kopp, Stefan Wirths, Martin R Müller, A novel flow cytometry-based assay to measure compromised B cell receptor signaling as a prognostic factor in chronic lymphocytic leukemia, Journal of Leukocyte Biology, Volume 108, Issue 6, Dec 2020, Pages 1851–1857, https://doi.org/10.1002/JLB.5TA0320-411RR
Close - Share Icon Share
Abstract
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults. In the past years, new therapeutic approaches (e.g., ibrutinib or venetoclax) have been established and greatly improved treatment of CLL. However, complete control or cure of the disease have not been reached so far. Thus, reliable prognostic markers are an imperative for treatment decisions. Recent studies have revealed an essential role for B cell receptor (BCR) signaling in the pathogenesis, prognosis, and therapy of CLL. A heterogeneous response to receptor stimulation with anti-IgM treatment culminating in different calcium flux capabilities has been demonstrated by several authors. However, the methods employed have not reached clinical application. Here, we report on a flow cytometry-based assay to evaluate calcium flux capabilities in CLL and demonstrate that compromised BCR signaling with diminished calcium flux is associated with a significantly better clinical outcome and progression free survival. In summary, our data strongly support the role of compromised BCR signaling as an important prognostic marker in CLL and establish a novel diagnostic tool for its assessment in clinical settings.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of malignant B cells. The disease generally affects elderly people and shows an indolent clinical course.1 Despite encouraging therapeutic advances, CLL still is considered an incurable disease.2 A number of prognostic markers such as IGHV mutational status, expression of ZAP-70, CD38, and β2-microglobulin as well as certain cytogenetic abnormalities (e.g., del17p) have been described.3 More recent studies have presented a new prognostic score (CLL-IPI) and novel genetic markers (e.g., NOTCH1 and TP53 mutations).4,5
While immunochemotherapy has been the standard first-line treatment of CLL for many years, novel therapies targeting B cell receptor (BCR) signaling such as ibrutinib and idelalisib play an increasingly important role.6 Along these lines, several recent studies have focused on altered signal transduction through the BCR and consecutively impaired calcium flux in CLL. Le Roy et al. demonstrated that in vitro stimulation of the BCR results in calcium flux and nuclear factor of activated T cells (NFAT) activation and could show that DNA binding capacity of NFAT2 correlates with clinical outcome.7 Another study has recently demonstrated that surface IgM expression and intracellular calcium mobilization are dependent on IGHV mutational status and DNA methylation.8 Multiple laboratories have furthermore characterized anergy, a state of BCR unresponsiveness and impaired calcium mobilization, as a hallmark of indolent CLL with favorable outcome.9,10 Previous studies have primarily analyzed calcium mobilization in PBMCs without gating for CLL cells or required a time-consuming isolation of CLL cells before the measurements. Furthermore, ratiometrics with FuraRed by calculation of the ratio between bound and unbound calcium has been established for PBMCs11 and provides several advantages, although has not been used in the context of CLL.12
In the current study, we have established a flow cytometry-based assay to evaluate intracellular calcium flux capabilities in primary CLL cells employing a cohort of 25 patients. The degree of calcium flux was subsequently correlated with well-established prognostic parameters and clinical outcome. Using a receiver operating characteristics (ROC) analysis, we defined a cutoff value to discriminate a responder from a nonresponder population with significant differences in progression-free survival (PFS). In summary, we provide a novel assay to quickly and reliably assess BCR signaling in CLL cells as a means to predict clinical outcome.
Methods
CLL samples
PBMCs of CLL patients (n = 25) or healthy voluntary blood donors (n = 5) were obtained by ficoll gradient centrifugation. Healthy controls consisted of a representative population for age and gender distribution. Patients’ characteristics are summarized in Table 1. All patients had been free of treatment for at least 2 weeks at the time of sample acquisition. A subpopulation of patients had already completed at least 1 treatment. All experiments were approved by the Ethics Committee of the University of Tübingen (574/2011 B02) and written informed consent was obtained from all patients.
Clinical and genetic characteristics of the responder and nonresponder CLL cohorts
| Characteristics . | CLL cohort (n = 25) . | Nonresponder (n = 11) . | Responder (n = 14) . | P-value . |
|---|---|---|---|---|
| Median age (years) | 66.8 (range 50–86) | 66.8 (range 50–79) | 66.9 (range 50–86) | 0.97c |
| Male sex - no. | 19 | 8 | 11 | 0.55a |
| Initial Binet stage | 0.08a | |||
| A | 21 | 11 | 10 | |
| B or C | 4 | 0 | 4 | |
| Binet stage at time of sample collection | 0.0078a | |||
| A | 18 | 11 | 7 | |
| B or C | 7 | 0 | 7 | |
| Hemoglobuline (g/dl) | 13.2 (range 8.2-15.9) | 13.9 (range 12–15.9) | 12.6 (range 8.2-15.9) | 0.16b |
| Platelet count (1000/µl) | 189 (range 7–360) | 217 (range 110–360) | 166 (range 7–257) | 0.24b |
| Leukocyte count (1000/µl) | 75 (range 14–230) | 48.8 (range 14–217) | 111 (range 15.9-230) | 0.027b |
| ZAP-70 positive | 2 (n = 6) | 0 (n = 2) | 2 (n = 4) | 0.47a |
| CD38 positive | 2 (n = 11) | 0 (n = 6) | 2 (n = 5) | 0.18a |
| High risk genetic aberration (17p,11q) or TP53 mutation | 4 (n = 19) | 1 (n = 8) | 3 (n = 11) | 0.60a |
| IGHV | 0.0098a | |||
| Mutated | 10 | 7 | 3 | |
| Unmutated | 7 | 0 | 7 | |
| β-2-microglobuline (mg/l) | 3.6 (range 1–10.8, n = 9) | 2.1 (range 1–3.6, n = 3) | 4.7 (range 2.1-10-8, n = 6) | 0.15b |
| Median number of treatments during time of disease | 1.8 (range 0–10) | 0.18 (range 0–1) | 2.6 (range 0–10) | 0.0008b |
| Characteristics . | CLL cohort (n = 25) . | Nonresponder (n = 11) . | Responder (n = 14) . | P-value . |
|---|---|---|---|---|
| Median age (years) | 66.8 (range 50–86) | 66.8 (range 50–79) | 66.9 (range 50–86) | 0.97c |
| Male sex - no. | 19 | 8 | 11 | 0.55a |
| Initial Binet stage | 0.08a | |||
| A | 21 | 11 | 10 | |
| B or C | 4 | 0 | 4 | |
| Binet stage at time of sample collection | 0.0078a | |||
| A | 18 | 11 | 7 | |
| B or C | 7 | 0 | 7 | |
| Hemoglobuline (g/dl) | 13.2 (range 8.2-15.9) | 13.9 (range 12–15.9) | 12.6 (range 8.2-15.9) | 0.16b |
| Platelet count (1000/µl) | 189 (range 7–360) | 217 (range 110–360) | 166 (range 7–257) | 0.24b |
| Leukocyte count (1000/µl) | 75 (range 14–230) | 48.8 (range 14–217) | 111 (range 15.9-230) | 0.027b |
| ZAP-70 positive | 2 (n = 6) | 0 (n = 2) | 2 (n = 4) | 0.47a |
| CD38 positive | 2 (n = 11) | 0 (n = 6) | 2 (n = 5) | 0.18a |
| High risk genetic aberration (17p,11q) or TP53 mutation | 4 (n = 19) | 1 (n = 8) | 3 (n = 11) | 0.60a |
| IGHV | 0.0098a | |||
| Mutated | 10 | 7 | 3 | |
| Unmutated | 7 | 0 | 7 | |
| β-2-microglobuline (mg/l) | 3.6 (range 1–10.8, n = 9) | 2.1 (range 1–3.6, n = 3) | 4.7 (range 2.1-10-8, n = 6) | 0.15b |
| Median number of treatments during time of disease | 1.8 (range 0–10) | 0.18 (range 0–1) | 2.6 (range 0–10) | 0.0008b |
Complete data set was not available for every patient. The statistical analysis was performed using aFisher’s exact test, bMann-Whitney test, or cStudent’s t-test.
Clinical and genetic characteristics of the responder and nonresponder CLL cohorts
| Characteristics . | CLL cohort (n = 25) . | Nonresponder (n = 11) . | Responder (n = 14) . | P-value . |
|---|---|---|---|---|
| Median age (years) | 66.8 (range 50–86) | 66.8 (range 50–79) | 66.9 (range 50–86) | 0.97c |
| Male sex - no. | 19 | 8 | 11 | 0.55a |
| Initial Binet stage | 0.08a | |||
| A | 21 | 11 | 10 | |
| B or C | 4 | 0 | 4 | |
| Binet stage at time of sample collection | 0.0078a | |||
| A | 18 | 11 | 7 | |
| B or C | 7 | 0 | 7 | |
| Hemoglobuline (g/dl) | 13.2 (range 8.2-15.9) | 13.9 (range 12–15.9) | 12.6 (range 8.2-15.9) | 0.16b |
| Platelet count (1000/µl) | 189 (range 7–360) | 217 (range 110–360) | 166 (range 7–257) | 0.24b |
| Leukocyte count (1000/µl) | 75 (range 14–230) | 48.8 (range 14–217) | 111 (range 15.9-230) | 0.027b |
| ZAP-70 positive | 2 (n = 6) | 0 (n = 2) | 2 (n = 4) | 0.47a |
| CD38 positive | 2 (n = 11) | 0 (n = 6) | 2 (n = 5) | 0.18a |
| High risk genetic aberration (17p,11q) or TP53 mutation | 4 (n = 19) | 1 (n = 8) | 3 (n = 11) | 0.60a |
| IGHV | 0.0098a | |||
| Mutated | 10 | 7 | 3 | |
| Unmutated | 7 | 0 | 7 | |
| β-2-microglobuline (mg/l) | 3.6 (range 1–10.8, n = 9) | 2.1 (range 1–3.6, n = 3) | 4.7 (range 2.1-10-8, n = 6) | 0.15b |
| Median number of treatments during time of disease | 1.8 (range 0–10) | 0.18 (range 0–1) | 2.6 (range 0–10) | 0.0008b |
| Characteristics . | CLL cohort (n = 25) . | Nonresponder (n = 11) . | Responder (n = 14) . | P-value . |
|---|---|---|---|---|
| Median age (years) | 66.8 (range 50–86) | 66.8 (range 50–79) | 66.9 (range 50–86) | 0.97c |
| Male sex - no. | 19 | 8 | 11 | 0.55a |
| Initial Binet stage | 0.08a | |||
| A | 21 | 11 | 10 | |
| B or C | 4 | 0 | 4 | |
| Binet stage at time of sample collection | 0.0078a | |||
| A | 18 | 11 | 7 | |
| B or C | 7 | 0 | 7 | |
| Hemoglobuline (g/dl) | 13.2 (range 8.2-15.9) | 13.9 (range 12–15.9) | 12.6 (range 8.2-15.9) | 0.16b |
| Platelet count (1000/µl) | 189 (range 7–360) | 217 (range 110–360) | 166 (range 7–257) | 0.24b |
| Leukocyte count (1000/µl) | 75 (range 14–230) | 48.8 (range 14–217) | 111 (range 15.9-230) | 0.027b |
| ZAP-70 positive | 2 (n = 6) | 0 (n = 2) | 2 (n = 4) | 0.47a |
| CD38 positive | 2 (n = 11) | 0 (n = 6) | 2 (n = 5) | 0.18a |
| High risk genetic aberration (17p,11q) or TP53 mutation | 4 (n = 19) | 1 (n = 8) | 3 (n = 11) | 0.60a |
| IGHV | 0.0098a | |||
| Mutated | 10 | 7 | 3 | |
| Unmutated | 7 | 0 | 7 | |
| β-2-microglobuline (mg/l) | 3.6 (range 1–10.8, n = 9) | 2.1 (range 1–3.6, n = 3) | 4.7 (range 2.1-10-8, n = 6) | 0.15b |
| Median number of treatments during time of disease | 1.8 (range 0–10) | 0.18 (range 0–1) | 2.6 (range 0–10) | 0.0008b |
Complete data set was not available for every patient. The statistical analysis was performed using aFisher’s exact test, bMann-Whitney test, or cStudent’s t-test.
Calcium measurements of CLL samples
Thawed CLL cells were incubated in 250 µl RPMI 1640 (Invitrogen) containing 5% FCS (Biochrom), 10 µg/ml FuraRed (Invitrogen), and 0.02% Pluronic F127 (Invitrogen) for 25 min at 30°C. Cell suspensions were subsequently diluted with 250 µl of medium containing 10% FCS and incubated at 37°C for another 10 min. Cells were then diluted with 1 ml, 0 mM Ca2+ Krebs-Ringer solution containing 10 mM Hepes, pH 7.0, 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 0.5 mM EGTA and washed with 1 ml, 0 mM Ca2+ Krebs-Ringer solution. Cells were subsequently incubated with CD19-PE-Cy7 (HIB19, 1:100) and CD5-APC (UCHT2, 1:100) (eBioscience) for 20 min. For the measurements, the cells were diluted with 200 µl 4 mM Ca2+ Krebs-Ringer solution and a baseline was recorded formarkers 60 s. Calcium movement was assessed after stimulation of the cells with 20 µg/ml αIgM (Southern Biotech). After 4 min of recording, 1 µM Ionomycin was added as a positive control. Increases in free intracellular Ca2+ were measured on a Canto II (BD) by excitation with a violet laser (406 nm) and a blue laser (488 nm) and appropriate filters. To determine the calcium flux, the ratio of bound and unbound (ratiometrics) FuraRed was calculated with FlowJo 10.1 software.
Statistical analysis
Data are displayed as boxplots with mean ± SEM, 25% or 75% quantiles and min/max whiskers. Statistical tests were used as applicable (Mann-Whitney test, Kruskal-Wallis-Wilcoxon test, Students t-test, or Fisher´s exact test). Statistical analyses were conducted using GraphPad Prism 8.1.0 and JMP® Pro (SAS Institute Inc., Version 14.2) software. Distribution of PFS was calculated by the Kaplan-Meier method. A Log-rank test was performed to test the difference of survival between the 2 groups. For predictive cutoff value estimation, we subgrouped calcium flux with respect to corresponding PFS times. The predictive value of calcium flux for PFS was evaluated by examining the area under the receiver-operator characteristic (ROC) curve using a confidence interval of 95% (using SPSS (SigmaStat, Version 21)). The Youden´s index was used as cut-off. P-values of <0.05 were considered statistically significant.
Results and Discussion
Compromised calcium mobilization upon BCR stimulation has previously been described as a marker for acquired anergy in B cells.9 To assess this in CLL patients, cells were gated for CD19 and CD5 positivity and compared with CD19+ B cells from healthy donors (Fig. 1A). Hereby, we prevented the measurement of calcium signals in non-CLL cells. While measuring calcium flux with visible wavelength excitation fluorescent indicators by flow cytometry in CLL has been previously described,7,8,13,14 detection of Ca2+ ratiometrics by FuraRed provides several advantages. It allows for an analysis with only 1 dye and can correct for artifactual changes in fluorescence due to variations in indicator dye loading, changes in equipment, auto fluorescence of cells, and effects of fluorescent bleaching.11,15,16
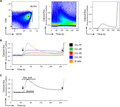
Calcium mobilization capacity of B cells and CLL cells. Flow cytometry analysis was done by FlowJo V 10.1 (A) Gating strategy of 1 representative patient for calcium flux measurements. CLL cells were gated for CD19+ and CD5+ positivity (left). Calcium flux was calculated with the ratio of bound to unbound FuraRed (middle) and the corresponding time kinetics was calculated (right). (B) Calcium kinetics of 1 representative heathy B cell donor and 4 representative CLL patients stimulated with 20 µg/ml αIgM (black arrow) and 1 µM ionomycin added as a positive control (dotted arrow). (C) Calcium flux kinetics were analyzed as described above. Baseline and maximum peak values were obtained by FlowJo V 10.1 software. Baseline calcium flux levels were subtracted from maximum peak values in each patient for further analysis. s = seconds
Ratiometric analysis of the basal calcium mobilization capacity of CLL cells shows no self-acting calcium kinetic characteristics over 1 min while application of soluble αIgM could increase calcium kinetics from baseline levels in almost all samples and the maximum peak slowly declined to baseline levels after 3 min (Fig. 1A). Thereafter, we evaluated the impact of αIgM treatment in B cells from healthy donors (n = 5). All normal CD19+ cells rapidly responded with calcium mobilization upon αIgM stimulation as shown in Fig. 1B for 1 representative donor. Furthermore, all B cells from healthy donors displayed a rapid decline in calcium kinetics over the observed time period. In contrast, circulating CLL cells (n = 25) responded differently to BCR stimulation. We were able to detect either no response or a diminished response, but in all cases the detected response was attenuated when compared to B cells from healthy donors (Fig. 1B). Interestingly, the calcium flux decline was also compromised and in most cases baseline levels were not reached after 240 s.
For further analysis, we defined the calcium flux as the difference between the maximum peak and the baseline level. The maximum peak was consistently reached 5 s after adding αIgM (Fig. 1C). Calcium flux capability was significantly reduced in CLL cells compared to normal B cells (Fig. 2A). Patients with a mutated IgHV mutational status exhibited a significantly attenuated calcium flux (mean 0.03) whereas patients with unmutated IgHV genes showed no attenuation of calcium flux (mean 0.13; Fig. 2B).

Calcium flux discriminates 2 cohorts with different clinical outcome. Calcium flux levels of CLL patients (n = 25) and B cells of healthy donors (n = 5) were determined by calcium mobilization assay. (A) Calcium flux levels are displayed for both groups (Mann-Whitney-test, box plots with min/max. (B) Calcium flux levels for mutated (m-IGHV) and unmutated (u-IGHV) IGHV status are shown (Mann-Whitney test, box plots with min/max. (C) Kaplan-Meier plot for each calcium flux quartile is shown. (D) The predictive value of calcium flux was evaluated by examining the area under the receiver-operator characteristic (ROC) curve using a confidence interval of 95%. All statistical tests were considered statistically significant when P < 0.05. Statistical analyses were computed using SigmaStat, Version 21 (SPSS). The estimated cutoff value was determined by the highest Youden’s index. Calcium flux above 0.085 was classified as responder to IgM stimulation. (E) Progression-free survival (PFS) of the 2 cohorts was depicted as a Kaplan-Meier plot
To determine the impact of calcium flux on progression-free survival (PFS), calcium flux levels of CLL patients were divided into quartiles according to their distribution. Patients in the fourth quartile (blue) showed a median PFS of 1 month. On the contrary, in the first quartile (black), almost no patient progressed to treatment requirement. Interestingly, PFS of the other 2 quartiles was ranked in between suggesting a constant increase in risk with higher calcium flux (Fig. 2C).
The predictive accuracy of calcium flux for progression free survival was confirmed statistically significant (AUC = 0.91, P < 0.001) by ROC analysis (Fig. 2D). Furthermore, the obtained value of the highest specificity and sensitivity (Youden´s index) was used as an optimized calcium flux cut-off. Patients with a calcium flux above 0.085 were defined as responders and below 0.085 as non-responders to αIgM treatment. Kaplan-Meier analysis demonstrated a significantly inferior progression-free survival for responders than nonresponders with a hazard ratio of 10.82 (Fig. 2E).
To assess the value of calcium flux at different time points, sequential samples of 4 patients were analyzed. Calcium flux remained stable over time in patients without treatment indication. One patient who received ibrutinib had a significant decline in calcium mobilization capability below the cutoff value of 0.085 at a later time point (Fig. 3). This is in line with the observation, that in vitro BTK inhibitor application to CLL cells results in a diminished αIgM response.17

Calcium flux levels of CLL patients (n = 4) were determined by calcium mobilization assay at 2 different time points at least 6 weeks apart. CLL #4 received ibrutinib after the first time point. Other patients (CLL #1–3) were only followed up after the first time point
Next, patients were grouped according to their αIgM response and clinical characteristics (Table 1). Interestingly, calcium flux correlated significantly with a higher Binet stage (P = 0.0078) and a higher white blood count (WBC) (48,800 vs. 110,000/µL, P = 0.027) which have both been reported as independent prognostic markers in CLL.18,19 In our data set, the impact of the αIgM response on PFS was superior to WBC and Binet stage (Table 2). While we could not detect a statistically significant correlation for high risk cytogenetics, a mutated IGHV status was clearly associated with the nonresponder cohort (P = 0.0098). More aggressive disease with an increased median number of treatments on the other hand was significantly associated with the responder cohort (P = 0.0008).
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model 1 | |||
| α-IgM response | 9.56 | 1.13-81.26 | 0.0038 |
| Leukocyte count (1000/µl) | 6.15 | 0.99-44.3 | 0.0578 |
| Model 2 | |||
| α-IgM response | 11.3 | 1.3-98.46 | 0.0083 |
| Binet stage (A vs. B/C) | 3.98 | 0.81-19.62 | 0.06 |
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model 1 | |||
| α-IgM response | 9.56 | 1.13-81.26 | 0.0038 |
| Leukocyte count (1000/µl) | 6.15 | 0.99-44.3 | 0.0578 |
| Model 2 | |||
| α-IgM response | 11.3 | 1.3-98.46 | 0.0083 |
| Binet stage (A vs. B/C) | 3.98 | 0.81-19.62 | 0.06 |
Two models have been tested each consisting of αIgM response and leukocyte count or Binet stage.
CI, confidence interval; PFS, progression free survival.
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model 1 | |||
| α-IgM response | 9.56 | 1.13-81.26 | 0.0038 |
| Leukocyte count (1000/µl) | 6.15 | 0.99-44.3 | 0.0578 |
| Model 2 | |||
| α-IgM response | 11.3 | 1.3-98.46 | 0.0083 |
| Binet stage (A vs. B/C) | 3.98 | 0.81-19.62 | 0.06 |
| Variable . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Model 1 | |||
| α-IgM response | 9.56 | 1.13-81.26 | 0.0038 |
| Leukocyte count (1000/µl) | 6.15 | 0.99-44.3 | 0.0578 |
| Model 2 | |||
| α-IgM response | 11.3 | 1.3-98.46 | 0.0083 |
| Binet stage (A vs. B/C) | 3.98 | 0.81-19.62 | 0.06 |
Two models have been tested each consisting of αIgM response and leukocyte count or Binet stage.
CI, confidence interval; PFS, progression free survival.
In summary, we describe a novel assay to determine calcium flux by flow cytometry in CLL. While measuring calcium flux by flow cytometry has been previously described,13 this study is the first ratiometric analysis by FuraRed detection in primary CLL cells.10 A main advantage of this approach when compared to single color staining with Fluo-3 or Fluo-4 is that it allows for less assay variations while the simultaneous measurement of other surface marker in the same test tube is not affected.11,15,16
Additionally, our data demonstrate that BCR responsiveness as assessed by this flow cytometry-based test is a critical prognostic factor in CLL. While CLL cells with mutated IGHV genes generally show an anergic phenotype with BCR unresponsiveness and an indolent clinical course,9 CLL cells with an unmutated IGHV status are responsive to BCR stimulation and exhibit more aggressive disease. Our data clearly demonstrate that CLL patients with an impaired response to BCR stimulation with attenuated calcium flux exhibit a significantly better clinical outcome than their BCR-responsive counterparts. Using ROC analysis, we were able to define a clear cutoff with respect to BCR responsiveness to define 2 cohorts with significantly different clinical outcomes. While our approach is entirely novel, our data are in line with the observations of multiple previous studies. However, further prospective studies are needed to verify this assay in a larger patient cohort. It has been demonstrated in recent studies that CD38 expression and IGHV mutational status have critical influence on BCR signaling in CLL cells.13 Another recent analysis has clearly shown that surface IgM expression and calcium signaling in CLL cells correlate with clinical outcome.8 Other authors have employed an in vitro assay to show that CLL samples can be discriminated into 2 groups with different clinical outcomes depending on calcium flux and the consecutive DNA binding capacity of the transcription factor NFAT2.7
Taken together, we here present a novel flow cytometry-based assay to determine BCR responsiveness in CLL cells. Our approach is fast and can be implemented in routine analysis of CLL patients. Furthermore, we show that this parameter is clinically relevant and can discriminate between patients with indolent and aggressive disease.
Authorship
J.S.H., M.M., and M.R.M. conceived and designed the experiments, and wrote the manuscript. J.S.H. and F.M.T. performed experiments. J.S.H., M.M., and C.H. performed data analysis. J.S.H., C.H., M.H., and D.D. obtained consent and were responsible for sample collection. S.W. and H.G.K. designed research and provided important advice. The manuscript was revised by all authors and before submitting the final version of the manuscript approval from every author was obtained.
Abbreviations
- AUC
area under the curve
- BCR
B cell receptor
- CLL
chronic lymphocytic leukemia
- IGHV
immunoglobulin heavy chain variable
- NFAT
nuclear factor of activated T cells
- PFS
Progression free survival
- ROC
Receiver operating characteristics
- s
seconds
- WBC
White blood count
Acknowledgments
This work was supported by the DFG grant MU 3340/1-1 und MU 3340/1-2 (M.R.M.), the Deutsche Krebshilfe grant 111134 (M.R.M.) and 70113496 (M.M.), a fortüne grant (M.R.M.) and a fortüne junior grant (M.M.) from the University of Tübingen and a scholarship from the Boehringer Ingelheim Fond (J.S.H.).
Disclosure
The authors declare no conflict of interest.
References



