-
PDF
- Split View
-
Views
-
Cite
Cite
Ting Shi, Tiantian Li, Xinru Jiang, Xin Jiang, Qingwen Zhang, Yuli Wang, Yaxing Zhang, Lixin Wang, Xiangyang Qin, Weidong Zhang, Yuejuan Zheng, Baicalin protects mice from infection with methicillin-resistant Staphylococcus aureus via alleviating inflammatory response, Journal of Leukocyte Biology, Volume 108, Issue 6, Dec 2020, Pages 1829–1839, https://doi.org/10.1002/JLB.3AB0820-576RRR
Close - Share Icon Share
Abstract
Sepsis was redefined as life-threatening organ dysfunction caused by a dysregulated host response to infection in 2016. One of its most common causes is Staphylococcus aureus, especially methicillin-resistant Staphylococcus aureus (MRSA), which leads to a significant increase in morbidity and mortality. Therefore, innovative and effective approaches to combat MRSA infection are urgently needed. Recently, host-directed therapy (HDT) has become a new strategy in the treatment of infectious diseases, especially those caused by antibiotic-resistant bacteria. Baicalin (BAI) is the predominant flavonoid and bioactive compound isolated from the roots of Radix Scutellariae (Huang Qin), a kind of traditional Chinese medicine. It has been reported that BAI exhibits multiple biological properties such as anti-oxidant, antitumor, and anti-inflammatory activities. However, the therapeutic role of BAI in MRSA infection is still unknown. In this study, it is found that BAI treatment inhibited the production of IL-6, TNF-α, and other cytokines from MRSA- or bacterial mimics-stimulated Mϕs and dendritic cells (DCs). BAI played an anti-inflammatory role by inhibiting the activation of ERK, JNK MAPK, and NF-κB pathways. Moreover, the serum level of TNF-α was decreased, whereas IL-10 was increased, in mice injected with MRSA. Furthermore, the bacterial load in livers and kidneys were further decreased by the combination of BAI and vancomycin (VAN), which might account for the amelioration of tissue damage. BAI reduced the high mortality rate caused by MRSA infection. Collectively, the results suggested that BAI may be a viable candidate of HDT strategy against severe sepsis caused by antibiotic-resistant bacteria such as MRSA.
Introduction
In recent years, the therapeutic outcome of sepsis has been improved a lot due to the early application of broad-spectrum antibiotics and organ support system.1 However, sepsis remains a major public health concern worldwide as a leading cause of morbidity, mortality, long-term disability, and healthcare costs.2 The nosocomial mortality for patients with sepsis is up to 32.8% in almost 30,000 patients, reported by the Surviving Sepsis Campaign (SSC), which is a global initiative aimed at improving survival in septic patients.3,4 According to the latest antimicrobial resistance surveillance program of China antimicrobial surveillance network (CHINET) in 2017, the detection rate of Staphylococcus aureus (S. aureus) was up to 31.9%, ranking number one in all Gram-positive (G+) bacteria. Notably, the national average detection rate of methicillin-resistant Staphylococcus aureus (MRSA) has been >30% since 2013.5 Generally speaking, antibiotics are the first choice in the treatment of infectious diseases caused by bacteria. However, with the abuse of antibiotics, the emergence of drug-resistant bacteria, such as MRSA, has become a global threat. Sepsis induced by MRSA has a worse prognosis because of its multi-resistance to a range of antibiotics.6,7 Due to the limited choices of effective antibiotics, alternative therapeutic approaches are urgently needed to treat sepsis resulting from MRSA infection. Host-directed therapy (HDT) is an emerging therapeutic strategy of infection, focusing on the immune response of host instead of pathogens.8
During the initiating stage of infection, proper inflammatory response contributes to the control of infection. After the recognition of bacteria, innate immune cells such as Mϕs and dendritic cells (DCs) are activated and secrete a series of chemokines, recruiting circulating monocytes and neutrophils to the sites of infection.9 Monocytes differentiate into Mϕs in situ and phagocytose bacteria together with neutrophils, leading to the limitation of foci. APCs, for example, Mϕs and DCs, digest bacteria and present their Ags to activate T or B cells, eventually leading to the elimination of invading pathogens. However, over-activated innate immune response induced by severe infection will lead to the overwhelming release of cytokines, resulting in cytokine storm.10,11 Cytokine storm is an excessive, aberrant, and non-effective host immune response that is associated with multiple-organ failure, which occur rapidly and deadly.10–12 Clinical studies have detected cytokine storm in patients with sepsis.10,11 Currently, the number of confirmed cases of the coronavirus disease 2019 (COVID-19) has been reported >20,000,000 worldwide. Poor outcomes in COVID-19 were reported to correlate with clinical and laboratory features of cytokine storm syndrome. In addition to vaccine development and approaches that directly target the pathogens, how to deal with the cytokine storm to prevent the immunopathology has become a major focus.13,14 Broad screening for cytokine storm, neutralizing mAbs against pro-inflammatory cytokines (e.g., IL-6), or small-molecule inhibitors of inflammation-related signaling pathways may be useful.14,15
As a major family of pattern recognition receptors (PRRs), TLRs can recognize their corresponding pathogen associated molecular patterns (PAMPs), initiating innate immune response in Mϕs and DCs. Among them, TLR2 is responsible for the recognition of some cell wall components of bacteria (e.g., S. aureus), such as peptidoglycan (PGN) or lipoproteins, evoking inflammatory response.16–19 Pam3CSK4, a synthetic tripalmitoylated lipopeptide that mimics the acylated amino terminus of lipoproteins, which represents the main PAMP of bacteria to stimulate immune cells through TLR2/TLR1 and evokes inflammatory response. Following the ligation of TLR2 by Pam3CSK4 or PGN, the intracellular adaptor myeloid differentiation protein 88 (MyD88) binds to the TIR domain of TLR2 and recruits IL-1 receptor associated kinases (IRAKs). Then, IRAKs are phosphorylated and associate with TNF receptor-associated factor 6 (TRAF6) to phosphorylate TGF-β-activated kinase 1 (TAK1) and IKKα/β respectively. Eventually, it activates the downstream signaling pathways such as extracellular signal regulated kinase 1/2 (ERK1/2), JNK, p38 MAPK, and NF-κB pathways. PI3K/Akt pathway is also involved in TLR2 signaling. The activation of signaling pathways leads to the production of pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-1β, and IL-12), chemokines (e.g., MCP-1), IFN (e.g., IFN-γ), and anti-inflammatory cytokine (e.g., IL-10).20–22
Traditional Chinese medicine (TCM) has been considered as a cornucopia of new drug discovery with HDT effect due to its abundant bioactive substances, such as micheliolide, ephedrine hydrochloride, etc.23–26 Baicalin (BAI) is the predominant flavonoid isolated from the roots of Scutellaria baicalensis (Huang Qin). It has been reported that BAI possesses many different pharmacological activities, including anti-oxidative,27 antitumor,28 and anti-inflammatory roles in LPS stimulation or the infection of Mycobacterium tuberculosis.29,30 Besides that, BAI could suppress the growth of S. aureus, biofilm formation, and expression of virulence-related factors, and exert protective roles against S. aureus infection.31 When combined with osthole, BAI protected mice from S. aureus pneumonia.32 Whether BAI has protective effects during infection caused by MRSA deserves further study. The present study demonstrated that BAI played an anti-inflammatory role in Pam3CSK4, PGN, or heat-killed MRSA (HK-MRSA)-induced inflammatory response. Furthermore, BAI played a protective role against MRSA infection. The synergistic protective effects of BAI and dexamethasone (DXM) or vancomycin (VAN) were also observed in septic mice. Therefore, BAI may be a promising immunomodulatory and/or anti-inflammatory drug candidate of HDT strategy to treat infectious diseases, even those caused by antibiotic-resistant bacteria like MRSA.
Materials and Methods
Reagents
BAI (molecular weight: 446.36, purity > 98%, the chemical structure is shown in Fig. 1A) was purchased from Tauto Biotech (Shanghai, China). Pam3CSK4 was purchased from InvivoGen (San Diego, CA, USA). VAN and DXM were purchased from the National Institute for Food and Drug Control (Beijing, China).
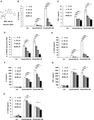
BAI suppresses the production of inflammatory mediators from Pam3CSK4- or PGN-stimulated Mϕs and DCs. (A) The chemical structure of BAI. (B–E) Primary peritoneal Mϕs (3.5 × 105/well) and (F–H) DCs (5 × 105/well) were seeded in 24-well plates respectively, and administrated with a range of concentrations of BAI with or without Pam3CSK4 (100 ng/mL) for 6 or 18 h. Cytokine expression was determined by ELISA and qRT-PCR. Values were means of 3 replicate determinations (n = 3) ± sd. Statistical significance was determined at *P < 0.05; **P < 0.01; and ***P < 0.001
Mice
Specific pathogen-free (SPF) C57BL/6J mice (age 4–8 weeks) were purchased from Joint Ventures Sipper BK Experimental Animal Co. (Shanghai, China). Infected mice were housed in a biosafety level 3 (BSL-3) containment animal facility. Animal welfare and experimental procedures were carried out in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All the animal experiments were approved by the Shanghai Public Health Clinical Center Laboratory Animal Welfare and Ethics Committee (permit number: 2019-A006-01).
Cell culture
Immortalized bone marrow-derived Mϕs (iBMDMs) were maintained in RPMI-1640 supplemented with 10% FBS and M-CSF (20 ng/ml). Thioglycolate-elicited mouse primary peritoneal Mϕs were obtained from female C57BL/6J mice (6-8 weeks of age) as described previously.33 After 2 h incubation, suspending cells were removed and the adherent cells were used as peritoneal Mϕs. Bone marrow cells were obtained from tibias and femurs of C57BL/6 mice, and then induced into DCs by GM-CSF (10 ng/ml) and IL-4 (1 ng/ml) for 5 constitutive days or induced into BMDMs by L929 conditional medium containing M-CSF.25
The strains of bacteria and their growth conditions
The methicillin-resistant Staphylococcus aureus (MRSA, HS488) was kindly supplied by the Institute of Antibiotics, Huashan Hospital, Fudan University and Key Laboratory of Clinical Pharmacology of Antibiotics (Shanghai, China) and grown in Luria-Bertani (1% Tryptone, 0.5% Yeast extract, 1% NaCl) broth at 37°C. HK-MRSA was obtained by heating in a water bath at 90°C for 30 min and then washed in sterile PBS before use.
Detection of cytokines and RNA quantification
The production of cytokines in the culture supernatants was measured by using ELISA kits (R&D Systems, Minneapolis, MN, USA). Detection of cytokines in serum was carried out by cytometric beads array (CBA) assay (BD Biosciences, San Jose, USA) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) analysis was conducted as previously.
Western blot analysis
Cells were lysed with M-PER™ Protein Extraction Reagent (Pierce, Rockford, IL) containing protease and phosphatase inhibitory cocktails (Calbiochem, San Diego, CA, USA). The protein concentration of each sample was determined by BCA assay (Pierce, Rockford, IL, USA). Equal amounts of extracts were loaded for 10% SDS-PAGE and transferred onto PVDF membranes. Membranes were subsequently probed with specific primary Abs (Cell Signaling Technology, Beverly, MA, USA) of interest followed by corresponding secondary Abs. β-Actin was used as an internal control.
Luciferase assays
NF-κB luciferase reporter assays were carried out as previously.25,34 Raw264.7 was transfected with plasmids encoding NF-κB luciferase and pRL-TK Renilla luciferase by utilizing jetPEI™ (Polyplus). After stimulated with Pam3CSK4 for 24 h, cells were harvested and luciferase activities were measured by using the Dual-Glo Luciferase Assay System (E2920, Promega). To exclude the influence of transfection efficiency, the results were normalized by division of Firefly luciferase activity with that of Renilla luciferase.
Bacterial load and histopathology of livers and kidneys in MRSA-challenged mice
Mice were infected intraperitoneally with a dose of MRSA (2 × 108 CFU/mouse) and treated with corresponding drugs. After 12 h, livers and kidneys were obtained and placed in sterile tubes containing 1 ml of 0.9% NaCl, respectively. Tenfold serial dilutions of the liver or kidney homogenates were plated on Luria-Bertani agar plates and incubated at 37°C for 12 h. The numbers of colony-forming units (CFU) were calculated and presented as log10 CFU/g. Liver and kidney specimens were fixed in 10% formalin. The tissues were sliced and stained with H&E. The histopathological changes were observed by using a Zeiss Imager M2 microscope equipped with an Axio CamHRc CCD camera (Carl Zeiss, Goettingen, Germany). The scoring criteria were in line with the degree of inflammation and necrosis of livers and kidneys.35,36
Statistic analysis
All the data were presented as means ± sd. Comparisons between 2 groups were performed using Student’s t-test analysis. Survival analysis was done by using the Log-Rank test. The survival curve was drawn by Graphpad Prism 8.0. Statistical significance was determined at *P < 0.05; **P < 0.01; and ***P < 0.001.
Results and Discussion
BAI at concentration range of 50–200 uM inhibits the production of inflammatory mediators in a dose-dependent manner from Pam3CSK4- or PGN-stimulated Mϕs and DCs
The innate immune cells were served as the first line of defense against invading pathogens. However, excessive activation of various immune cells, such as Mϕs and DCs, resulting in the aggressive production of inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-12, etc.), was a hallmark of sepsis.10,11 Thus, we examined whether BAI may regulate the inflammatory responses in mouse Mϕs and DCs induced by Pam3CSK4. First, to exclude the possibility of drug-induced toxicity, cell proliferation assay was conducted in mouse Mϕs and DCs. As Supplementary Figure 2 showed, BAI (0–100 μM) did not affect the viability of all examined Mϕs and DCs within 48 h (P > 0.05). High concentrations of BAI (150 or 200 μM) did not affect the viability of all examined cells at 24 h (P > 0.05) but had limited inhibition of cellular growth on BMDMs and DCs at 48 h (P < 0.01). For in vitro experiments, mouse Mϕs and DCs were stimulated with Pam3CSK4 (100 ng/ml) and different concentrations of BAI simultaneously for 6 or 18 h. The results showed that BAI significantly reduced the production of IL-6, TNF-α, IL-1β, and IL-12p70 in Pam3CSK4-stimulated mouse primary peritoneal Mϕs (Fig. 1B-E). Similarly, BAI decreased the expression of the pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β) in Pam3CSK4-induced Raw264.7 in a dose-dependent manner (Supplementary Fig. 3). We also found that BAI decreased the production of IL-6, TNF-α, and IL-12p70 following the stimulation of Pam3CSK4 in DCs (Fig. 1F-H).
PGN, the main component of G+ and G− bacterial cell wall, is another bacterial PAMP and a ligand of TLR2 that induces inflammatory response in immune cells. To explore the regulative role of BAI on PGN-evoked innate immune response, mouse primary peritoneal Mϕs were stimulated simultaneously with PGN (25 μg/ml) and BAI. Data showed that BAI reduced the expression of pro-inflammatory cytokine IL-6, TNF-α, and IL-1β (Supplementary Fig. 4).
Collectively, BAI down-regulated bacterial components or mimics (Pam3CSK4 or PGN) induced inflammatory response by suppressing the production of pro-inflammatory cytokines in a dose-dependent manner in mouse Mϕs and DCs.
BAI reduces the production of IL-6 and TNF-α triggered by heat-killed MRSA in peritoneal Mϕs and immortalized bone marrow-derived Mϕs
Heat-killed bacteria maintain their structure and PAMPs that can elicit immune response. Meanwhile, it avoids the influence of bacterial propagation. To better identify the regulatory roles of BAI in inflammatory response during bacterial infection, Mϕs were stimulated by HK-MRSA instead of PAMP mimics. Data showed that BAI inhibited the secretion of IL-6 and TNF-α in a dose-dependent manner in HK-MRSA-stimulated primary peritoneal Mϕs (Fig. 2A and B), as well as in iBMDMs (Fig. 2C and D). Moreover, after 24 h stimulation of HK-MRSA, the protein levels of IL-6 or TNF-α in the supernatants were reduced by >60% with the treatment of BAI (P < 0.001) in iBMDMs. These results indicated that BAI down-regulated HK-MRSA-induced inflammatory response.
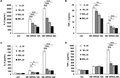
BAI reduces the production of IL-6 and TNF-α from HK-MRSA-stimulated peritoneal Mϕs and iBMDMs. Primary peritoneal Mϕs (3.5 × 105/well) and iBMDMs (2 × 105/well) were seeded into plates and incubated overnight respectively. Cells were administrated with different concentrations of BAI for 12 or 24 h with the stimulation of HK-MRSA (MOI = 10). The secretion of IL-6 and TNF-α was detected in the supernatants of primary peritoneal Mϕs (A, B) or iBMDMs (C, D). Values were means of 3 replicate determinations (n = 3) ± sd. Statistical significance was determined at *P < 0.05; **P < 0.01; and ***P < 0.001
BAI inhibits the activation of ERK, JNK, and NF-κB signaling pathways with the stimulation of Pam3CSK4 or HK-MRSA
As shown previously, BAI played an anti-inflammatory role with the stimulation of Pam3CSK4, PGN, or HK-MRSA. As a commonly used ligand for TLR2, Pam3CSK4 activates ERK, JNK, p38 MAPKs, NF-κB, and PI3K/Akt signaling pathways, all of which account for the production of the downstream cytokines. To further dissect the molecular mechanisms under the immunoregulatory role of BAI, the phosphorylation of the main mediators in these signaling pathways were detected by Western blot. As shown in Fig. 3A and B, BAI dramatically down-regulated Pam3CSK4-elicited phosphorylation of ERK, JNK, IKKα/β, ΙκΒα, and p65 of NF-κB in Raw264.7. Correspondingly, Pam3CSK4-induced activity of NF-κB luciferase reporter gene was decreased by BAI in Raw264.7 (Fig. 3C). Furthermore, we tested whether BAI could inhibit the activation of MAPKs and NF-κB signaling pathways evoked by HK-MRSA in primary Mϕs (BMDMs). In line with the results of Pam3CSK4 stimulation in Raw264.7, BAI also markedly reduced the expression of pERK, pJNK, pIKKα/β, and pp65 of NF-κB upon HK-MRSA stimulation in BMDMs (Fig. 3D and E).

BAI inhibits the activation of ERK, JNK and NF-κB signalling pathways with the stimulation of Pam3CSK4 or HK-MRSA. (A and B) Raw264.7 cells (1 × 106/well) were seeded in 6-well plates overnight and stimulated with Pam3CSK4 (100 ng/ml) with or without BAI (100 μM) for the indicated time periods. The phosphorylation of MAPK and NF-κB pathways were confirmed by Western blot. (C) Raw264.7 was co-transfected with NF-κB luciferase reporter plasmid and pRL-TK-Renilla-luciferase plasmid. After 30 h, cells were stimulated with Pam3CSK4 (100 ng/ml) for 24 h, and the luciferase activities were measured. The NF-κB luciferase activities were presented as fold increase. Values were means of 3 replicate determinations (n = 3) ± sd. (D and E) BMDMs (1.5 × 106/well) were seeded in 6-well plates overnight and stimulated with HK-MRSA (MOI = 10) with or without BAI (100 μM) for the indicated time periods. The phosphorylation of MAPK and NF-κB pathways in were confirmed by Western blot. Similar results were obtained in 3 independent experiments. Statistical significance was determined at **P < 0.01 and ***P < 0.001
PI3K/Akt signaling pathway also contributed to the production of inflammatory cytokines after TLR2 ligation. Phosphorylation of Akt at Ser473 was a significant indicator of PI3K/Akt pathway activation. The isoforms of p70 S6 kinase (p70S6K), eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), and glycogen synthase kinase (GSK)-3β represent the crucial downstream kinases of Akt. The results showed that BAI slightly inhibited the phosphorylation of Akt (Ser473) and its downstream signaling molecule p70S6K (Thr389) with the stimulation of Pam3CSK4 (Supplementary Fig. 5A), but had no inhibitory effects on those induced by HK-MRSA in BMDMs (Supplementary Fig. 5B).
Taken together, BAI influenced the activation of MAPKs and NF-κB in Pam3CSK4 or HK-MRSA-stimulated Mϕs. And the inhibition of ERK, JNK, and IKKα/β phosphorylation largely accounted for the immunoregulatory roles of BAI.
BAI decreases the secretion of serum TNF-α and increases IL-10 level in MRSA-challenged mice
The overwhelming expression of pro-inflammatory cytokines is a typical character of SIRS and usually results in organ dysfunction with subsequent multiple organ failure, and even death.10,11 Chemokines (such as MCP-1) and inflammatory cytokines (such as IL-12 and IFN-γ) contributed to self-amplifying signaling cascade. The level of serum inflammatory mediators was associated with the severity of septic patients. Thus, the secretion of serum cytokines in MRSA-challenged mice was examined by CBA methods. MRSA-induced peritonitis mouse model was carried out by intraperitoneal injection of MRSA (2 × 108 CFU/mouse). Since VAN was the first choice of antibiotics for MRSA infection in clinic,37–39 it was chosen as a positive control in our mouse model. Besides that, DXM was also a common drug to cure sepsis and septic shock in clinic. In the sera of MRSA-challenged mice, the production of TNF-α was decreased (P < 0.05), while IL-10 was increased (P < 0.05) by more than 4-folds by BAI treatment (Fig. 4B and F). The level of serum inflammatory cytokines in mice was down-regulated by DXM or VAN alone (Fig. 4A-F). Interestingly, the synergetic effects of BAI and VAN in the down-regulation of MCP-1 and IFN-γ were detected (Fig. 4D and E).
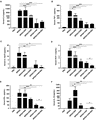
BAI decreases the serum level of TNF-α and increases IL-10 level in MRSA-challenged mice. C57BL/6J mice were intraperitoneally infected with MRSA (2 × 108 CFU/mouse) and treated with BAI (100 mg/kg), DXM (7 mg/kg), and VAN (110 mg/kg). Blood samples were collected, clotted, and centrifuged at 4°C (3500 rpm, 25 min) to obtain serum samples after 12 h. The serum levels of IL-6 (A), TNF-α (B), IL-12p70 (C), MCP-1 (D), IFN-γ (E), and IL-10 (F) were measured by cytometric bead array (CBA) assay. Data were expressed as mean ± sd (N = 5/group). Statistical significance was determined at *P < 0.05; **P < 0.01; and ***P < 0.001
In conclusion, BAI treatment alone decreased TNF-α expression and increased IL-10 secretion in vivo. What’s more, the synergistic role of BAI and VAN decreased the secretion of MCP-1 and IFN-γ in infective murine model.
The combination of BAI and VAN reduces bacterial load in organs and alleviates liver and kidney injury in MRSA-infected mice
During sepsis, livers and kidneys are considered to be the vulnerable organs.10,11 Bacterial load and pathological injury in these organs are considered as 2 aspects associated with the severity of sepsis. Therefore, the effects of BAI on bacterial load and histopathological changes in MRSA-infected mice were examined. As shown in Fig. 5A and B, the bacterial loads in livers and kidneys were significantly reduced by DXM or VAN treatment (P < 0.01). Although BAI alone had no effects on the bacterial burden in MRSA-challenged mice, there was a synergetic role of BAI and VAN in decreasing the bacterial load in livers and kidneys.

The combination of BAI and VAN reduces bacterial load in organs and alleviates liver and kidney injury in MRSA-infected mice. C57BL/6J mice were intraperitoneally injected with MRSA (2 × 108 CFU/mouse) and treated with BAI (100 mg/kg), DXM (7 mg/kg), and VAN (110 mg/kg). Twelve hours later, the livers and kidneys were collected. Bacterial loads in livers (A) and kidneys (B) were calculated by the Log10 CFU/g. (C) H&E staining of liver or kidney sections were from the indicated group (200×). The representative results from 3 independent experiments are shown. (D and E) Liver and kidney injury were scored by observing the morphological structure. Data were expressed as mean ± sd (N = 5/group). Statistical significance was determined at *P < 0.05 and **P < 0.01
The histopathological damages of livers and kidneys were also investigated. H&E staining of the liver biopsy section from PBS-treated group exhibited a normal histological structure without pathological changes (Fig. 5C–E). Significant inflammatory symptoms such as infiltration of inflammatory cells and disordered array of hepatic plates were observed in MRSA-infected mice. Compared with MRSA-infected group, mice treated with DXM and VAN separately exhibited less infiltration of inflammatory cells and milder cellular injury. Additionally, the combined treatment of BAI and VAN had a synergistic protective effect on liver injury in MRSA-challenged mice. The similar synergistic protection of BAI and VAN was observed in kidneys. These results indicated that BAI combined with VAN played a synergistic protective role in MRSA-infected mice.
BAI improves the survival status of mice challenged by MRSA
The survival analysis was carried out to determine the protective role of BAI against septic shock induced by MRSA. PBS or BAI treatment alone had no significant adverse effects and did not cause mortality during the observation period (data not shown). According to the usage of antibiotics in clinic, VAN is utilized as a sensitive antibiotic for MRSA infection. Additionally, glucocorticoids are usually used in the treatment of severe sepsis.40 As shown in Fig. 6, the mortality rate was 100% in MRSA-infected septic murine model. Thirty percent of mice in BAI-treated group survived (P < 0.05). No mortality was observed in VAN treatment group or the combined treatment group (VAN + BAI group). Although the survival rate in DXM-treated group had no significant increase, the combined treatment of DXM and BAI protected 50% of mice against lethal MRSA infection, which suggested a synergistic role between DXM and BAI (P < 0.01). Moreover, the mice in DXM + BAI treatment group exhibited significant recovery with ameliorated clinical symptoms (such as lethargy, hunched posture and piloerection) compared to those in DXM treatment group. Moreover, the bactericidal effect of BAI was determined by using the minimal inhibitory concentration (MIC) test, which indicated that BAI had no direct antibacterial effects on MRSA within the examined concentrations (0.125-128 μg/ml; data not shown). Whether BAI could promote the phagocytosis of bacteria by phagocytes in vivo, accounting for the decrease of bacterial burden in organs needs to be further demonstrated.

BAI improves the survival status of mice challenged by MRSA. C57BL/6 J mice were challenged with lethal dose of MRSA (6 × 108 CFU/mouse, i.p.) and treated with VAN (110 mg/kg), DXM (7 mg/kg), BAI (100 mg/kg), or their combination. Survival status of different groups were recorded for 7 consecutive days. N = 20/group. Data were analyzed using Log-Rank test and survival curve was generated by GraphPad Prism 8.0 software. Statistical significance was determined at *P < 0.05; **P < 0.01; and ***P < 0.001.
In conclusion, BAI inhibited the production of pro-inflammatory factors (IL-6, TNF-α, IL-1β, and IL-12p70) induced by Pam3CSK4, PGN, or HK-MRSA in Mϕs and DCs. In MRSA-challenged mice, BAI decreased the serum level of TNF-α. Impressively, BAI increased the level of anti-inflammatory cytokine IL-10 by 4.4-folds. The anti-inflammatory role of BAI in vivo was not equal to its effects shown in vitro might because the in vivo circumstance is composed of different types of cells, thus, it is much more complicated than 1 single cell type in vitro. Whether and how BAI influences the role of other immune cells deserve to be demonstrated. BAI had a protective role against MRSA infection, and the combination of BAI and DXM instead of DXM treatment alone was demonstrated to play a protective role in MRSA-induced septic mice. The synergistic role of BAI and VAN could not be exhibited because VAN offered 100% protection in MRSA infected mouse model in our study, but the synergistic role of them decreased the bacterial load of livers and kidneys, alleviating the histologic damage of these organs, indicating that the combination of BAI and VAN might reduce sequelae. Although the strain of MRSA in our study is sensitive to VAN, the reduced susceptibility of MRSA to VAN was reported recently in clinic,41,42 indicating that VAN might not always be effective for the treatment of MRSA infection. It is noteworthy that anti-microbial resistance (AMR), a tough problem worldwide, is evolutionary and changeable, so it might not always be so easy to find a suitably effective antibiotic as AMR continues to develop in the future. Thus, it is worthy and valuable to explore an alternative therapeutic strategy (also called Plan B) or adjuvant drug candidates besides antibiotics. Host-directed therapy (HDT) is a new strategy different from antibiotics to treat infectious diseases from the aspect of host which has an advantage of treating infection caused by antibiotic-resistant bacteria. In this study, we demonstrated BAI does not have a direct effect on MRSA itself, but it down-regulates the overactivated inflammatory response caused by MRSA infection, offering protection to important organs and decreasing the mortality rate. Thus, BAI may be a promising immunomodulatory and/or anti-inflammatory agent of HDT strategy for the management of infection caused by antimicrobial resistant bacteria (e.g., MRSA).
Authorship
T.S. and T.T.L. are joint first authors of this study. Y.J.Z., W.D.Z., and X.Y.Q. are joint senior authors of the current study and designed the experiments. T.S., T.T.L., X.R.J., X.J., Q.W.Z., Y.L.W., Y.X.Z., and L.X.W. performed the experiments. T.S. and T.T.L. analyzed the data. T.S., Y.J.Z., W.D.Z., and X.Y.Q. wrote the manuscript. All authors read and approved the manuscript. T.S. and T.T.L. contributed equally to this work.
Abbreviations
- BAI
baicalin
- DCs
dendritic cells
- DXM
dexamethasone
- HDT
host-directed therapy
- iBMDMs
immortalized bone marrow-derived Mϕs
- MRSA
methicillin-resistant Staphylococcus aureus
- PGN
peptidoglycan
- SIRS
systemic inflammatory response syndrome
- VAN
vancomycin
Acknowledgments
The authors would like to thank Prof. Xiaojian Wang (Zhejiang University) for sharing iBMDM cells. The authors also thank Prof. Huazhang An (Second Military Medical University) for providing plasmids. The authors also thank Miss Naomi Ong for language editing. This study was supported by National Key Research and Development Program of China (2020YFC0845400, 2017YFC1700200, 2017YFC1702000), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Education, National Natural Science Foundation of China (81471537), Novel coronavirus pneumonia emergency tackling project at SHUTCM (2019YJ06-03) and Interdisciplinary Project of “Clinical Immunology of Traditional Chinese Medicine” in Shanghai (30304113598).
Disclosure
The authors declare no conflicts of interest.
References
Supplementary data
Supporting Information
Supporting Information
Supporting Information
Supporting Information



