-
PDF
- Split View
-
Views
-
Cite
Cite
Ying Zheng, Lei Chai, Yanhui Fan, You-Qiang Song, Kwan-Yat Zee, Wen Wei Tu, Lijian Jin, Wai Keung Leung, Th2 cell regulatory and effector molecules single nucleotide polymorphisms and periodontitis, Journal of Leukocyte Biology, Volume 108, Issue 5, Nov 2020, Pages 1641–1654, https://doi.org/10.1002/JLB.4MA0720-698RR
Close - Share Icon Share
Abstract
To investigate the association between T helper 2 (Th2) cell regulatory and effector molecules’ genetic polymorphisms and periodontitis. Single nucleotide polymorphisms (SNPs) of 11 Th2 cell regulatory or effector molecules genes (CD28, CTLA4, IL4, IL5, IL6, IL9, IL10, IL13, IL4R, GATA3, STAT6, and rs1537415; total 130 SNPs) were studied in Chinese nonsmokers (163 periodontitis-free controls, 141 periodontitis patients) using Sequenom iPlex assays. SNPs potentially associated with periodontitis (adjusted allelic P < 0.1) in this cross-sectional study were further investigated via meta-analysis. Allele G of rs4553808 in promoter of CTLA4 was more frequently detected in periodontitis than controls (P < 0.005), but did not remain significant after age and gender adjustment. Haplotype (GTT) in a block of three CTLA4 SNPs (rs4553808, rs16840252, rs5742909) was significantly associated with periodontitis. Meta-analysis of SNPs identified indicated allele T of CTLA4 rs5742909 (3 studies; 461 control, 369 periodontitis) and allele G of IL6 rs1800796 (18 studies; 2760 control, 2442 periodontitis) were significantly associated with periodontitis (OR = 1.44 and OR = 1.30, respectively). Within limitations of this study, a haplotype of CTLA4 concerning Th2 cell regulation, may be associated with periodontitis in Chinese nonsmokers followed. Meta-analysis indicated rs5742909 of CTLA4 and rs1800796 of IL6 appeared significantly associated with periodontitis.
1 Introduction
T helper 2 (Th2) cells have been suggested to play role in the development of periodontitis.1,2 They influence human humoral immunity via modulation of B cell IgG and IgE secretion. T helper 1 (Th1) cells, on the other hand, promote macrophage activation and delayed hypersensitivity, hence influencing cell-mediated immune responses. There are some hypotheses suggested that the imbalance between Th1 and Th2 cells and their related cytokines production may be associated with periodontitis.1,2 If the subgingival biofilm continuously irritate the periodontal tissue, B cells and plasma cells would be the major immune cell types recruited in the advanced lesions, implying a more significant role for Th2 cells in periodontal defense.3 Th2 cells were regarded as agents to ameliorate periodontal disease symptoms, because less Th2 type cytokines (IL-4 and IL-6) were detected in the disease tissue.4,5 The controversy remained for decades, but many studies supported or implied Th2 cells may play an important role in periodontitis.
Caused by mixed opportunistic bacterial infection, periodontal disease expression/progression could be modified by smoking, diabetes, stress, and genetic factors.6,7 Interests in genetic predisposition of periodontitis grew over last couple of decades.7 An important gene reported in the first genome-wide association study (GWAS) on previously named aggressive periodontitis (AP), or periodontitis in young adults,8,9 was related to Th2 cells. It was reported that a single nucleotide polymorphism (SNP) rs1537415 in glycosyltransferase 6 domain containing 1 gene would encode a transcription factor binding site with reduced globin transcription factor binding protein 3 (GATA-3) attachment affinity and the SNP is significantly associated with AP.10 GATA-3 is considered the master switch required for the development of Th2 cell11 and its mRNA expression appeared significantly higher in healthy gingiva than in advanced periodontitis lesions.12 Although the GWAS study was on young adults with periodontitis, it is possible that periodontitis of all ages in general may share similar/same genetic risk indicator. However, a recent meta-analysis on periodontitis GWAS, including the aforementioned report, with a sample size of 45,651 stated an insignificant association between rs1537415 and periodontitis.13 The same paper also reported low heritability of periodontitis, a common limitation for GWAS study on complex traits.14
In brief, Th1 cells exhibit proinflammatory responses and can enhance the destructive pathway in periodontal tissues. Th2 cells and T regulatory (Treg) cells, on the other hand, associate with anti-inflammatory responses and immune suppressive properties, which can control or attenuate periodontal disease development. Treg is a protective T cell subset to prevent tissue damage in the periodontal environment.15 We hypothesized that hereditary acquired inadequate Th2 responses may associate with increased risk for periodontitis and such genetic predisposition could be detectable from periodontitis patients via a candidate gene approach.
The differentiation of Th2 cells involved many regulatory molecules, including cluster of differentiation 28 (CD28), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), IL-4, IL-4 receptor (IL-4R), GATA-3, and STAT6 (Supporting Information Fig. S1). Th2 cells-produced effector molecules mainly include IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13.16–18
A few studies investigated the association between periodontitis and genetic polymorphisms of genes coding for the aforementioned molecules, including CD28, CTLA4, IL-4, IL-6, IL-4R, IL-10, and IL-13.7,19,20 However, only limited SNPs concerning these gene regions were covered in the previous investigations. Here we carried out a candidate gene study on periodontitis, based upon the aforementioned hypothesis, to investigate the association between periodontal disease and SNPs of genes known to control or regulate Th2 cell differentiation (CD28, CTLA4, IL-4R, GATA3, STAT6) and Th2 cell effector molecules production (IL-4, IL-5, IL-6, IL-9, IL-10, IL-13) in Chinese nonsmokers. We postulated that the same SNPs reported by Song et al.21 and Zhao et al.22 would be significantly associated with periodontitis in the cohorts followed.
Based upon the results of this study, we also undertook a meta-analysis of any relevant Th2 regulatory SNPs, tentatively identified in the current study to be potentially associated with periodontitis, in an attempt to improve the strength of findings observed in the current study.
2 Materials and Methods
2.1 Participants
We computed the study sample size using a web browser program, Genetic Association Study Power Calculato r2, which was derived from CaTS power calculator.24 Taking rs1537415 as a reference SNP, to reach a power of 95%, with a disease prevalence of 40%,25 a disease allele frequency of 38.8%, and a genotype relative risk of 1.59,10 the anticipated sample size should be 130 cases and 130 controls.
Patients’ records, orthopantomogram (OPG) plus other available radiographs were screened within 1 mo of first attendance to a dental hospital. Potential eligible subjects were invited to attend a clinical examination. Roughly one control case (gender and age matched) was recruited for each test case included.26,27
Chinese, nonsmokers, >35 yr old, who might be periodontitis free or have periodontitis (based on OPG on first attendance26,27) but otherwise healthy were invited for periodontal examination. Demographic information and medical and dental histories were obtained from patients’ records, supplemented by information obtained during the day of the clinical examination. Race and ethnicity were self-reported, with a participant being considered Chinese if his or her biologic parents, grandparents, and great grandparents were all reported to be ethnic Chinese.26,27 Smoking history was self-reported; patients who currently smoked or who had quit within 12 mo were considered to be smokers and those who had never smoked or who had quit for more than 12 mo were considered to be nonsmokers. Smokers were excluded. For periodontitis subjects, scans of their OPG had to show >50% alveolar bone loss at >30% of proximal sites measured by a trained examiner using Schei ruler, with each tooth contributing two sites: mesial and distal.28 After OPG screening, clinical periodontal examination by another designated examiner had to confirm the presence of at least two teeth in each quadrant with probing pocket depth (PPD) ≥5 mm and bleeding on probing (BOP). Periodontitis-free participants or controls were recruited as someone with no PPD > 4 mm and no gum recession >2 mm in any site, and had no history of tooth loss due to periodontal diseases.26,27 Over the 3 yr study period (2005–2008), 304 Chinese participants, including 163 periodontitis-free controls and 141 periodontitis patients fulfilling all selection criteria, consented and with acceptable DNA quality/quantity from blood sample (Section 2.2) were recruited in Prince Philip Dental Hospital, Hong Kong (Fig. 1). The strengthening the reporting of observational studies in epidemiology guidelines were followed. All participants were systemic healthy and were nonsmokers (Table 1). Written informed consent was obtained from all participants. The study was approved by Ethics Committee, Faculty of Dentistry, The University of Hong Kong (1/8/12d).
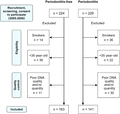
Flow diagram showing participants selection process in the candidate genes study
| . | Periodontitis-free n = 163 . | Periodontitis n = 141 . | Test . | P-value . |
|---|---|---|---|---|
| Age in years | 44.2 ± 6.4 | 45.2 ± 5.6 | t | 0.16 |
| (35–72) | (35–59) | |||
| Gender | ||||
| Male | 64 | 50 | χ2 | 0.38 |
| Female | 99 | 91 | ||
| Standing teeth | 27.3 ± 2.0 | 25.1 ± 2.9 | t | <0.05 |
| (22–32) | (16–28) | |||
| Percentage sites with radiographic | ||||
| Bone loss ≥50%a | 0.0 ± 0.0 | 43.7 ± 14.2 | – | – |
| Percentage sites probing attachment level ≥5 mm | 0.0 ± 0.0 | 51.7 ± 28.8 | – | – |
| BOP% | ND | 74.4 ± 25.5 | – | – |
| . | Periodontitis-free n = 163 . | Periodontitis n = 141 . | Test . | P-value . |
|---|---|---|---|---|
| Age in years | 44.2 ± 6.4 | 45.2 ± 5.6 | t | 0.16 |
| (35–72) | (35–59) | |||
| Gender | ||||
| Male | 64 | 50 | χ2 | 0.38 |
| Female | 99 | 91 | ||
| Standing teeth | 27.3 ± 2.0 | 25.1 ± 2.9 | t | <0.05 |
| (22–32) | (16–28) | |||
| Percentage sites with radiographic | ||||
| Bone loss ≥50%a | 0.0 ± 0.0 | 43.7 ± 14.2 | – | – |
| Percentage sites probing attachment level ≥5 mm | 0.0 ± 0.0 | 51.7 ± 28.8 | – | – |
| BOP% | ND | 74.4 ± 25.5 | – | – |
Data presented as mean ± sd (range in parenthesis) or as absolute numbers; ND, not determined; and BOP, bleeding on probing.
Panoramic proximal alveolar bone loss measured using Schei ruler.28
| . | Periodontitis-free n = 163 . | Periodontitis n = 141 . | Test . | P-value . |
|---|---|---|---|---|
| Age in years | 44.2 ± 6.4 | 45.2 ± 5.6 | t | 0.16 |
| (35–72) | (35–59) | |||
| Gender | ||||
| Male | 64 | 50 | χ2 | 0.38 |
| Female | 99 | 91 | ||
| Standing teeth | 27.3 ± 2.0 | 25.1 ± 2.9 | t | <0.05 |
| (22–32) | (16–28) | |||
| Percentage sites with radiographic | ||||
| Bone loss ≥50%a | 0.0 ± 0.0 | 43.7 ± 14.2 | – | – |
| Percentage sites probing attachment level ≥5 mm | 0.0 ± 0.0 | 51.7 ± 28.8 | – | – |
| BOP% | ND | 74.4 ± 25.5 | – | – |
| . | Periodontitis-free n = 163 . | Periodontitis n = 141 . | Test . | P-value . |
|---|---|---|---|---|
| Age in years | 44.2 ± 6.4 | 45.2 ± 5.6 | t | 0.16 |
| (35–72) | (35–59) | |||
| Gender | ||||
| Male | 64 | 50 | χ2 | 0.38 |
| Female | 99 | 91 | ||
| Standing teeth | 27.3 ± 2.0 | 25.1 ± 2.9 | t | <0.05 |
| (22–32) | (16–28) | |||
| Percentage sites with radiographic | ||||
| Bone loss ≥50%a | 0.0 ± 0.0 | 43.7 ± 14.2 | – | – |
| Percentage sites probing attachment level ≥5 mm | 0.0 ± 0.0 | 51.7 ± 28.8 | – | – |
| BOP% | ND | 74.4 ± 25.5 | – | – |
Data presented as mean ± sd (range in parenthesis) or as absolute numbers; ND, not determined; and BOP, bleeding on probing.
Panoramic proximal alveolar bone loss measured using Schei ruler.28
2.2 DNA isolation and genotyping
Ten milliliters of venous blood were collected from each participant and stored in tubes containing ethylene-diamine-tetra-acetic acid at −70°C before DNA extraction. Genomic DNA was extracted using a QIAamp DNA mini-kit according to the manufacture’s instruction and were stored at −70°C before genotyping. All DNA samples were checked before genetic analysis, and those with insufficient quantity and/or quality for genotyping (<10 ng/μl, or A/280 ratio not within the range of 1.7–2.0, or showing DNA degradation upon electrophoresis) were discarded.
In total, 135 SNPs of CD28, CTLA4, GATA3, STAT6, IL4, IL4R, IL5, IL6, IL9, IL10, and IL13 were selected for genotyping (Supporting Information Table S1), including rs1537415, the GATA-3 binding site associated with AP.10 All the samples were genotyped by MassARRAY iPlex Gold assay (Sequenom, San Diego, CA, USA).
2.3 SNP selection
By using the HapMap Genome Browser release #2729 (phases 1, 2, and 3—merged genotypes and frequencies), SNPbrowser software version 4.0 (Applied Biosystems, Foster City, CA, USA) and the dbSNP database in the US National Center for Biotechnology Information website,30 the SNPs of CD28, CTLA4, GATA3, STAT6, IL4, IL4R, IL5, IL6, IL9, IL10, and IL13 to be analyzed in this study were selected according to the following criteria: (i) tagging SNPs were selected by the SNP Tagging Wizard of SNPbrowser (haplotype r2 ≥ 80% and minor allele frequency [MAF] ≥0.05 in Han Chinese population in Beijing, China [CHB]) and tag SNP picker of HapMap Genome Browser (tagger multimarker r2 ≥ 80% and MAF ≥ 0.05 in CHB population); (ii) SNPs in coding regions were selected directly on SNPbrowser (in CHB population); (iii) SNPs in regulatory regions were selected if their published MAFs were more than 0.1; (iv) SNPs in introns were selected only if they were adjacent to exons within 100 bp and if their published MAFs were also more than 0.1; and (v) SNPs of the 11 genes of interest previously reported to be associated with periodontitis.
2.4 Genotyping
For genotyping, an online assay design software (MassARRAY Assay Design Suite, Sequenom) was used to design PCR and single-base extension primers.31 rs1537415 was designed as priority. Within the 135 SNPs, five SNPs were failed to design because of the technical limitation of iPlex platform (Supporting Information Tables S1 and S2).
For each 96-well plate of assay, there were 89 wells for samples, five for random duplicates, one water control well and one blank well. Four of the duplicate check samples and six randomly selected samples for each sample plate were ran on 1% agarose gel and the bands were required to be intact after the electrophoresis. PCR amplification, the shrimp alkaline phosphatase treatment, and the primer extension reactions were performed according to the manufacture’s instruction of iPLEX Gold assay (Sequenom). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was used to read the signals and detect the genotypes. Online annotation tool, annotate variation, was used to annotate the SNPs.
2.5 Quality control
Before being considered suitable for further statistical analysis, the 130 SNPs were screened according to the following three criteria: (i) SNP call rate > 0.8; (ii) MAF > 0.1; and (iii) Hardy-Weinberg equilibrium (HWE) test showing P ≥ 0.01 in the periodontitis-free group.
Twenty samples were duplicated for each SNP during genotyping and the concordance rate was 99.8%. One SNP (rs13306436, a downstream variant) in IL6 has no call on one of two alleles in any individual and was excluded (Supporting Information Table S1). Eleven SNPs with call rate less than 0.8. Out of the remaining 118 SNPs, 29 were with MAF less than 0.1 or with only one genotype detected hence were excluded from further analysis. After filtering by MAF and call rate, three SNPs with HWE P-value <0.01 in the periodontitis-free group was detected and one was excluded except rs1537415 and rs231775, which were reported to be associated with periodontitis in previous studies,10,32,33 were included for further analysis. Together with 70 of the 130 SNPs, which passed the subsequent quality control tests, 72 SNPs were analyzed (Supporting Information Tables S2 and S3).
2.6 Meta-analysis
As SNPs nominally implicated (P < 0.1) may represent true disease risk loci,34 SNPs with adjusted P-value (allelic) <0.1 in the current periodontitis association analysis were identified. A systematic search was performed by searching electronic biomedical databases, including PubMed, Web of Science, Wanfang Data, and China National Knowledge Infrastructure. All articles published on or before December 31, 2019 were searched in these databases. The name of the molecules encoded by the genes potentially associated was used in the search. The key words used for search were “Cytotoxic T-Lymphocyte Associated Protein 4″ or “CTLA-4″ and “periodontitis” and “polymorphism”; “interlukin-4″ or “IL-4″ and “periodontitis” and “polymorphism”; “interlukin-4 receptor” or “IL-4R” and “periodontitis” and “polymorphism”; or “interlukin-6″ or “IL-6″ and “periodontitis” and “polymorphism.” The eligibility criteria were cross-sectional human case-control studies with periodontal healthy individuals as controls. Reports analyzing the association between the SNPs of interest (adjusted allelic P < 0.1) identified in the current cross-sectional study and periodontitis were selected. No language restriction was set. The following data were extracted from the studies directly: the authors, funding source, the year of publication, the country, the ethnicity of the population, the sample size of the cases, and controls with different genotypes and allele types. The related references in the articles or relevant publications citing the selected articles were also read and included if relevant.
Possibilities of data available from GWAS on periodontitis was also explored. The related literature search followed the same protocol described above with key words as “periodontitis” and “GWAS” or “genome wide association study.” The final results were verified in GWAS Catalog,35 an open GWAS database summaries published GWAS. One recent GWAS meta-analysis on periodontitis13 included and deposited most if not all GWAS on periodontitis data available in the Gene-Lifestyle Interactions in Dental Endpoints (GLIDE) consortium and the corresponding data were extracted accordingly.36
The screening and selection of the finally included studies followed preferred reporting items for systematic reviews and meta-analyses (PRISMA).37 We also conducted a risk of bias assessment on the included studies, which is a common procedure for ordinary meta-analyses but is seldom performed in genetic risk meta-analyses.
2.7 Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) Statistics 23.0 (Chicago, IL, USA) together with PLINK 1.90 when appropriate. Differences between the distributions of sex among periodontitis-free and periodontitis individuals were tested with χ2 statistic. Differences between cases and controls in number of standing teeth and age were assessed with t-test.
The allele types and genotypes of each suitable SNP single marker were screened initially by χ2 test. To avoid false-negative findings on the SNPs with intermediate frequency or effects, a less-stringent significance level was used at beginning (P < 0.005).38 To eliminate random SNP association, haplotype analysis was carried out on the potentially associated region. For the logistic regression, an adaptive permutation test was performed by PLINK 1.90 to correct against multiple comparisons.39 Full mode of PLINK was performed, including Cochran-Armitage trend test assuming codominant mode (2 degrees of freedom), genotypic test (2 degrees of freedom), dominant gene action test (1 degree of freedom), recessive gene action test (1 degree of freedom), and allelic test. Fisher exact test was used when there was any cell less than five. Odds ratios (ORs) and 95% confidence intervals (CIs) were determined from the result of allelic test.
The association of disease and haplotype in the block of these SNPs were analyzed. The linkage disequilibrium (LD) analysis was performed by Haploview 4.2.
Review Manager 5.3 was used for meta-analysis. Random effect model was used when the number of studies was more than 4 or I square was more than 50% in case the P-value of heterogeneity was less than 0.05, otherwise fixed effect model was chosen as default. Subgroup analysis was performed in different populations. Funnel plots were drawn under fixed effect model to identify publication bias. The evaluation of bias of the studies included were following Cochrane collaboration’s tool.40
3 Results
The demography and clinical profile of the participants were shown in Table 1 and there was no significant difference between cases and controls concerning age and gender.
3.1 Association study of Th2 related gene polymorphisms
DNA samples from all 304 nonsmoking participants were genotyped and analyzed. After filtering by MAF (<0.1), call rate (<0.8), and HWE P-value (<0.01). Seventy out of the 130 candidate SNPs passed the quality control tests. Adding rs231775 (HWE with P < 0.01 but found significantly associated with periodontitis in other populations32,33) and rs1537415 (AP-associated SNP10 but with P-value of HWE < 0.01), a total of 72 SNPs were analyzed (Supporting Information Table S1). The distribution of genotypes and alleles of the 72 SNPs analyzed were elaborated in Supporting Information Table S2.
Cochran-Armitage trend test under the heredity mode of codominant showed rs4553808 appeared significantly associated with periodontitis (P = 0.0042), but such observation did not remain significant after age and gender adjustment.
Four SNPs (rs4553808, rs16840252, rs5742909, and rs3087243) in CTLA4 with P < 0.05 in the Cochran-Armitage trend test were analyzed on LD. rs4553808, rs16840252, and rs5742909 from the region of CTLA4 were closely linked (r2 = 0.98) and located in the regulatory regions (Fig. 2). The haplotype (GTT) in a block of the three CTLA4 SNPs above was significantly associated with periodontitis, after adjustment against age and gender (Table 2).
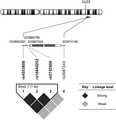
Pairwise linkage disequilibrium (LD) of the four single nucleotide polymorphisms (SNP) associated with periodontitis in the cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) gene (rs4553808, rs16840252, rs5742909, and rs3087243). The enlarged region at middle of the figure highlighted the relative location of the four SNPs along chromosome 2 at 2q33. The numbers above the bar are the corresponding chromosome positions. rs4553808, rs16840252, rs5742909 located in a promoter region upstream of, whereas rs3087243 is located downstream of CTLA4. Diamonds in the haplotype blocks represent pairwise LD (r2) between all SNPs assessed; as shown in the figure key, the darker the diamond, the stronger the LD between the SNPs
| Haplotype . | Periodontitis freea n = 162b . | Periodontitisa n = 140b . | χ2 P . | Adjusted P c . |
|---|---|---|---|---|
| CTLA4 | ||||
| 27 (8.3) | 42 (15.0) | 0.006 | 0.007 |
| 297 (91.7) | 238 (85.0) |
| Haplotype . | Periodontitis freea n = 162b . | Periodontitisa n = 140b . | χ2 P . | Adjusted P c . |
|---|---|---|---|---|
| CTLA4 | ||||
| 27 (8.3) | 42 (15.0) | 0.006 | 0.007 |
| 297 (91.7) | 238 (85.0) |
Data presented as absolute numbers; percentage in parenthesis
Haplotype count: numbers of alleles with haplotypes.
One case failed genotyping on SNP rs5742909 and one periodontitis-free participant with haplotype ATC were excluded.
Logistic regression with the adjustment for age and gender. The significant level was set at P < 0.01.
| Haplotype . | Periodontitis freea n = 162b . | Periodontitisa n = 140b . | χ2 P . | Adjusted P c . |
|---|---|---|---|---|
| CTLA4 | ||||
| 27 (8.3) | 42 (15.0) | 0.006 | 0.007 |
| 297 (91.7) | 238 (85.0) |
| Haplotype . | Periodontitis freea n = 162b . | Periodontitisa n = 140b . | χ2 P . | Adjusted P c . |
|---|---|---|---|---|
| CTLA4 | ||||
| 27 (8.3) | 42 (15.0) | 0.006 | 0.007 |
| 297 (91.7) | 238 (85.0) |
Data presented as absolute numbers; percentage in parenthesis
Haplotype count: numbers of alleles with haplotypes.
One case failed genotyping on SNP rs5742909 and one periodontitis-free participant with haplotype ATC were excluded.
Logistic regression with the adjustment for age and gender. The significant level was set at P < 0.01.
The AP-associated SNP, rs1537415,10 did not appear to be associated with periodontitis in the current case-control investigation.
3.2 Meta-analysis
Nine SNPs presented adjusted allelic P-value < 0.1, including rs4553808, rs16840252, rs5742909, and rs3087243 of CTLA4; rs2243290 and rs2243291 of IL4, rs1800796, rs2066992, and rs2069852 of IL6 (Supporting Information Table S3). No candidate gene study was available regarding association between rs4553808, rs16840252, or rs3087243 of CTLA4; rs2243290 or rs2243291 of IL4; and rs2066992 or rs2069852 of IL6 with periodontitis. The downloaded data files regarding periodontitis GWAS studies36 from GLIDE consortium were consulted. Unfortunately, the data set only contain summary statistics of the SNPs analyzed. It did not include allelic/genetic data for individual cohort followed. Such data are needed for the present meta-analysis; hence incorporating the related GWAS data for further investigation is considered impossible. At the end, meta-analysis of the aforementioned seven SNPs or incorporation of GWAS data into the meta-analyses of rs5742909 or rs1800796 was not carried out.
Four papers32,33,41,42 were identified reporting CTLA4 candidate genes and periodontitis by key words search, but only two papers32,41 involved rs5742909; hence the flow of the selection on rs5742909 relevant articles was not illustrated in graphic presentation. Reports citing and cited by the included studies were looked up and no additional relevant study was detected.
For rs5742909 of CTLA4, the included Iranian study surveyed 218 controls and 126 cases32 and the later Chinese study included 80 controls and 103 periodontitis patients.41 The bias assessment of the included studies was detailed in Supporting Information Figures S2 and S3. Data meta-analysis on rs5742909 and periodontitis with current report and the two studies available (461 controls and 369 cases) showed significant association between allele T of the former and periodontitis (Table 3 and Fig. 3).
Meta-analysis of association between CTLA4 rs5742909, IL6 rs1800796 and periodontitisa
| SNPs/Ethnicity . | OR . | CI (95%) . | P-value . | Study groups . | Sample size (n) . |
|---|---|---|---|---|---|
| CTLA4 rs5742909 | 1.44 | 1.07–1.94 | 0.02 | 3 | 830 |
| IL6 rs1800796 | |||||
| All | 1.30 | 1.11–1.52 | 0.0009 | 18 | 5202 |
| Asian | 1.25 | 1.08–1.45 | 0.003 | 15 | 3974 |
| Chinese | 1.35 | 1.17–1.56 | <0.0001 | 11 | 3365 |
| Caucasian | 2.12 | 1.25–3.58 | 0.005 | 2 | 717 |
| SNPs/Ethnicity . | OR . | CI (95%) . | P-value . | Study groups . | Sample size (n) . |
|---|---|---|---|---|---|
| CTLA4 rs5742909 | 1.44 | 1.07–1.94 | 0.02 | 3 | 830 |
| IL6 rs1800796 | |||||
| All | 1.30 | 1.11–1.52 | 0.0009 | 18 | 5202 |
| Asian | 1.25 | 1.08–1.45 | 0.003 | 15 | 3974 |
| Chinese | 1.35 | 1.17–1.56 | <0.0001 | 11 | 3365 |
| Caucasian | 2.12 | 1.25–3.58 | 0.005 | 2 | 717 |
CI: confidence interval; OR: odds ratio; and SNP: single nucleotide polymorphism.
CTLA4 rs5742909: T vs. C allele; C: reference allele; IL6 rs1800796: G vs. C allele; C: reference allele.
Meta-analysis of association between CTLA4 rs5742909, IL6 rs1800796 and periodontitisa
| SNPs/Ethnicity . | OR . | CI (95%) . | P-value . | Study groups . | Sample size (n) . |
|---|---|---|---|---|---|
| CTLA4 rs5742909 | 1.44 | 1.07–1.94 | 0.02 | 3 | 830 |
| IL6 rs1800796 | |||||
| All | 1.30 | 1.11–1.52 | 0.0009 | 18 | 5202 |
| Asian | 1.25 | 1.08–1.45 | 0.003 | 15 | 3974 |
| Chinese | 1.35 | 1.17–1.56 | <0.0001 | 11 | 3365 |
| Caucasian | 2.12 | 1.25–3.58 | 0.005 | 2 | 717 |
| SNPs/Ethnicity . | OR . | CI (95%) . | P-value . | Study groups . | Sample size (n) . |
|---|---|---|---|---|---|
| CTLA4 rs5742909 | 1.44 | 1.07–1.94 | 0.02 | 3 | 830 |
| IL6 rs1800796 | |||||
| All | 1.30 | 1.11–1.52 | 0.0009 | 18 | 5202 |
| Asian | 1.25 | 1.08–1.45 | 0.003 | 15 | 3974 |
| Chinese | 1.35 | 1.17–1.56 | <0.0001 | 11 | 3365 |
| Caucasian | 2.12 | 1.25–3.58 | 0.005 | 2 | 717 |
CI: confidence interval; OR: odds ratio; and SNP: single nucleotide polymorphism.
CTLA4 rs5742909: T vs. C allele; C: reference allele; IL6 rs1800796: G vs. C allele; C: reference allele.
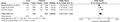
Odds ratios (ORs) and 95% confidence intervals (CI) of individual studies and pooled data for the association between the T allele of the CTLA4 rs5742909 and periodontitis. Data were synthesized with fixed effects meta-analysis with OR and 95% CIs being calculated
Twenty articles were found reporting rs1800796 of IL6 and periodontitis.20,43-61 Special attention was paid upon the full text and reported absolute values. Regarding articles on rs1800796 of IL6 and periodontitis, 2 of the 20 studies were suspected reporting data from almost same cohort,49,52 and only the recent report with a bigger sample size was included in the final analysis.52 Two groups of studies on rs1800796 were using exactly the same cohort,20,,46,51, 55 that is, same control/case counts, so only data from the later reports was used.20,51 Reports citing and cited by the included studies were looked up and no additional relevant study was detected. As a result, a total of 17 studies20,43-45,47,48,50-54,56-61 together with data of the current study were finally included in the meta-analysis (Fig. 4, Supporting Information Table S4; controls = 2760, periodontitis = 2442). The bias assessment was detailed in Appendix (Supporting Information Figs. S4 and S5).
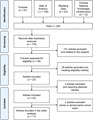
Flow diagram showing the selection process of the published IL6 studies included in the meta-analysis. The number of publications (n) in each stage is labeled. Seventeen studies plus results of current study were included in the meta-analysis
The 17 included papers plus the current study on rs1800796 of IL6 and periodontitis comprised 13 studies on east Asians (including 11 on Chinese), 1 on central Asians (Iranians), 1 on south Asians (Indians), 1 on Caucasians, and 1 study investigated a multicultural population, including Asians, Caucasians, and Africans (Supporting Information Table S4). When analyzing data from all included studies (Supporting Information Table S4, Fig. 5), G allele of rs1800796 appeared to be associated with increased risk of periodontitis (P = 0.0009, OR = 1.30). Meta-analysis stratified by ethnicity showed the G allele remained significant both in Caucasians (P = 0.003, OR = 2.15) and in East Asians with periodontitis (P < 0.0001, OR = 1.35). When considering only the 11 studies on Chinese, significantly more rs1800796 G allele were reported in the periodontitis group than controls (P = 0.0002, OR = 1.33) (Fig. 5).

Forest plot on 18 studies for the association between the G allele of the IL6 rs1800796 and periodontitis in (A) all studies; (B) subgroup of East Asians; (C) subgroup of Caucasians; (D) subgroup of Chinese. Data were synthesized with fixed effects meta-analysis with odds ratios (ORs) and 95% confidence intervals (CI) being calculated. Pooled effect estimates are indicated by diamonds, with I2 and significance of the overall effect being given. When the number of studies was more than four or the I2 was more than 50% in case P-value of heterogeneity less than 0.05, OR and P-value were calculated using random effect model
In general, studies included in the meta-analyses presented different levels of bias. Unknown risk of selection bias and performance bias exist as the criteria for the control or nonperiodontitis group were not clearly defined and the concordance test on the genotyping was not mentioned (Supporting Information Figs. S2 and S4). Smokers or participants with diabetes might be included in the data in three studies on rs1800796 where exact details were lacking, which presented as unknown or high risk of selection bias (Supporting Information Fig. S4). Although smoking as a confounding factor was adjusted in one of the studies,52 the data of the nonsmokers was not defined, so both smokers and nonsmokers were included in the meta-analysis, which indicate a high risk of bias (Supporting Information Fig. S4).
4 Discussion
Th2 cell has been reported to be associated with periodontitis,3 but the genetic risk indicators of Th2 cell-related genes have not been thoroughly investigated. This study investigated a selection of SNPs from 11 genes, which are closely related with Th2 cells or their function regulation. The current candidate gene study indicated G allele of rs4553808 appeared significantly associated with periodontitis (P = 0.0042) but not after age and gender adjustment (Supporting Information Table S3; allelic P = 0.0073, genotypic P = 0.0280 compare to predetermined P < 0.005). The observation indicated further study with a bigger sample size is needed to confirm the association between rs4553808 polymorphism and periodontitis.
Four CTLA4 SNPs, that is, rs4553808, rs16840252, rs5742909 (upstream), and rs3087243 (CT60, downstream) were found to qualify as periodontitis risk loci (P = 0.0073, 0.0106, 0.0099 or 0.0213, respectively; Supporting Information Table S3). Further investigations with bigger sample size are needed to confirm the above observations. It was, however, noteworthy that rs3087243 was previously reported in GWAS to be associated with several autoimmune diseases.62
The haplotype GTT in a block of these SNPs from CTLA4 promoter region (rs4553808, rs16840252, rs5742909) was significantly enriched in Chinese periodontitis patients (15.0%) compared to the periodontitis-free controls (8.3%). The association between rs5742909 and periodontitis was supported by the meta-analysis of candidate gene data that follows (Table 3). CTLA-4 is encoded by a gene located on chromosome 2q33 and it is a key T cell down-regulation molecule. The closely linked three SNPs located in the same promoter region of CTLA4, which indicated a lower possibility of false positive result or positive result by chance. The current observation implied fine mapping and functional study of the CTLA4 promoter especially at the rs4553808, rs16840252, or rs5742909 haplotype GTT region might potentially shed light on the related Th2 regulation biology and hence the corresponding periodontitis risks in humans.
In attempt to control periodontal inflammation hence the possibility of reverting the former to health, a series of chemical signals need to be activated to down-regulate inflammatory cytokines, attenuate immune cell trafficking, and induce immune cell apoptosis and clearance.63 The current meta-analysis based on candidate gene studies data indicated rs5742909 T allele at promoter region of CTLA4 was associated with periodontitis (Table 3). CTLA-4 (CD152) is an important negative regulator of T cell activation, which transiently express on the surface of T cells. Within periodontal tissue, CTLA-4 can suppress proliferation of T-cells in response to periodontopathogens.64 Therefore Tregs expressing inhibitory molecule CTLA-4 are supposed to attenuate inflammatory responses against periodontopathogens or antigens.32,42
Despite the available GWAS data did not provide genetic information of each cohort hence prevented further meta-analysis in relation to SNPs of interests detectable in the current investigation, a Supporting Information Table S5 is prepared to summarize the stated P concerning the nine SNPs with adjusted allelic P < 0.1 reported in the present study. It is noteworthy that Shungin et al.13 reported none of the nine SNPs, including rs5742909 or rs1800796, was associated with periodontitis.
Some previous studies tested the association between periodontal disease and CTLA4 SNPs. A missense variant in a coding region, rs231775, was tested in studies on periodontitis but the results were controversial.32,33,65 Result from the current investigation showed the SNP was not significantly associated with periodontitis (P = 0.464; Supporting Information Table S3). The current rs231775 HWE test indicated P < 0.01, demonstrating the SNP may be influenced by selection, genetic drift, mutation. or other factors in the Chinese cohort followed. G allele of rs231775 was the minor allele in Iranian (28%)32 and in Brazilian controls (27%),33 but was major allele in Chinese control of the current study (72.9%). The latter, was consistent with the data reported on the Chinese population of HapMap. The present observation perhaps indicated genetic variance of the rs231775 among different populations. Similar to report by Houshmand and coworkers,32 CTLA4 upstream SNP rs733618 was not associated with periodontitis (P = 0.658). A recent study reported the G allele of rs56102377, a 3 prime untranslated region variant, might be regulated by miR-105, which caused a down-regulation of CTLA4.41 rs56102377 was not included in the current study, because the published MAF in the 1000 Genome and HapMap was <0.05 at the time of study design. The implication of rs56102377 in periodontitis therefore warrants further investigation.
Limited published reports could be included in our candidate gene data-based meta-analysis on CTLA4 as few studies investigated periodontitis and rs4553808, rs16840252, rs5742909, or rs3087243. At the end, only an SNPs in the CTLA4 upstream region, rs5742909, could be considered. The SNP of interest was studied in Iranian and Chinese.32,41 Interestingly, both earlier studies independently reported no association between the SNP and periodontitis. However, after combining their data with the current investigation (461 control, 369 periodontitis), allele T of rs5742909 appeared significantly associated with periodontitis (Fig. 3, Table 3). Putting the observation together, more investigations regarding association and/or functional studies related to rs5742909 polymorphism need to be carried out.
Only two of nine potential periodontitis risk loci identified from this candidate gene study, that is, rs5742909 of CTLA4 discussed above or rs1800796 of IL6, published reports with usable data. Seventeen reports (Supporting Information Table S4) were available related to IL6 rs1800796 and periodontitis, together with the present study consists of a candidate gene database of 2760 control and 2442 periodontitis patients. In line with previous reports, the current meta-analysis indicated significant association between allele G of IL6 rs1800796 and periodontitis (Fig. 5A). The observation remained significant even if the data was segregated in subgroups of East Asians, Caucasians, or Chinese (Fig. 5B–D).
Having dual roles, IL-6 acts as either pro- or anti-inflammatory signaling agents.66,67 At the initial phase of periodontitis, the gram-negative pathogens and their lipopolysaccharides activates transcription of proinflammatory cytokines in affected host cells via the NFκB pathway,68–70 resulting in IL-1, IL-6, and TNF-α production and related matrix metalloproteinase activation leading to degradation of periodontal extracellular matrix and bone resorption through increased RANKL secretion.71,72 IL-6 activates transcription mediated by nuclear factor of activated T cells leading to production of IL-4 by naïve CD4+ T cells and their differentiation into effector Th2 cells.18 Because IL-6 is abundantly produced by antigen-presenting cells, it is also a likely source of early Th1/Th2 control during CD4+ T cell activation.18,73 On the other hand, being an anti-inflammatory cytokine, at the later stage of an inflammatory process, IL-6 can inhibit the synthesis of proinflammatory cytokines such as IL-1, TNF-α, and increase the production of anti-inflammatory signals by Th2 cells.74 Periodontitis patients appeared to express higher serum and salivary IL-6 level than controls75,76 might indicate perhaps a modulatory role of the cytokine at situation when chronic periodontal inflammation is established. In fact, rs1800796 located in the promoter region of IL6, which contributes to the functional regulation of IL-6 expression.77
The effect of G allele of rs1800796 on IL6 transcriptional activity and expression level unfortunately remained controversial.78–82 The current meta-analysis reported association between G allele of rs1800796 with periodontitis (Table 3). Provided rs1800796 G allele in vivo would inference IL6 expression in human periodontal tissue, which in theory it could, the importance of such polymorphism as one possible genetic periodontitis risk is reinforced. More studies are however, warranted to elucidate the role of rs1800796 in periodontal inflammation/defense.
Indeed, a meta-analysis published in 2013 analyzed eight reports on rs1800796 and periodontitis indicated that the SNP might be associated with increased risk of periodontitis in Europeans.21 Interestingly, frequencies of G allele at rs1800796 appeared very different among the two Caucasian studies, being 5%48 or 94%.43 Four Chinese studies were included in the Song et al.21 meta-analysis; however, two were suspected to report cohorts from the same or almost same source and we excluded the duplication in the current investigation (please refer to Section 3.2, “Meta-analysis”).
A recent meta-analysis included 18 studies as well as the suspected duplicated ones from two groups of studies20,,46,51, 55 exploring relationship between rs1800796 and periodontitis reported significant association between the two, be that Asians only or all subjects included.22 The present report, excluding the suspected duplicated data, adding data from two latest studies20,51 and current report confirmed such observation. However, because of putting central Asian participants’ data under Caucasians, Zhao et al.22 reported rs1800796 did not associate with periodontitis in the latter.
The meta-analysis contained studies with different levels of bias, so the result should be considered with caution. Like a recent report,83 we adopted in part the PRISMA checklist to qualitatively assess the possibility of bias in the current meta-analysis in order to assist readers’ appreciation of the results observed.
One of the limitations of the current study is that some common risk indicators especially that of socioeconomic status, education, among others, were not recorded. These data were known to associate with periodontitis84 and hence perhaps best be adjusted during the data analysis.
Genes of TCR were not investigated in this study because of the high complexity. There is no candidate gene study to date on associations between TCR genetic polymorphisms and periodontitis. However, SNPs in the genes of TCR complex were reported to be associated with other diseases, such as type 1 diabetes.85 The latest periodontitis GWAS meta-analysis by Shungin et al.13 (n = 45,651), together with an earlier report by Munz and coworkers86 (n = 25,003) in the same year, revealed that a SNPs rs11084095 of SIGLEC5 (sialic acid binding Ig-like lectin 5, Siglec-5 or CD170) remained the only one SNP significantly associated with periodontitis (P = 1.3 × 10−9 or 5.0 × 10−8, respectively). Siglec-5 is an inhibitory receptor with expression in various myeloid immune cells and may mediate Src homology region 2 domain-containing tyrosine phosphatase (SHP)-1/-2 dependent signaling. SHP-1/-2, two cytoplasmic protein tyrosine phosphatases, are critical regulators of T-cell by inhibiting TCR signaling, including not only Th1/2 cells, but also other T cells, such as Treg and thymic T cells.87 The association between periodontitis and SNPs in the region coding for different TCR segments, that is, constant segment or variable segment, could be studied in the future.
From the available 13 GWASs10,88-99 and 2 GWAS meta-analyses,13,86 none except one13 provided a full set of association study result summary (n = 45,651) online.36 Only one GWAS88 reported a SNP, that is, rs11084095 of SIGLEC5, which was found to be associated with periodontitis after GWAS meta-analyses.13,86 The authors, nonetheless, cautioned against heterogeneity introduced by the different approaches to periodontitis classification, different patterns of periodontal treatment, and varying distributions of age in some of the cohorts followed or gene–environment interactions not accounted for in the study design.13 Regarding the nine SNPs identified to be of interest in the current candidate gene study, none was associated with periodontitis from the Shungin et al. meta-analysis (Supporting Information Table S5).13 The low number of significant locus (n = 1) identified so far from both GWAS meta-analyses is in fact is in agreement theoretically, with the total GWAS sample size available.100
In general, GWAS screened the whole genome for the disease associated genetic polymorphisms without a hypothesis, whereas candidate gene studies focus on some specific genes based on the potential biologic function/mechanism, such as Th2 regulation upon periodontitis in the current study. Theoretically, as a hypothesis-free approach, GWAS should be able to identify more disease associated genetic polymorphisms and reveal the heritability of the disease. However, compared to twins/family studies, GWAS on periodontitis detected a lower heritability estimate of 0.07 for combined definitions of periodontitis, increasing with disease severity.101 The atheoretical GWAS approach, until present moment, not able to capture all variation due to variable nucleotide tandem repeat polymorphisms (VNTRs), nor does it capture all variation due to copy number variations. Being atheoretical, the nature of GWAS and large number of potential main effects/interactions forced substantial corrections are needed for GWAS suggest that even genuine effects will be “corrected” away or overlooked.102 In contrast to GWAS, candidate gene approach study specific alleles of genes that are hypothesized to be related to a phenotype. Candidate genes may be VNTRs with well-known functional impact, SNPs that have emerged from prior GWAS studies, among others. It enables theory driven investigation between SNPs and particular polymorphisms taking into considerations of potential environmental interactions, that is, a broader network of physiologic and contextual findings, allows for stronger hypotheses based research.101
A systematic review compared the SNPs on cancer-associated genes identified via GWAS vs. candidate gene approach.103 The study analyzed a database with research since 2000 and found GWAS reported 269 significant associations, whereas candidate gene methods reported 349. Only 7.1% (41 of total 577) of associations were found significantly associated with cancer in both methods and with similar effect sizes. Therefore, this study group was not overtly surprised regarding the lack of concordance between the current candidate-gene-based CTLA4 and IL6 results vs. the corresponding data reported from recent GWAS meta-analyses.
There appears yet insufficient evidence to support the exclusive role of Th2 cells in pathogenesis of periodontitis, because neither CTLA-4 nor IL-6 is produced or works exclusively in Th2 cells. CTLA-4 in general is a negative immune modulator expressed by many T cells, including Treg and T helper cells, whereas IL-6 does not only work on Th2 cells through IL-4 but also has inhibitory effect on IL-1 and TNF-α. A recent study focused on risk indicators related to recurrent pregnancy loss, postulated that CTLA-4 up-regulates TGF-β1 production from endometrial epithelial cells, which in turn modulates IL-6 expression thereby positively influencing maturation of naïve CD4+ T cells to Tregs thus reduces pregnancy failure.104 However, further functional studies should be carried out on the expression and effect of CTLA4 and IL6 on Th2 cells from subjects with or without periodontitis. More genetic studies, including candidate gene approach, on CTLA4 should be carried out to identify possible periodontitis associating SNPs, to clarify the limited observations made so far.
5 Conclusion
Among the 11 Th2 related genes, a haplotype in a block of 3 SNPs (rs4553808, rs16840252, and rs5742909) from CTLA4, the most important inhibitory receptor on Th2 cell, which modulates cell activation and development of the latter, appeared significantly associated with chronic periodontitis in a nonsmoking southern Chinese cohort. The present candidate gene study results did not indicate IL6 rs1800796 associate with periodontitis in the Chinese cohort followed. Nevertheless, the following candidate gene-based meta-analysis on rs5742909 of CTLA4 and rs1800796 of IL6 provided another opportunity to reassess all relevant studies involved and demonstrated the potential association between CTLA4 rs5742909 or IL-6 rs1800796 and the susceptibility of periodontitis. Functional studies should be performed to validate the effect of disease-associated SNPs and haplotype reported in this study and periodontitis in the future. As CTLA-4 and IL-6 are not exclusively expressed in Th2 cells, the current study is not sufficient to clarify the role of the genetic polymorphisms on Th2 cells of affected individuals on the pathogenesis of periodontitis.
Authorship
The authors contributed in the following manner: study conception and design: W.K.L., Y-Q.S., and Y.Z.; institutional review board submission: L.C., K-Y.Z., W.K.L., Y-Q.S., and Y.Z.; blood sampling and analysis: L.C. and Y.Z.; patient recruitment: L.C., K-Y.Z., L.J., W.K.L., and Y.Z.; acquisition of data: L.C., W.W.T., Y.F., and Y.Z.; statistical analysis: W.K.L., Y.F., Y-Q.S., and Y.Z.; interpretation of data: W.K.L., W.W.T., Y.F., Y-Q.S., and Y.Z.; manuscript preparation: W.K.L., W.W.T., Y-Q.S., and Y.Z.; and critical manuscript revision: W.K.L., Y-Q.S., and Y.Z. All authors approved the final version of the manuscript.
Abbreviations
- AP
aggressive periodontitis
- BOP
bleeding on probing
- CD
cluster of differentiation
- CHB
Han Chinese population in Beijing, China
- CI
confidence interval
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- GATA-3
globin transcription factor binding protein 3
- GLIDE
Gene-Lifestyle Interactions and Dental Endpoints
- GWAS
genome-wide association study
- HWE
Hardy-Weinberg equilibrium
- LD
linkage disequilibrium
- MAF
minor allele frequency
- OPG
orthopantomogram
- OR
odds ratio
- PPD
probing pocket depth
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- SHP
Src homology region 2 domain-containing tyrosine phosphatase
- SIGLEC5
sialic acid binding Ig-like lectin 5
- SNP
single nucleotide polymorphism
- Th1
T helper 1 cell
- Th2
T helper 2 cell
- Treg
regulatory T cell
- VNTR
variable nucleotide tandem repeat polymorphisms
Acknowledgments
This study is substantially supported by The University of Hong Kong Research and Conference Grant (10206044, 10207348) and Health and Medical Research Fund of the Hong Kong Special Administrative Region, China (02132216).
Disclosures
The authors declare no conflicts of interest.
References



