-
PDF
- Split View
-
Views
-
Cite
Cite
Takeshi Terashima, Kenichi Harada, Taro Yamashita, Diagnosis, clinical characteristics, and treatment of combined hepatocellular-cholangiocarcinoma, Japanese Journal of Clinical Oncology, Volume 55, Issue 4, April 2025, Pages 327–333, https://doi.org/10.1093/jjco/hyaf029
Close - Share Icon Share
Abstract
The concept and definition of combined hepatocellular-cholangiocarcinoma (cHCC-CCA), an extremely rare condition accounting for only 1% of all primary liver cancers, has shifted in recent years. The latest World Health Organization Classification (fifth edition) includes two types of cHCC-CCAs, (i) the classical type described in the previous edition, which contains a mixture of distinctly differentiated components of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) and (ii) intermediate cell carcinoma wherein all cells comprising the tumor express both hepatocellular and cholangiocellular features. However, the pathogenesis of cHCC-CCA, including its origins, remains controversial even among experts. Treatment strategies for cHCC-CCA in clinical practice have been determined based on imaging findings, tumor markers, and pathologically predominant tumor components for either HCC or ICC, suggesting that cHCC-CCA has yet to be been established as an independent disease entity. As with HCC and ICC, the treatment strategy for HCC-CCA involves initially considering resectability. Although systemic therapy has been considered for patients unsuitable for local treatment, no prospective clinical trials have evaluated the efficacy and safety of systemic therapy for cHCC-CCA, which could explain the lack of a standard of care. In recent years, however, studies have demonstrated the efficacy of immune checkpoint inhibitors for HCC and ICC, with therapeutic results having been reported for cHCC-CCA. Hence, further accumulation of cases is expected to facilitate the establishment of a consensus on treatment strategies in the near future.
Introduction
The liver is one of the major sites for the development of malignancy, with primary liver cancer being the leading cause of death according to cancer site [1]. Although hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancers, the proportion of intrahepatic cholangiocarcinoma (ICC) has been increasing in recent years [2]. Other histologic types of primary liver cancer include combined hepatocellular-cholangiocarcinoma (cHCC-CCA), fibrolamellar carcinoma, and hepatic epithelioid hemangioendothelioma; however, these histologic types are extremely infrequent, accounting for only 1% of all primary liver cancers [3,4]. The existence of cHCC-CCA, which consists of both HCC and ICC components, has been recognized for quite some time [5]; however, comprehensive reviews of its diagnosis, management, and treatment have been limited given its infrequent occurrence and dynamic disease concepts or classifications. Here, we introduce the concept and definition of cHCC-CCA based on the latest WHO Classification (fifth edition) and provide an overview of its diagnosis, clinical characteristics, and treatment.
Definition and subclassification of cHCC-CCA
Allen et al. initially defined cHCC-CCA as the simultaneous existence of HCC and ICC within the same liver, subsequently categorizing the condition into the following three types: [1] the double cancer type wherein both HCC and ICC are located in separate areas in the same liver; [2] the combined type wherein a single tumor contains both clearly differentiated HCC components and ICC components, each with its own region; and [3] the mixed type wherein a single tumor contains a mixture of distinct differentiated components of HCC and ICC components that cannot be separated into distinct regions [6]. Goodman et al. grouped cHCC-CCA tumors into the following three subclassifications: type 1 (collision tumors) wherein both distinctly differentiated HCC and ICC components are present within a single tumor, each with its own region; type 2 (transitional tumors) wherein both distinctly differentiated HCC and ICC components are present within a single tumor but are not divided into regions; and type 3 (fibrolamellar tumors) histologically characterized by carcinoma cells with abundant acidophilic granular sporulation arranged in a planar or sheet-like pattern, with an increase in vitellinated connective tissue showing a stratified structure between them but with a pseudoadenoductal structure with mucus production [7].
The latest edition of the WHO Classification (i.e. the fifth edition) had been published in 2019 [8] as a revision to the fourth edition [9] after a consensus paper [10]. In the previous edition, cHCC-CCA tumors were largely categorized into the classical type and subtypes with stem cell features, with the latter being further divided into the typical subtype, intermediate-cell subtype, and cholangiolocellular subtype [9]. In the current fifth edition, subtypes with stem-cell features were abolished; cholangiolocellular carcinoma was classified as a subtype of intrahepatic bile duct carcinoma (small duct type) [8]; and the double cancer type and combined type in the Allen classification, as well as the collision tumors and fibrolamellar tumors in the Goodman classification, were also excluded from this category. Hence, the WHO Classification assumes that only distinctly differentiated HCC and ICC components are present within a single tumor but not divided into regions, which corresponds to the WHO Classification of Classical Types (fourth edition) (Fig. 1A). Intermediate cell carcinoma, which was listed as the intermediate type, was retained in the fifth edition, further characterizing it as a tumor wherein all cells express both hepatocyte and cholangiocyte features (Fig. 1B) [11].
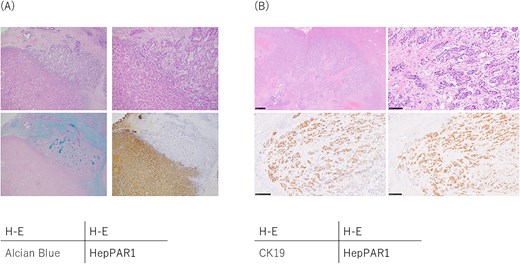
Pathological findings of combined hepatocellular-cholangiocarcinoma. (A) Classical type. The upper right area shows the cholangiocarcinoma component and the lower left area shows the hepatocellular carcinoma component, and these components are mixed in this tumor and cannot be separated into distinct regions. Immunohistochemical staining revealed that the cholangiocarcinoma components were positive for Alcian blue and CK19 (not shown) staining, whereas the hepatocellular carcinoma component were positive for and HepPAR1 staining. (B) Intermediate cell carcinoma. Intermediate cell carcinoma is composed of cells that exhibit characteristics of both hepatocellular carcinoma and cholangiocarcinoma. Immunohistochemical staining revealed these tumor cells were positive for both CK19 and HepPAR1.
Epidemiology of cHCC-CCA
The prevalence of cHCC-CCA has varied widely in published studies [3,4,12–15] given that the disease concept and subclassification of cHCC-CCA has been successively changed recently, as mentioned earlier, and that it is rarely diagnosed based on pathological findings prior to treatment, unlike most other tumors that are diagnosed based on imaging findings and clinical features.
The 23rd nationwide follow-up survey of primary liver cancer in Japan recorded 20 853 newly diagnosed cases of primary liver cancer (14 745 males and 6108 females) from 516 centers nationwide. Among these cases, 263 (188 males and 75 females) were diagnosed with cHCC-CCA based on clinical findings, accounting for only 1.3% of all primary liver cancers [16]. Although the mentioned survey reported on the prevalence of cHCC-CCA in one of the largest cohort of patients, only 39 of these patients were diagnosed based on pathological findings, whereas the others were diagnosed based on imaging findings and clinical features. Hence, the exact prevalence and number of deaths from cHCC-CCA diagnosed according to the latest edition of the WHO Classification remain unclear.
Clinical characteristics
Evidently, caution must be exercised when interpreting the results of previous epidemiological studies given the low disease frequency and changing diagnostic criteria. Studies have long noted that chronic liver disease caused by hepatitis B virus or hepatitis C virus is often associated with HCC and ICC [15,17]. In fact, the 23rd nationwide follow-up survey of primary liver cancer in Japan found that 21.0%, 50.4%, and 32.0% of the cases presented with hepatitis B surface antigen positivity, HBc antibody positivity, and HCV antibody positivity, respectively, with such values being closer to those in patients with HCC than in patients with ICC [16]. However, these data were collected from newly diagnosed cases in 2014 and 2015, and the rapid change in the etiology of HCC since then mainly due to the successful development of direct-acting anti-viral agents for hepatitis C virus [18] suggests that the same trend may be observed in cHCC-CCA. Other clinical characteristics include the Child–Pugh classification (A/B/C) of 81.5%/15.3%/3.2%, tumor marker AFP (<10 ng/ml/10 ng/ml to 399 ng/ml/400 ng/ml) of 37%/37%/26%, PIVKA-II (<40 mAU/ml/40 ml to 499 mAU/ml/500 mAU/ml or more) of 47%/28%/25%, CEA (<5.0 ng/ml/5.0 ng/ml to 9.9 ng/ml/10.0 ng/ml or more) of 66%/18%/16%, and CA19–9 (<37 U/ml/37 U/ml to 299 U/ml/300 U/ml or more) of 55%/27%/18% (Table 1) [16]. It looked like that in cHCC-CCA, AFP and PIVKA-II were positive at similar rate as in HCC, and CEA and CA19–9 were positive at similar rate as in ICC; however, it is important to note that most diagnoses of cHCC-CCA were not based on pathological findings in this cohort as described below. Therefore, these data do not necessarily mean that most patients with pathologically diagnosed cHCC-CCA have elevated levels of both tumor markers for HCC, such as AFP/PIVKA-II, and ICC, such as CEA/CA19–9, and it could be interpreted as the current situation where cHCC-CCA may be diagnosed without pathological confirmation if both tumor markers are elevated.
Serum tumor marker levels in hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and combined hepatocellular-cholangiocarcinoma (adopted with modifications from reference [14].
| . | Hepatocellular carcinoma . | Intrahepatic cholangiocarcinoma . | Combined hepatocellular-cholangiocarcinoma . |
|---|---|---|---|
| AFP (ng/ml) | (n = 16 514) | (n = 978) | (n = 225) |
| <10 | 8160 (49.4%) | 826 (84.5%) | 104 (46.2%) |
| 10 ≤ , ≤ 199 | 4938 (29.9%) | 114 (11.7%) | 68 (30.2%) |
| 200 ≤ , ≤ 399 | 599 (3.6%) | 16 (1.6%) | 15 (6.7%) |
| 400≤ | 2817 (17.1%) | 22 (2.2%) | 38 (16.9%) |
| PIVKA-II (mAU/ml) | (n = 16 097) | (n = 865) | (n = 220) |
| <40 | 6018 (37.4%) | 727 (84.0%) | 112 (50.9%) |
| 40 ≤ , ≤ 99 | 2326 (14.4%) | 53 (6.1%) | 36 (16.4%) |
| 100 ≤ , ≤ 499 | 2779 (17.2%) | 34 (3.9%) | 30 (13.6%) |
| 499≤ | 4974 (30.9%) | 51 (5.9%) | 42 (19.1%) |
| CEA (ng/ml) | (n = 9083) | (n = 1350) | (n = 178) |
| <5.0 | 6980 (76.8%) | 852 (63.1%) | 124 (69.7%) |
| 5.0 ≤ , ≤ 9.9 | 1698 (18.7%) | 204 (15.1%) | 31 (17.4%) |
| 10.0 ≤ , ≤ 19.9 | 276 (3.0%) | 81 (6.0%) | 13 (7.3%) |
| 20.0≤ | 129 (1.4%) | 213 (15.8%) | 10 (5.6%) |
| CA19–9 (U/ml) | (n = 8147) | (n = 1325) | (n = 170) |
| <37 | 6468 (79.4%) | 473 (35.7%) | 102 (60.0%) |
| 37 ≤ , ≤ 99 | 1225 (15.0%) | 220 (16.6%) | 28 (16.5%) |
| 100 ≤ , ≤ 299 | 317 (3.9%) | 163 (12.3%) | 16 (9.4%) |
| 300≤ | 137 (1.7%) | 469 (35.4%) | 24 (14.1%) |
| . | Hepatocellular carcinoma . | Intrahepatic cholangiocarcinoma . | Combined hepatocellular-cholangiocarcinoma . |
|---|---|---|---|
| AFP (ng/ml) | (n = 16 514) | (n = 978) | (n = 225) |
| <10 | 8160 (49.4%) | 826 (84.5%) | 104 (46.2%) |
| 10 ≤ , ≤ 199 | 4938 (29.9%) | 114 (11.7%) | 68 (30.2%) |
| 200 ≤ , ≤ 399 | 599 (3.6%) | 16 (1.6%) | 15 (6.7%) |
| 400≤ | 2817 (17.1%) | 22 (2.2%) | 38 (16.9%) |
| PIVKA-II (mAU/ml) | (n = 16 097) | (n = 865) | (n = 220) |
| <40 | 6018 (37.4%) | 727 (84.0%) | 112 (50.9%) |
| 40 ≤ , ≤ 99 | 2326 (14.4%) | 53 (6.1%) | 36 (16.4%) |
| 100 ≤ , ≤ 499 | 2779 (17.2%) | 34 (3.9%) | 30 (13.6%) |
| 499≤ | 4974 (30.9%) | 51 (5.9%) | 42 (19.1%) |
| CEA (ng/ml) | (n = 9083) | (n = 1350) | (n = 178) |
| <5.0 | 6980 (76.8%) | 852 (63.1%) | 124 (69.7%) |
| 5.0 ≤ , ≤ 9.9 | 1698 (18.7%) | 204 (15.1%) | 31 (17.4%) |
| 10.0 ≤ , ≤ 19.9 | 276 (3.0%) | 81 (6.0%) | 13 (7.3%) |
| 20.0≤ | 129 (1.4%) | 213 (15.8%) | 10 (5.6%) |
| CA19–9 (U/ml) | (n = 8147) | (n = 1325) | (n = 170) |
| <37 | 6468 (79.4%) | 473 (35.7%) | 102 (60.0%) |
| 37 ≤ , ≤ 99 | 1225 (15.0%) | 220 (16.6%) | 28 (16.5%) |
| 100 ≤ , ≤ 299 | 317 (3.9%) | 163 (12.3%) | 16 (9.4%) |
| 300≤ | 137 (1.7%) | 469 (35.4%) | 24 (14.1%) |
Serum tumor marker levels in hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and combined hepatocellular-cholangiocarcinoma (adopted with modifications from reference [14].
| . | Hepatocellular carcinoma . | Intrahepatic cholangiocarcinoma . | Combined hepatocellular-cholangiocarcinoma . |
|---|---|---|---|
| AFP (ng/ml) | (n = 16 514) | (n = 978) | (n = 225) |
| <10 | 8160 (49.4%) | 826 (84.5%) | 104 (46.2%) |
| 10 ≤ , ≤ 199 | 4938 (29.9%) | 114 (11.7%) | 68 (30.2%) |
| 200 ≤ , ≤ 399 | 599 (3.6%) | 16 (1.6%) | 15 (6.7%) |
| 400≤ | 2817 (17.1%) | 22 (2.2%) | 38 (16.9%) |
| PIVKA-II (mAU/ml) | (n = 16 097) | (n = 865) | (n = 220) |
| <40 | 6018 (37.4%) | 727 (84.0%) | 112 (50.9%) |
| 40 ≤ , ≤ 99 | 2326 (14.4%) | 53 (6.1%) | 36 (16.4%) |
| 100 ≤ , ≤ 499 | 2779 (17.2%) | 34 (3.9%) | 30 (13.6%) |
| 499≤ | 4974 (30.9%) | 51 (5.9%) | 42 (19.1%) |
| CEA (ng/ml) | (n = 9083) | (n = 1350) | (n = 178) |
| <5.0 | 6980 (76.8%) | 852 (63.1%) | 124 (69.7%) |
| 5.0 ≤ , ≤ 9.9 | 1698 (18.7%) | 204 (15.1%) | 31 (17.4%) |
| 10.0 ≤ , ≤ 19.9 | 276 (3.0%) | 81 (6.0%) | 13 (7.3%) |
| 20.0≤ | 129 (1.4%) | 213 (15.8%) | 10 (5.6%) |
| CA19–9 (U/ml) | (n = 8147) | (n = 1325) | (n = 170) |
| <37 | 6468 (79.4%) | 473 (35.7%) | 102 (60.0%) |
| 37 ≤ , ≤ 99 | 1225 (15.0%) | 220 (16.6%) | 28 (16.5%) |
| 100 ≤ , ≤ 299 | 317 (3.9%) | 163 (12.3%) | 16 (9.4%) |
| 300≤ | 137 (1.7%) | 469 (35.4%) | 24 (14.1%) |
| . | Hepatocellular carcinoma . | Intrahepatic cholangiocarcinoma . | Combined hepatocellular-cholangiocarcinoma . |
|---|---|---|---|
| AFP (ng/ml) | (n = 16 514) | (n = 978) | (n = 225) |
| <10 | 8160 (49.4%) | 826 (84.5%) | 104 (46.2%) |
| 10 ≤ , ≤ 199 | 4938 (29.9%) | 114 (11.7%) | 68 (30.2%) |
| 200 ≤ , ≤ 399 | 599 (3.6%) | 16 (1.6%) | 15 (6.7%) |
| 400≤ | 2817 (17.1%) | 22 (2.2%) | 38 (16.9%) |
| PIVKA-II (mAU/ml) | (n = 16 097) | (n = 865) | (n = 220) |
| <40 | 6018 (37.4%) | 727 (84.0%) | 112 (50.9%) |
| 40 ≤ , ≤ 99 | 2326 (14.4%) | 53 (6.1%) | 36 (16.4%) |
| 100 ≤ , ≤ 499 | 2779 (17.2%) | 34 (3.9%) | 30 (13.6%) |
| 499≤ | 4974 (30.9%) | 51 (5.9%) | 42 (19.1%) |
| CEA (ng/ml) | (n = 9083) | (n = 1350) | (n = 178) |
| <5.0 | 6980 (76.8%) | 852 (63.1%) | 124 (69.7%) |
| 5.0 ≤ , ≤ 9.9 | 1698 (18.7%) | 204 (15.1%) | 31 (17.4%) |
| 10.0 ≤ , ≤ 19.9 | 276 (3.0%) | 81 (6.0%) | 13 (7.3%) |
| 20.0≤ | 129 (1.4%) | 213 (15.8%) | 10 (5.6%) |
| CA19–9 (U/ml) | (n = 8147) | (n = 1325) | (n = 170) |
| <37 | 6468 (79.4%) | 473 (35.7%) | 102 (60.0%) |
| 37 ≤ , ≤ 99 | 1225 (15.0%) | 220 (16.6%) | 28 (16.5%) |
| 100 ≤ , ≤ 299 | 317 (3.9%) | 163 (12.3%) | 16 (9.4%) |
| 300≤ | 137 (1.7%) | 469 (35.4%) | 24 (14.1%) |
Diagnosis
Similar to HCC, cHCC-CCA is often diagnosed incidentally during ordinal surveillance examinations in patients with chronic liver disease or imaging examinations performed for other diseases. Imaging tests, such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography often used with contrast agents have also been performed for qualitative diagnosis [19–21]. Wells et al. reported the following four imaging patterns for cHCC-CCA: Type 1, HCC type with diffuse or mosaic-like early staining and washout; Type 2, ICC type with ring-shaped early staining, washout or fading in the portal or delayed phase, and occasionally delayed staining in the central region; Type 3, ICC type with ring-shaped early staining and persistent staining until the late phase; and Type 4, mixed staining with early and delayed staining within the same tumor [21]. The mentioned study reported that among the 29 cases, 23 (79%) presented with the Type 2 pattern, whereas two cases (7%) each presented with Type 1, Type 3, and Type 4 patterns. Moreover, ‘target appearance,’ including ring staining in the early contrast phase, washout around the mass, central staining in the delayed phase, diffusion-restricted findings at the edge of the mass on diffusion-weighted imaging, and high signal intensity at the center during the transition phase or hepatocellular phase of gadolinium ethoxybenzyl diethlenetriamine pentaacetic acid contrast-enhanced MRI, was useful for differentiating between cHCC-CCA and HCC [22,23].
Given that imaging findings typical of cHCC-CCA have yet to be identified, pathological confirmation is essential for an accurate diagnosis. In other words, tumor biopsies enable accurate diagnosis and treatment selection, and the accumulation of such cases contributes to the development and establishment of standard of care. As a starting point, noting ‘atypical’ findings with regard to radiological findings or tumor markers is necessary to determine the indication for tumor biopsy [24]. For example, in cases wherein both HCC- and adenocarcinoma-specific tumor markers are elevated, increased CA19–9 levels despite imaging findings suggesting HCC or persistent staining into the late phase on dynamic CT or MRI should warrant aggressive tumor biopsy to confirm the pathological findings. In particular, although tumor biopsy is generally contraindicated for HCC nodules due to the risk of complications, such as bleeding, tumor biopsy should be strongly considered in patients with atypical HCC findings based on imaging results and tumor marker values. The National Comprehensive Cancer Network Clinical Practice Guideline in Oncology does not necessarily require biopsy for patients with classical radiological findings with consistent tumor marker levels, or who are candidate for curative resection or transplant; however, in addition to pathological confirmation being mandatory in the diagnosis for patients with advanced stage, it is also recommended that repeat biopsies be considered to ascertain the dominant histology at recurrence in patients with metastatic or locally-advanced recurrence after a prior resection or local therapies for cHCC-CCA. Moreover, a repeat biopsy at tumor progression may be warranted to reassess dominant histology of a progressing lesion, especially if there are discordant areas of response and progression. As described below, personalized medicine based on genetic alterations is being developed in both HCC and ICC, given their potential use for future comprehensive genomic profiling studies.
Treatment strategies for cHCC-CCA
Given that cHCC-CCA is an extremely rare condition, only a few reports have examined the advantages and disadvantages of the available treatment options [13]. Most of the reports to date have selected treatment according to the presence of either HCC or ICC. Resection is the most commonly reported treatment for cHCC-CCA [25–27], perhaps due to the high rates of cHCC-CCA diagnosed through pathological analysis of resected specimens. These studies reported that recurrence rates for cHCC-CCA remained high even after radical resection and that the prognosis of patients with cHCC-CCA was poorer than that of patients with HCC. However, some studies have suggested that patients with cHCC-CCA had a better prognosis than did patients with ICC, whereas others suggested the opposite. Regardless, evaluating the resectability of the tumor has been the first step in determining the treatment for cHCC-CCA, with resection being preferentially considered among those with acceptable tumor factors and residual liver functional reserve (Fig. 2). Several other studies have reported on the outcomes of local therapies other than resection, such as liver transplantation [28–30], percutaneous local therapy for small tumors [31], or transarterial chemoembolization for hypervascularized tumors [32–34]. Their findings suggested that treatment for cHCC-CCA was based more on the presence of HCC rather than ICC provided that tumor spread was limited.
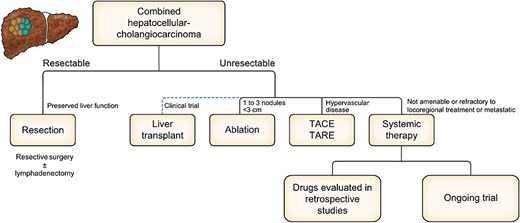
Proposal of treatment strategies for combined hepatocellular-cholangiocarcinoma on an EASL position paper.
The 23rd nationwide follow-up survey of primary liver cancer in Japan revealed that among the 263 patients diagnosed with cHCC-CCA, 187 (71.1%), 5 (1.9%), 21 (8.0%), 7 (2.7%), 20 (7.6%), and 21 (8.0%) received hepatic resection or liver transplantation, local therapy, hepatic arterial embolization, hepatic arterial chemotherapy, systemic treatment, and no treatment, respectively [16]. However, as mentioned earlier, cHCC-CCA is rarely diagnosed before treatment among patients who did not undergo resection. Moreover, several cases who undergo resection with a preoperative diagnosis of either HCC or ICC are subsequently pathologically diagnosed with cHCC-CCA, with such cases possibly including those with double cancer, collision tumors, or fibrolamellar tumors. Generally, treatment for cHCC-CCA is selected according to the treatment strategy for HCC or ICC based on clinical judgment from imaging findings, tumor markers, and pathologically predominant components in the tumor, which suggests that cHCC-CCA has yet to be established as an independent disease entity.
Systemic treatment for cHCC-CCA
To date, no prospective clinical trials have evaluated the efficacy and safety of specific regimens for cHCC-CCA, and the few retrospective studies have mostly included varying treatment regimens [35–42] (Table 2). The 23rd nationwide follow-up survey of primary liver cancer in Japan revealed that 15, 8, and six patients received sorafenib, gemcitabine, and cisplatin as systemic treatment [16], indicating that molecular targeted drugs have been administered according to HCC or cytotoxic agents according to ICC. In recent years, however, several studies have demonstrated the efficacy of regimens including immune checkpoint inhibitors (ICIs) for both HCC and ICC [43–46], which can now be used in clinical practice. A study from South Korea found that 25 of 101 patients with cHCC-CCA who received systemic treatment and were selected to receive ICI either alone or in combination with other agents obtained a response rate of 20%, median progression-free survival (PFS) of 3.5 months, and median overall survival (OS) of 8.3 months [41]. A study from France on 16 patients with cHCC-CCA treated with atezolizumab and bevacizumab reported that three of nine patients (33%) who received the aforementioned combination as first-line treatment achieved a median PFS of 3 months and a median OS of 13 months [42]. Similarly, Satake et al., who reported a detailed case report of six patients with cHCC-CCA receiving atezolizumab and bevacizumab combination therapy in Japan, showed that three patients achieved a radiological response [47]. Based on these results, a prospective study is currently underway in Japan to evaluate the efficacy of atezolizumab and bevacizumab combination therapy in patients with unresectable cHCC-CCA (jRCTs031220099). The mentioned study aims to determine the response rate as its primary endpoint and plans to include 12 patients. Meanwhile, a clinical trial on gemcitabine and cisplatin in combination with atezolizumab or atezolizumab and bevacizumab is also underway (NCT05211323). This study sets PFS as its primary endpoint and plans to include 88 patients. Apparently, the former study provides the standard treatments for advanced HCC, whereas the latter provides a combination therapy involving gemcitabine, cisplatin, and immune-check point inhibitors is the standard of care for advanced ICC. Although the results of these trials would not be available for many years, we hope that their findings would facilitate the development of therapeutics for cHCC-CCA.
Reports containing more than 10 cases of combined hepatocellular-cholangiocarcinoma who received systemic treatment (adopted with modifications from reference [2].
| Author . | Country . | Published year, (Reference) . | Included period . | Number of patients . | Systemic treatment . | ORR (%) . | mPFS (months) . | mOS (months) . |
|---|---|---|---|---|---|---|---|---|
| Saimon | France | 2017, [35] | 2008 to 2017 | 30 | Gemcitabine+platinum | 28.6 | 9.0 | 16.2 |
| Kobayashi | Japan | 2018, [36] | 2002 to 2015 | 36 | Gemcitabine+cisplatin, fluorouracil+cisplatin, sorafenib, others | 5.6 | 2.8 | 8.9 |
| Trikalinos | United states of America | 2018, [37] | 1999 to 2006 | 68 | Gemcitabine +5-FU, gemcitabine + platinum | 0–24.3 | 4.8–8.0 | 9.6–11.7 |
| Kim | South Korea | 2021, [38] | 1999 to 2015 | 99 | Sorafenib, cytotoxic agents | 14.1 | 3.8 | 10.6 |
| Gigante | France | 2022, [39] | 2009 to 2020 | 83 | Platinum, molecular target agents, others | 13 | 4 | 12 |
| Pomej | EU | 2023, [40] | 2003 to 2022 | 44 | Cytotoxic agents, others | 6.8 | 3.0–3.2 | 4.6 |
| Jang | South Korea | 2023, [41] | 2015 to 2021 | 25 | Immune checkpoint inhibitors | 20.0 | 3.5 | 8.3 |
| Gigante | France | 2023, [42] | 2020 to 2022 | 16 | Atezolizumab+bevacizumab | 33.3 | 3 | 13 |
| Author . | Country . | Published year, (Reference) . | Included period . | Number of patients . | Systemic treatment . | ORR (%) . | mPFS (months) . | mOS (months) . |
|---|---|---|---|---|---|---|---|---|
| Saimon | France | 2017, [35] | 2008 to 2017 | 30 | Gemcitabine+platinum | 28.6 | 9.0 | 16.2 |
| Kobayashi | Japan | 2018, [36] | 2002 to 2015 | 36 | Gemcitabine+cisplatin, fluorouracil+cisplatin, sorafenib, others | 5.6 | 2.8 | 8.9 |
| Trikalinos | United states of America | 2018, [37] | 1999 to 2006 | 68 | Gemcitabine +5-FU, gemcitabine + platinum | 0–24.3 | 4.8–8.0 | 9.6–11.7 |
| Kim | South Korea | 2021, [38] | 1999 to 2015 | 99 | Sorafenib, cytotoxic agents | 14.1 | 3.8 | 10.6 |
| Gigante | France | 2022, [39] | 2009 to 2020 | 83 | Platinum, molecular target agents, others | 13 | 4 | 12 |
| Pomej | EU | 2023, [40] | 2003 to 2022 | 44 | Cytotoxic agents, others | 6.8 | 3.0–3.2 | 4.6 |
| Jang | South Korea | 2023, [41] | 2015 to 2021 | 25 | Immune checkpoint inhibitors | 20.0 | 3.5 | 8.3 |
| Gigante | France | 2023, [42] | 2020 to 2022 | 16 | Atezolizumab+bevacizumab | 33.3 | 3 | 13 |
ORR, objective response rate; PFS, progression-free survival; OS, overall survival
Reports containing more than 10 cases of combined hepatocellular-cholangiocarcinoma who received systemic treatment (adopted with modifications from reference [2].
| Author . | Country . | Published year, (Reference) . | Included period . | Number of patients . | Systemic treatment . | ORR (%) . | mPFS (months) . | mOS (months) . |
|---|---|---|---|---|---|---|---|---|
| Saimon | France | 2017, [35] | 2008 to 2017 | 30 | Gemcitabine+platinum | 28.6 | 9.0 | 16.2 |
| Kobayashi | Japan | 2018, [36] | 2002 to 2015 | 36 | Gemcitabine+cisplatin, fluorouracil+cisplatin, sorafenib, others | 5.6 | 2.8 | 8.9 |
| Trikalinos | United states of America | 2018, [37] | 1999 to 2006 | 68 | Gemcitabine +5-FU, gemcitabine + platinum | 0–24.3 | 4.8–8.0 | 9.6–11.7 |
| Kim | South Korea | 2021, [38] | 1999 to 2015 | 99 | Sorafenib, cytotoxic agents | 14.1 | 3.8 | 10.6 |
| Gigante | France | 2022, [39] | 2009 to 2020 | 83 | Platinum, molecular target agents, others | 13 | 4 | 12 |
| Pomej | EU | 2023, [40] | 2003 to 2022 | 44 | Cytotoxic agents, others | 6.8 | 3.0–3.2 | 4.6 |
| Jang | South Korea | 2023, [41] | 2015 to 2021 | 25 | Immune checkpoint inhibitors | 20.0 | 3.5 | 8.3 |
| Gigante | France | 2023, [42] | 2020 to 2022 | 16 | Atezolizumab+bevacizumab | 33.3 | 3 | 13 |
| Author . | Country . | Published year, (Reference) . | Included period . | Number of patients . | Systemic treatment . | ORR (%) . | mPFS (months) . | mOS (months) . |
|---|---|---|---|---|---|---|---|---|
| Saimon | France | 2017, [35] | 2008 to 2017 | 30 | Gemcitabine+platinum | 28.6 | 9.0 | 16.2 |
| Kobayashi | Japan | 2018, [36] | 2002 to 2015 | 36 | Gemcitabine+cisplatin, fluorouracil+cisplatin, sorafenib, others | 5.6 | 2.8 | 8.9 |
| Trikalinos | United states of America | 2018, [37] | 1999 to 2006 | 68 | Gemcitabine +5-FU, gemcitabine + platinum | 0–24.3 | 4.8–8.0 | 9.6–11.7 |
| Kim | South Korea | 2021, [38] | 1999 to 2015 | 99 | Sorafenib, cytotoxic agents | 14.1 | 3.8 | 10.6 |
| Gigante | France | 2022, [39] | 2009 to 2020 | 83 | Platinum, molecular target agents, others | 13 | 4 | 12 |
| Pomej | EU | 2023, [40] | 2003 to 2022 | 44 | Cytotoxic agents, others | 6.8 | 3.0–3.2 | 4.6 |
| Jang | South Korea | 2023, [41] | 2015 to 2021 | 25 | Immune checkpoint inhibitors | 20.0 | 3.5 | 8.3 |
| Gigante | France | 2023, [42] | 2020 to 2022 | 16 | Atezolizumab+bevacizumab | 33.3 | 3 | 13 |
ORR, objective response rate; PFS, progression-free survival; OS, overall survival
Future direction
As described earlier, clinical treatment strategies for cHCC-CCA have generally been determined based on imaging findings, tumor markers, and the pathologically predominant components in the tumor, in accordance with the treatment strategy for either HCC or ICC. The main reason for this lack of uniformity in treatment strategies for cHCC-CCA is the exceptional infrequency of this disease entity, as well as its characteristics that complicate diagnosis before treatment, making it difficult to develop large-scale studies. Another reason is that current criteria do not depend on the amount (percentage) of each component when both HCC and ICC components are found. In other words, a patient whose tumor is largely composed of HCC components is diagnosed with cHCC-CCA despite only having a few ICC components. Therefore, a treatment similar to that for HCC is expected to be selected in such cases. Conversely, patients whose tumors largely consist of ICC components are also diagnosed with cHCC-CCA despite having a limited amount of HCC components, with such patients ideally receiving treatment similar to that for ICC. After all, the treatment strategies for these patients have already been determined and do not require the development of treatment strategies specific to cHCC-CAA. One study reported that cHCC-CCA with an ICC component of <10% can be considered equivalent to HCC in terms of radiological findings and clinical course [48]. Although further research is needed to determine the minimum cutoff value, defining the presence of either component based on who much they exceed a certain level, similar to the MiNEN [49], may be useful in establishing disease concepts and facilitating treatment development. Alternatively, reclassifying most cHCC-CCA as HCC-like or ICC-like based on deep learning-based phenotyping may be possible [50]. If so, cHCC-CCA need not be an independent disease entity, at least in terms of treatment strategy.
In recent years, pathological elucidation, including genetic abnormalities, has also emerged, with some studies reporting on the genetic landscape of cHCC-CCA [51–54]. Understanding the genetic abnormalities observed in cHCC-CCA may contribute to the differential diagnosis of HCC and ICC or the establishment of a treatment strategy; however, genetic abnormalities associated with cHCC-CCA contain features of both HCC and ICC, although such results were expected. At present, personalized medicine based on genetic alterations is limited in the field of HCC [55], and the significance of comprehensive genomic profiling for cHCC-CCA in clinical practice may be restricted to indications for molecularly targeted therapies that target specific molecules recognized in ICC, such as FGFR2 or IDH1 [56].
Conclusions
The concept and definition of cHCC-CCA have shifted quite frequently, with the latest version of the WHO Classification published only 5 years ago finally establishing them. As mentioned in this review, it is difficult to make a diagnosis of cHCC-CCA based on radiological findings or pathological findings from a specimen obtained by needle biopsy, Unfortunately, these are limitations in the accurate diagnosis of cHCC-CCA and are the main reason that prevents the development and establishment of standard of care especially for the patients with advanced stage. The current situation is that treatment strategies is considered in accordance with HCC or ICC based on incomplete information including these imaging or pathological findings as well as tumor marker. If this situation continues, in which a unique treatment strategy for cHCC-CCA may not be necessary, the disease concept of cHCC-CCA itself will become meaningless. To tackle these difficulties, treatment selection based on the proportion of HCC and ICC components or genetic characteristics may be one of the future treatment strategies, but most importantly, we must recognize the existence of this extremely rare prevalent disease, understand its many limitations, and work with multiple investigators to initiate treatment selection based on a common rationale. Several previous reports on cHCC-CCA must require review, and new evidence must be accumulated. A consensus on treatment strategies is expected through the accumulation of cases in the future.
Abbreviations
CT: Computed tomography
ESCAT: ESMO Scale for Clinical Actionability of molecular Targets.
ICI: Immune checkpoint inhibitors.
MRI: Magnetic resonance imaging.
OS: Overall survival.
PFS: Progression-free survival.
Acknowledgements
None.
Conflict of interest: Takeshi Terashima received lecture fees from Chugai Pharmaceutical Co., Ltd and AstraZeneca plc.
Funding
The authors have no financial support.


