-
PDF
- Split View
-
Views
-
Cite
Cite
Chikako Funasaka, Yoichi Naito, Shota Kusuhara, Takehiro Nakao, Hiromichi Nakajima, Megumi Kawamoto, Kaede Baba, Kanako Mamishin, Chihiro Kondoh, Kenichi Harano, Nobuaki Matsubara, Ako Hosono, Tomoaki Sasaki, Toshikatsu Kawasaki, Toru Mukohara, Clinical features of CDK4/6 inhibitor-related interstitial lung disease in patients with breast cancer: a case series study, Japanese Journal of Clinical Oncology, Volume 53, Issue 2, February 2023, Pages 105–114, https://doi.org/10.1093/jjco/hyac168
Close - Share Icon Share
Abstract
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors are the standard treatment for advanced hormone receptor-positive breast cancer. Although interstitial lung disease is a rare (1–3.3%) but serious adverse event associated with CDK4/6 inhibitors, the incidence of interstitial lung disease in Japanese patients in the real world and the risk factors of interstitial lung disease are not clear.
We retrospectively investigated the incidence of interstitial lung disease in 224 patients with advanced breast cancer who received CDK4/6 inhibitors at our hospital between 31 January 2017 and 31 January 2021. The correlation of age (>50 vs ≤50 years), presence or absence of previous history of interstitial lung disease, lung metastasis, smoking history and chest radiation with the development of interstitial lung disease was evaluated.
In total, 177 cases received palbociclib, 39 cases received abemaciclib and 8 cases received both palbociclib and abemaciclib, constituting a palbociclib group (n = 185) and an abemaciclib group (n = 47). At a median observation period of 607 days, 8.0% (18/224) cases (13 definite and 5 probable cases) had interstitial lung disease; 6.5% (12/185) of palbociclib-treated and 13% (6/47) of abemaciclib-treated cases. The median time to interstitial lung disease onset was 178 (range, 14–750) days. There was no significant correlation between the background factors studied and the development of interstitial lung disease.
The frequency of CDK4/6 inhibitor-induced interstitial lung disease was higher than that reported in clinical trials. We did not identify any risk factors for the development of interstitial lung disease in this study, and thus, larger studies that include patient predisposition are required.
Introduction
Breast cancer is the most prevalent malignancy among women worldwide, affecting 2 088 849 people in 2018, and has a consistently increasing prevalence (1). Although breast cancer is curable when diagnosed during its early stages, metastatic or distantly recurrent breast cancer is incurable and accounts for 626 679 yearly deaths worldwide (1).
For hormone receptor-positive human epidermal growth factor 2 (HER2)-negative metastatic or recurrent breast cancer, endocrine therapy combined with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors are recommended as the first- or second-line systemic therapy (2,3). CDK4/6 inhibitors are molecular targeted agents that inhibit CDKs, preventing the formation of CDK4/6–cyclin D complexes. Cyclin D is involved in the regulation of the cell cycle, and thus, CDK4/6 inhibitors arrest the progression of the cell cycle and produce anti-tumor effects (4). Currently, palbociclib and abemaciclib are approved for the treatment of metastatic or recurrent breast cancer in Japan and are used in combination with aromatase inhibitors or fulvestrant.
CDK4/6 inhibitors have been shown to have promising efficacy with favorable safety profiles that allow patients to maintain their quality of life in the clinical trial setting (5). The frequent adverse events associated with CDK4/6 inhibitors include myelosuppression and gastrointestinal symptoms, but these are generally manageable (6). Conversely, one rare but serious adverse event is interstitial lung disease (ILD) (7). In a combined analysis of the PALOMA-1, PALOMA-2, and PALOMA-3 trials, 1.0% of patients treated with palbociclib had ILD, 0.1% of which was grade 3 or higher, but no deaths were reported (8). For abemaciclib, in the MONARCH-2 and MONARCH-3 trials, 2.0–5.2% of patients had ILD, 0.7–1.2% had grade 3 or higher and 0.5% died with ILD (9). In the Food and Drug Administration Adverse Event Reporting System (FAERS), 2.1% of reported ILD was caused by abemaciclib and 0.3% by palbociclib or ribociclib, another CDK4/6 inhibitor. Of note, 52% of these CDK4/6 inhibitor-related ILD cases were reported from Asia/Japan, and 72% were in patients aged 65 years or older (10). In Japan, 14 cases of serious ILD caused by abemaciclib were reported after marketing, and 3 of them resulted in death, and a safety bulletin was issued (11).
Lung injury caused by anti-cancer drugs is generally associated with aged patients, a history of interstitial pneumonia and smoking history (12). Consistently, risk factors for ILD caused by molecular targeted drugs in lung cancer patients include age, smoking history and the presence of pre-existing ILD (13). However, no reports have evaluated the risk factors for drug-induced ILD with CDK4/6 inhibitors.
To address this gap in our knowledge, we retrospectively investigated the clinical features of CDK4/6-related ILD in advanced breast cancer patients.
Patients and methods
Patients
This study was a retrospective observational case series. We analyzed the records of 224 patients with metastatic or recurrent breast cancer who were treated with CDK4/6 inhibitors, i.e. palbociclib or abemaciclib, from January 2017 to January 2021 at the National Cancer Center Hospital East, Japan. Patients’ data were collected from medical records.
Data evaluation and statistical analysis
We investigated the presence and characteristics of ILD in these patients. CDK4/6 inhibitor-related ILD was defined as pulmonary disease newly occurring or deteriorating during treatment with a CDK4/6 inhibitor and included reported events of ILD as well as pneumonitis, pulmonary fibrosis, bronchiolitis, lung infiltration, radiation pneumonitis and that requiring differentiation of these. ILD was classified as definite or probable CDK4/6 inhibitor-related ILD. Definite CDK4/6 inhibitor-related ILD was determined by radiographic patterns of ILD classified as follows: (i) acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS); (ii) non-specific interstitial pneumonia (NSIP); (iii) cryptogenic organizing pneumonia (OP); (iv) acute eosinophilic pneumonia and (v) hypersensitivity pneumonia (HP) (14–16). The probable cases were determined as those with scope for a differential diagnosis of infectious pneumonia, lymphangitis carcinomatosis or radiation pneumonitis unrelated to CDK4/6 inhibitors. A diagnosis of ILD was obtained by initially referring to the imaging study report on the medical chart, which was then confirmed by a radiologist (T.Sasaki) with expertise in thoracic and oncologic imaging. The severity of ILD as an adverse event was assessed using the Common Toxicity Criteria for Adverse Events, version 5.0.
We used the χ2 and Fisher tests to evaluate whether age (>50 vs ≤50 years), presence or absence of a previous history of ILD, lung metastasis, smoking history, perioperative chemotherapy, treatment lines (1st, 2nd and 3rd lines or later) and chest radiation were correlated with the development of ILD. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics (17).
Ethics
The Institutional Review Board (IRB) of the National Cancer Center approved this study (IRB number 2017-431), which was conducted in accordance with the principles stated in Japan’s Ethics Guidelines for Epidemiological Research. The IRB waived the requirement for obtaining written informed consent from the study’s subjects.
Results
Patients’ backgrounds, incidence of ILD and outcomes
Between January 2017 to January 2021, 177 cases received palbociclib, 39 cases received abemaciclib and 8 cases received both palbociclib and abemaciclib, constituting a palbociclib group (n = 185) and an abemaciclib group (n = 47). All but 5 patients received palbociclib and/or abemaciclib in the practice setting. The median observation period was 607 days. Patient characteristics are summarized in Table 1. Median age was 64 years, and 191 (85%) cases were over 50 years old. In total, 35% received CDK4/6 inhibitors as first-line therapy and 65% received them as second-line or later. Eighty-nine cases (39%) received radiotherapy including that of the chest wall.
| Characteristics . | Number of patients (n = 224) . |
|---|---|
| age | |
| median (range) | 64 (33–88) |
| ECOG PS, n (%) | |
| - 0 or 1 | 215 (96.0%) |
| - ≥2 | 9 (4.0%) |
| treatment lines | |
| - 1st line | 78 (35%) |
| - 2nd line | 63 (28%) |
| - 3rd line or later | 83 (37%) |
| CDK4/6 inhibitor | |
| - Palbociclib | 177 (79%) |
| - Abemaciclib | 39 (17%) |
| -both | 8 (4%) |
| Combination endcrine therapy | |
| - AI (LET, ANA) | 100 (45%) |
| - FUL | 102 (46%) |
| - Other | 22 (10%) |
| Metastatic site | |
| lung | 87 (38%) |
| liver | 66 (29%) |
| lymph node | 77 (34%) |
| bone | 140 (63%) |
| Denovo Stage IV | 68 (30%) |
| Previous history of ILD | 7 (3%) |
| Smoking history | |
| Current/past smoker | 43 (19%) |
| never | 181 (81%) |
| Radiotherapy to chest wall | 89 (39%) |
| Perioperative chemotherapy | |
| Yes | 92 (41%) |
| No | 124 (55%) |
| Unknown | 8 (4%) |
| Characteristics . | Number of patients (n = 224) . |
|---|---|
| age | |
| median (range) | 64 (33–88) |
| ECOG PS, n (%) | |
| - 0 or 1 | 215 (96.0%) |
| - ≥2 | 9 (4.0%) |
| treatment lines | |
| - 1st line | 78 (35%) |
| - 2nd line | 63 (28%) |
| - 3rd line or later | 83 (37%) |
| CDK4/6 inhibitor | |
| - Palbociclib | 177 (79%) |
| - Abemaciclib | 39 (17%) |
| -both | 8 (4%) |
| Combination endcrine therapy | |
| - AI (LET, ANA) | 100 (45%) |
| - FUL | 102 (46%) |
| - Other | 22 (10%) |
| Metastatic site | |
| lung | 87 (38%) |
| liver | 66 (29%) |
| lymph node | 77 (34%) |
| bone | 140 (63%) |
| Denovo Stage IV | 68 (30%) |
| Previous history of ILD | 7 (3%) |
| Smoking history | |
| Current/past smoker | 43 (19%) |
| never | 181 (81%) |
| Radiotherapy to chest wall | 89 (39%) |
| Perioperative chemotherapy | |
| Yes | 92 (41%) |
| No | 124 (55%) |
| Unknown | 8 (4%) |
PS, performance status; CDK4/6, cyclin-dependent kinase 4/6; AI, aromatase inhibitor; LET, letrozole; ANA, anastrozole; FUL, fulvestrant; ILD,interstitial lung disease.
| Characteristics . | Number of patients (n = 224) . |
|---|---|
| age | |
| median (range) | 64 (33–88) |
| ECOG PS, n (%) | |
| - 0 or 1 | 215 (96.0%) |
| - ≥2 | 9 (4.0%) |
| treatment lines | |
| - 1st line | 78 (35%) |
| - 2nd line | 63 (28%) |
| - 3rd line or later | 83 (37%) |
| CDK4/6 inhibitor | |
| - Palbociclib | 177 (79%) |
| - Abemaciclib | 39 (17%) |
| -both | 8 (4%) |
| Combination endcrine therapy | |
| - AI (LET, ANA) | 100 (45%) |
| - FUL | 102 (46%) |
| - Other | 22 (10%) |
| Metastatic site | |
| lung | 87 (38%) |
| liver | 66 (29%) |
| lymph node | 77 (34%) |
| bone | 140 (63%) |
| Denovo Stage IV | 68 (30%) |
| Previous history of ILD | 7 (3%) |
| Smoking history | |
| Current/past smoker | 43 (19%) |
| never | 181 (81%) |
| Radiotherapy to chest wall | 89 (39%) |
| Perioperative chemotherapy | |
| Yes | 92 (41%) |
| No | 124 (55%) |
| Unknown | 8 (4%) |
| Characteristics . | Number of patients (n = 224) . |
|---|---|
| age | |
| median (range) | 64 (33–88) |
| ECOG PS, n (%) | |
| - 0 or 1 | 215 (96.0%) |
| - ≥2 | 9 (4.0%) |
| treatment lines | |
| - 1st line | 78 (35%) |
| - 2nd line | 63 (28%) |
| - 3rd line or later | 83 (37%) |
| CDK4/6 inhibitor | |
| - Palbociclib | 177 (79%) |
| - Abemaciclib | 39 (17%) |
| -both | 8 (4%) |
| Combination endcrine therapy | |
| - AI (LET, ANA) | 100 (45%) |
| - FUL | 102 (46%) |
| - Other | 22 (10%) |
| Metastatic site | |
| lung | 87 (38%) |
| liver | 66 (29%) |
| lymph node | 77 (34%) |
| bone | 140 (63%) |
| Denovo Stage IV | 68 (30%) |
| Previous history of ILD | 7 (3%) |
| Smoking history | |
| Current/past smoker | 43 (19%) |
| never | 181 (81%) |
| Radiotherapy to chest wall | 89 (39%) |
| Perioperative chemotherapy | |
| Yes | 92 (41%) |
| No | 124 (55%) |
| Unknown | 8 (4%) |
PS, performance status; CDK4/6, cyclin-dependent kinase 4/6; AI, aromatase inhibitor; LET, letrozole; ANA, anastrozole; FUL, fulvestrant; ILD,interstitial lung disease.
In total, 18/224 (8.0%) cases, including definite and probable cases, experienced CDK4/6 inhibitor-related ILD. Clinical symptoms related to ILD were dyspnea (n = 4), cough (n = 1), fever (n = 1) and sputum (n = 2). Ten cases showed no symptoms, but one had decreased blood oxygen saturation.
Thirteen (5.8%) of these patients had CT findings that were determined to be definite CDK4/6 inhibitor-related ILD: six cases, OP; three cases, NSIP; two cases, HP and two cases, AIP/ARDS (Table 2). Five were determined to be probable cases, two cases were indicated to be bacterial pneumonia and one case each was indicated to be lineal atelectasis, lymphangitis and radiation pneumonitis (Table 2). Twelve ILD cases (8 definite and 4 probable) occurred in the palbociclib group (12/185, 6.5%), and 6 cases (5 definite and 1 probable) in the abemaciclib group (6/47, 13%) (Table 3).
| No. of Case . | Abemaciclib(A) or Palbociclib(P) . | Combind Endocrine therapy . | treatment line . | age . | Performance status . | lung metastasis . | Initial grade . | Worst grade . | pre-exisiting ILD . | smoking . | Radiation including chest . | abnormality of CT scan . | Pre-exisiting CT abnormality . | Treatment . | outcome . | re-challenge CDK 4/6 inhibitor . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | LET | 2 | 82 | 1 | No | 1 | 1 | No | past smorker | Yes | Bacterial pneumonia | none | discontinuation, antibiotics | recover | Yes |
| 2 | P | LET | 1 | 74 | 1 | No | 1 | 1 | No | No | No | NSIP | none | PSL, antibiotics | recover | No |
| 3 | P | LET | 1 | 71 | 1 | Yes | 3 | 4 | No | No | No | OP | multiple lung metastases | mPSL pulse therapy, cycloposphamide | recover | No |
| 4 | P | ANA | 1 | 89 | 1 | Yes | 3 | 5 | No | No | No | AIP/ARDS | pleural dissemination | PSL | dead | No |
| 5 | P | LET | 1 | 80 | 1 | Yes | 1 | 1 | No | No | No | Bacterial pneumonia | none | discontinuation, antibiotics | recover | No |
| 6 | P | FUL | 1 | 77 | 1 | No | 2 | 2 | No | No | No | HP | old Tb | PSL | recover | No |
| 7 | A | FUL | 2 | 51 | 1 | Yes | 1 | 1 | No | No | No | OP | none | discontinuation | recover | No |
| 8 | P | FUL | 1 | 55 | 1 | No | 1 | 1 | No | No | No | linear atelectasis | none | discontinuation | recover | No |
| 9 | A | LET | 1 | 69 | 0 | Yes | 1 | 2 | No | No | No | OP | multiple lung metastases | PSL, antibiotics | recover | No |
| 10 | P | LET | 5 | 83 | 1 | Yes | 1 | 1 | No | No | No | NSIP | multiple lung metastases | discontinuation, antibiotics | recover | Yes |
| 11 | A | LET | 6 | 66 | 0 | Yes | 1 | 1 | No | No | Yes | OP | small lung meta | PSL | recover | No |
| 12 | P | FUL | 2 | 68 | 1 | Yes | 1 | 1 | No | No | No | lymphangitis | lymphangitis | discontinuation | recover | No |
| 13 | A | FUL | 8 | 69 | 1 | No | 2 | 2 | No | No | No | NSIP | NSIP | PSL | recover | No |
| 14 | A | FUL | 3 | 65 | 1 | No | 1 | 1 | No | No | Yes | OP | none | discontinuation | recover | No |
| 15 | A | FUL | 2 | 43 | 0 | No | 1 | 1 | No | unknown | Yes | OP | none | discontinuation | recover | No |
| 16 | P | LET | 7 | 82 | 1 | Yes | 1 | 1 | No | unknown | Yes | Radiation pneumonitis | multiple lung metastases | discontinuation | recover | Yes |
| 17 | P | LET | 3 | 74 | 1 | No | 2 | 2 | No | No | Yes | HP | mild bronchiolitis | PSL | recover | No |
| 18 | P | NA | 1 | 63 | 1 | Yes | 2 | 5 | No | No | No | AIP/ARDS | multiple lung metastases | mPSL pulse therapy | dead | No |
| No. of Case . | Abemaciclib(A) or Palbociclib(P) . | Combind Endocrine therapy . | treatment line . | age . | Performance status . | lung metastasis . | Initial grade . | Worst grade . | pre-exisiting ILD . | smoking . | Radiation including chest . | abnormality of CT scan . | Pre-exisiting CT abnormality . | Treatment . | outcome . | re-challenge CDK 4/6 inhibitor . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | LET | 2 | 82 | 1 | No | 1 | 1 | No | past smorker | Yes | Bacterial pneumonia | none | discontinuation, antibiotics | recover | Yes |
| 2 | P | LET | 1 | 74 | 1 | No | 1 | 1 | No | No | No | NSIP | none | PSL, antibiotics | recover | No |
| 3 | P | LET | 1 | 71 | 1 | Yes | 3 | 4 | No | No | No | OP | multiple lung metastases | mPSL pulse therapy, cycloposphamide | recover | No |
| 4 | P | ANA | 1 | 89 | 1 | Yes | 3 | 5 | No | No | No | AIP/ARDS | pleural dissemination | PSL | dead | No |
| 5 | P | LET | 1 | 80 | 1 | Yes | 1 | 1 | No | No | No | Bacterial pneumonia | none | discontinuation, antibiotics | recover | No |
| 6 | P | FUL | 1 | 77 | 1 | No | 2 | 2 | No | No | No | HP | old Tb | PSL | recover | No |
| 7 | A | FUL | 2 | 51 | 1 | Yes | 1 | 1 | No | No | No | OP | none | discontinuation | recover | No |
| 8 | P | FUL | 1 | 55 | 1 | No | 1 | 1 | No | No | No | linear atelectasis | none | discontinuation | recover | No |
| 9 | A | LET | 1 | 69 | 0 | Yes | 1 | 2 | No | No | No | OP | multiple lung metastases | PSL, antibiotics | recover | No |
| 10 | P | LET | 5 | 83 | 1 | Yes | 1 | 1 | No | No | No | NSIP | multiple lung metastases | discontinuation, antibiotics | recover | Yes |
| 11 | A | LET | 6 | 66 | 0 | Yes | 1 | 1 | No | No | Yes | OP | small lung meta | PSL | recover | No |
| 12 | P | FUL | 2 | 68 | 1 | Yes | 1 | 1 | No | No | No | lymphangitis | lymphangitis | discontinuation | recover | No |
| 13 | A | FUL | 8 | 69 | 1 | No | 2 | 2 | No | No | No | NSIP | NSIP | PSL | recover | No |
| 14 | A | FUL | 3 | 65 | 1 | No | 1 | 1 | No | No | Yes | OP | none | discontinuation | recover | No |
| 15 | A | FUL | 2 | 43 | 0 | No | 1 | 1 | No | unknown | Yes | OP | none | discontinuation | recover | No |
| 16 | P | LET | 7 | 82 | 1 | Yes | 1 | 1 | No | unknown | Yes | Radiation pneumonitis | multiple lung metastases | discontinuation | recover | Yes |
| 17 | P | LET | 3 | 74 | 1 | No | 2 | 2 | No | No | Yes | HP | mild bronchiolitis | PSL | recover | No |
| 18 | P | NA | 1 | 63 | 1 | Yes | 2 | 5 | No | No | No | AIP/ARDS | multiple lung metastases | mPSL pulse therapy | dead | No |
LET, letrozole; FUL, fulvestrant; PSL, prednisolone; mPSL, methyl predonisolone; ILD, interstitial lung disease. HP, hypersensitivity pneumonia; OP, organizing pneumonia; NSIP, non-specific interstitial pneumonia; AIP/ARDS, acute interstitial pneumonia/acute respiratory distress syndrome.
| No. of Case . | Abemaciclib(A) or Palbociclib(P) . | Combind Endocrine therapy . | treatment line . | age . | Performance status . | lung metastasis . | Initial grade . | Worst grade . | pre-exisiting ILD . | smoking . | Radiation including chest . | abnormality of CT scan . | Pre-exisiting CT abnormality . | Treatment . | outcome . | re-challenge CDK 4/6 inhibitor . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | LET | 2 | 82 | 1 | No | 1 | 1 | No | past smorker | Yes | Bacterial pneumonia | none | discontinuation, antibiotics | recover | Yes |
| 2 | P | LET | 1 | 74 | 1 | No | 1 | 1 | No | No | No | NSIP | none | PSL, antibiotics | recover | No |
| 3 | P | LET | 1 | 71 | 1 | Yes | 3 | 4 | No | No | No | OP | multiple lung metastases | mPSL pulse therapy, cycloposphamide | recover | No |
| 4 | P | ANA | 1 | 89 | 1 | Yes | 3 | 5 | No | No | No | AIP/ARDS | pleural dissemination | PSL | dead | No |
| 5 | P | LET | 1 | 80 | 1 | Yes | 1 | 1 | No | No | No | Bacterial pneumonia | none | discontinuation, antibiotics | recover | No |
| 6 | P | FUL | 1 | 77 | 1 | No | 2 | 2 | No | No | No | HP | old Tb | PSL | recover | No |
| 7 | A | FUL | 2 | 51 | 1 | Yes | 1 | 1 | No | No | No | OP | none | discontinuation | recover | No |
| 8 | P | FUL | 1 | 55 | 1 | No | 1 | 1 | No | No | No | linear atelectasis | none | discontinuation | recover | No |
| 9 | A | LET | 1 | 69 | 0 | Yes | 1 | 2 | No | No | No | OP | multiple lung metastases | PSL, antibiotics | recover | No |
| 10 | P | LET | 5 | 83 | 1 | Yes | 1 | 1 | No | No | No | NSIP | multiple lung metastases | discontinuation, antibiotics | recover | Yes |
| 11 | A | LET | 6 | 66 | 0 | Yes | 1 | 1 | No | No | Yes | OP | small lung meta | PSL | recover | No |
| 12 | P | FUL | 2 | 68 | 1 | Yes | 1 | 1 | No | No | No | lymphangitis | lymphangitis | discontinuation | recover | No |
| 13 | A | FUL | 8 | 69 | 1 | No | 2 | 2 | No | No | No | NSIP | NSIP | PSL | recover | No |
| 14 | A | FUL | 3 | 65 | 1 | No | 1 | 1 | No | No | Yes | OP | none | discontinuation | recover | No |
| 15 | A | FUL | 2 | 43 | 0 | No | 1 | 1 | No | unknown | Yes | OP | none | discontinuation | recover | No |
| 16 | P | LET | 7 | 82 | 1 | Yes | 1 | 1 | No | unknown | Yes | Radiation pneumonitis | multiple lung metastases | discontinuation | recover | Yes |
| 17 | P | LET | 3 | 74 | 1 | No | 2 | 2 | No | No | Yes | HP | mild bronchiolitis | PSL | recover | No |
| 18 | P | NA | 1 | 63 | 1 | Yes | 2 | 5 | No | No | No | AIP/ARDS | multiple lung metastases | mPSL pulse therapy | dead | No |
| No. of Case . | Abemaciclib(A) or Palbociclib(P) . | Combind Endocrine therapy . | treatment line . | age . | Performance status . | lung metastasis . | Initial grade . | Worst grade . | pre-exisiting ILD . | smoking . | Radiation including chest . | abnormality of CT scan . | Pre-exisiting CT abnormality . | Treatment . | outcome . | re-challenge CDK 4/6 inhibitor . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | LET | 2 | 82 | 1 | No | 1 | 1 | No | past smorker | Yes | Bacterial pneumonia | none | discontinuation, antibiotics | recover | Yes |
| 2 | P | LET | 1 | 74 | 1 | No | 1 | 1 | No | No | No | NSIP | none | PSL, antibiotics | recover | No |
| 3 | P | LET | 1 | 71 | 1 | Yes | 3 | 4 | No | No | No | OP | multiple lung metastases | mPSL pulse therapy, cycloposphamide | recover | No |
| 4 | P | ANA | 1 | 89 | 1 | Yes | 3 | 5 | No | No | No | AIP/ARDS | pleural dissemination | PSL | dead | No |
| 5 | P | LET | 1 | 80 | 1 | Yes | 1 | 1 | No | No | No | Bacterial pneumonia | none | discontinuation, antibiotics | recover | No |
| 6 | P | FUL | 1 | 77 | 1 | No | 2 | 2 | No | No | No | HP | old Tb | PSL | recover | No |
| 7 | A | FUL | 2 | 51 | 1 | Yes | 1 | 1 | No | No | No | OP | none | discontinuation | recover | No |
| 8 | P | FUL | 1 | 55 | 1 | No | 1 | 1 | No | No | No | linear atelectasis | none | discontinuation | recover | No |
| 9 | A | LET | 1 | 69 | 0 | Yes | 1 | 2 | No | No | No | OP | multiple lung metastases | PSL, antibiotics | recover | No |
| 10 | P | LET | 5 | 83 | 1 | Yes | 1 | 1 | No | No | No | NSIP | multiple lung metastases | discontinuation, antibiotics | recover | Yes |
| 11 | A | LET | 6 | 66 | 0 | Yes | 1 | 1 | No | No | Yes | OP | small lung meta | PSL | recover | No |
| 12 | P | FUL | 2 | 68 | 1 | Yes | 1 | 1 | No | No | No | lymphangitis | lymphangitis | discontinuation | recover | No |
| 13 | A | FUL | 8 | 69 | 1 | No | 2 | 2 | No | No | No | NSIP | NSIP | PSL | recover | No |
| 14 | A | FUL | 3 | 65 | 1 | No | 1 | 1 | No | No | Yes | OP | none | discontinuation | recover | No |
| 15 | A | FUL | 2 | 43 | 0 | No | 1 | 1 | No | unknown | Yes | OP | none | discontinuation | recover | No |
| 16 | P | LET | 7 | 82 | 1 | Yes | 1 | 1 | No | unknown | Yes | Radiation pneumonitis | multiple lung metastases | discontinuation | recover | Yes |
| 17 | P | LET | 3 | 74 | 1 | No | 2 | 2 | No | No | Yes | HP | mild bronchiolitis | PSL | recover | No |
| 18 | P | NA | 1 | 63 | 1 | Yes | 2 | 5 | No | No | No | AIP/ARDS | multiple lung metastases | mPSL pulse therapy | dead | No |
LET, letrozole; FUL, fulvestrant; PSL, prednisolone; mPSL, methyl predonisolone; ILD, interstitial lung disease. HP, hypersensitivity pneumonia; OP, organizing pneumonia; NSIP, non-specific interstitial pneumonia; AIP/ARDS, acute interstitial pneumonia/acute respiratory distress syndrome.
| CDK4/6 inhibitor . | Grade . | 1 . | 2 . | 3 . | 4 . | 5 . | Total . | (%) . |
|---|---|---|---|---|---|---|---|---|
| Number of patients | ||||||||
| Abemaciclib (n = 47) | 4 | 2 | 0 | 0 | 0 | 6 | 13% | |
| Palbociclib (n = 185) | 7 | 2 | 0 | 1 | 2 | 12 | 6.5% |
| CDK4/6 inhibitor . | Grade . | 1 . | 2 . | 3 . | 4 . | 5 . | Total . | (%) . |
|---|---|---|---|---|---|---|---|---|
| Number of patients | ||||||||
| Abemaciclib (n = 47) | 4 | 2 | 0 | 0 | 0 | 6 | 13% | |
| Palbociclib (n = 185) | 7 | 2 | 0 | 1 | 2 | 12 | 6.5% |
| CDK4/6 inhibitor . | Grade . | 1 . | 2 . | 3 . | 4 . | 5 . | Total . | (%) . |
|---|---|---|---|---|---|---|---|---|
| Number of patients | ||||||||
| Abemaciclib (n = 47) | 4 | 2 | 0 | 0 | 0 | 6 | 13% | |
| Palbociclib (n = 185) | 7 | 2 | 0 | 1 | 2 | 12 | 6.5% |
| CDK4/6 inhibitor . | Grade . | 1 . | 2 . | 3 . | 4 . | 5 . | Total . | (%) . |
|---|---|---|---|---|---|---|---|---|
| Number of patients | ||||||||
| Abemaciclib (n = 47) | 4 | 2 | 0 | 0 | 0 | 6 | 13% | |
| Palbociclib (n = 185) | 7 | 2 | 0 | 1 | 2 | 12 | 6.5% |
Only 2 of 18 cases developed CDK4/6 inhibitor-related ILD with a pre-existing interstitial lung abnormality of the same kind (lymphangitis in case 12 and NSIP in case 13 in Table 2), and the others had no or only unrelated abnormalities in the baseline chest CT scan. Most of the patients who experienced ILD were over 50 years old.
The severity of ILD is detailed in Table 3. There were two grade 5 events (death from ILD) in the palbociclib group with an AIP/ARDS pattern and no grade 3 or greater event in the abemaciclib group. The mortality rate from ILD was 11% among patients diagnosed with ILD and 0.9% among all patients who received CDK 4/6 inhibitors.
The median time to onset of ILD was 178 (14–750) days (Fig. 1). There was no particular trend from the time to onset of ILD. ILD management involved administration of corticosteroids (n = 9), discontinuation of CDK4/6 inhibitor (n = 9), antibiotics (n = 5) and administration of cyclophosphamide (n = 1) (Table 2).
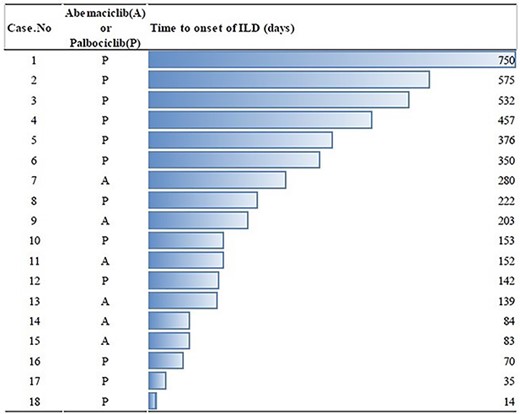
Three patients (case 1, case 10 and case 16 in Table 2) re-started CDK4/6 inhibitor after recovery from ILD. All these patients received palbociclib and experienced grade 1 ILD and recovered quickly from their ILD by discontinuing the CDK4/6 inhibitor. The CDK 4/6 inhibitors were restarted at the same dose as those before the grade 1 ILDs (75 mg in case 1, 125 mg in case 10 and 100 mg in case 16). The time to restarting CDK4/6 inhibitor was 13 days (case 16), 29 days (case 1) and 35 days (case 10). During the follow-up period from the restarting of CDK4/6 inhibitor (505 days in case 1, 120 days in case 10 and 205 days in case 16), no recurrence of ILD occurred among these patients.
Assessment of risk factors of CDK4/6 inhibitor-related ILD
We evaluated the correlation of CDK4/6-related ILD with age (>50 vs ≤50 years), presence or absence of a previous history of ILD, lung metastasis, smoking history, perioperative chemotherapy, treatment lines (1st, 2nd and 3rd line or later) and chest radiation. There was no relationship between ILD and these factors (Table 4). We did not assess the multivariate analysis because there was a small number of ILD events (n = 18).
| . | Occur of CDK4/6 inhibitor related ILD . | . | |
|---|---|---|---|
| Risk factor . | Yes . | No . | P-value . |
| age | Number of cases | ||
| >50 | 17 | 174 | 0.49 |
| <50 | 1 | 32 | |
| pre-exsisting ILD | |||
| Yes | 0 | 7 | 1 |
| No | 18 | 199 | |
| Lung metastasis | |||
| Yes | 10 | 77 | 0.14 |
| No | 8 | 129 | |
| Smoking history | |||
| Yes | 3 | 40 | 1 |
| No | 15 | 166 | |
| Radiation including chest | |||
| Yes | 6 | 83 | 0.62 |
| No | 12 | 123 | |
| Treatment lines | |||
| 1stline | 8 | 70 | 0.71 |
| 2ndline | 4 | 59 | |
| 3rdline or later | 6 | 77 | |
| Perioperative chemotherapy | |||
| Yes | 5 | 87 | 0.22 |
| No | 13 | 111 | |
| . | Occur of CDK4/6 inhibitor related ILD . | . | |
|---|---|---|---|
| Risk factor . | Yes . | No . | P-value . |
| age | Number of cases | ||
| >50 | 17 | 174 | 0.49 |
| <50 | 1 | 32 | |
| pre-exsisting ILD | |||
| Yes | 0 | 7 | 1 |
| No | 18 | 199 | |
| Lung metastasis | |||
| Yes | 10 | 77 | 0.14 |
| No | 8 | 129 | |
| Smoking history | |||
| Yes | 3 | 40 | 1 |
| No | 15 | 166 | |
| Radiation including chest | |||
| Yes | 6 | 83 | 0.62 |
| No | 12 | 123 | |
| Treatment lines | |||
| 1stline | 8 | 70 | 0.71 |
| 2ndline | 4 | 59 | |
| 3rdline or later | 6 | 77 | |
| Perioperative chemotherapy | |||
| Yes | 5 | 87 | 0.22 |
| No | 13 | 111 | |
| . | Occur of CDK4/6 inhibitor related ILD . | . | |
|---|---|---|---|
| Risk factor . | Yes . | No . | P-value . |
| age | Number of cases | ||
| >50 | 17 | 174 | 0.49 |
| <50 | 1 | 32 | |
| pre-exsisting ILD | |||
| Yes | 0 | 7 | 1 |
| No | 18 | 199 | |
| Lung metastasis | |||
| Yes | 10 | 77 | 0.14 |
| No | 8 | 129 | |
| Smoking history | |||
| Yes | 3 | 40 | 1 |
| No | 15 | 166 | |
| Radiation including chest | |||
| Yes | 6 | 83 | 0.62 |
| No | 12 | 123 | |
| Treatment lines | |||
| 1stline | 8 | 70 | 0.71 |
| 2ndline | 4 | 59 | |
| 3rdline or later | 6 | 77 | |
| Perioperative chemotherapy | |||
| Yes | 5 | 87 | 0.22 |
| No | 13 | 111 | |
| . | Occur of CDK4/6 inhibitor related ILD . | . | |
|---|---|---|---|
| Risk factor . | Yes . | No . | P-value . |
| age | Number of cases | ||
| >50 | 17 | 174 | 0.49 |
| <50 | 1 | 32 | |
| pre-exsisting ILD | |||
| Yes | 0 | 7 | 1 |
| No | 18 | 199 | |
| Lung metastasis | |||
| Yes | 10 | 77 | 0.14 |
| No | 8 | 129 | |
| Smoking history | |||
| Yes | 3 | 40 | 1 |
| No | 15 | 166 | |
| Radiation including chest | |||
| Yes | 6 | 83 | 0.62 |
| No | 12 | 123 | |
| Treatment lines | |||
| 1stline | 8 | 70 | 0.71 |
| 2ndline | 4 | 59 | |
| 3rdline or later | 6 | 77 | |
| Perioperative chemotherapy | |||
| Yes | 5 | 87 | 0.22 |
| No | 13 | 111 | |
Instructive cases of CDK4/6 inhibitor-related ILD
Case 3 (Table 2) was a 71-year-old woman who had de novo stage IV breast cancer with multiple lung and bone metastases. She started letrozole in May 20XX and received radiotherapy at 30 Gy/10 fractions for the left breast and spine for palliative purposes. In April 20XX + 1, palbociclib 125 mg/day was added to letrozole. She continued the combination treatment with two interruptions of palbociclib from May 20XX + 2 to July 20XX + 2 and from July 20XX + 3 to September 20XX + 3 for orthopedic surgeries. A dry cough appeared a few days after the resumption of treatment on September 3 in 20XX + 3. This continued and worsened, and shortness of breath then appeared. After 3 weeks from the resumption of palbociclib, the shortness of breath worsened at rest and a fever of 37.8°C appeared. She visited our clinic and was urgently hospitalized. Chest X-ray and CT scan showed ground-glass opacities in the bilateral lobes with an OP pattern (Fig. 2B). Two days after admission, she received methylprednisolone (mPSL) pulse therapy (1 g/body/day for 3 days) and then was maintained with PSL 1 mg/kg on the subsequent days. Despite this treatment, her respiratory condition clearly worsened, and we administered cyclophosphamide (500 mg/body). After 4 days from the administration of cyclophosphamide, a second mPSL pulse therapy was administered. After these therapies, her respiratory status improved (Fig. 2E) and she was discharged 24 days after hospitalization.
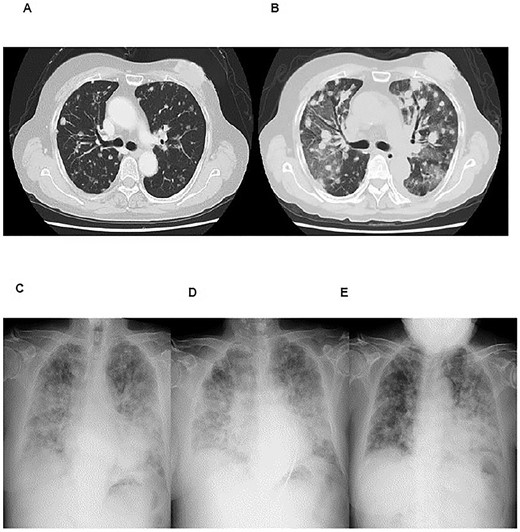
Imaging studies of case 3. Chest CT scans at 1 year before (A) and at onset (B) of ILD (organizing pneumonia). Chest X-rays at the onset of ILD (C), after mPSL pulse therapy (D), and after cyclophosphamide and second mPSL pulse therapy (E).
Case 4 (Table 2) was an 89-year-old woman who had left breast cancer and who had initially undergone mastectomy at the age of 69 years. She had not received radiotherapy. She had recurrence with left pleural effusion and a chest wall tumor and started to receive palbociclib plus anastrozole from October 20XX. Her disease was stabilized with the treatment, and no abnormalities were identified by chest X-ray examination at her visit to our clinic on 28 December 20XX + 1. After 1 week from the last visit, she visited our emergency room complaining about rapidly worsening dyspnea. Chest X-ray and CT scan showed a bilateral interstitial shadow (AIP/ARDS pattern) (Fig. 3). She was hospitalized and started to receive PSL at 0.5 mg/kg. Three days after hospitalization, her oxygen needs increased and then mPSL pulse therapy (1 g/body/day for 3 days) was started. Despite the treatment, she fell into respiratory failure and died 10 days after hospitalization.
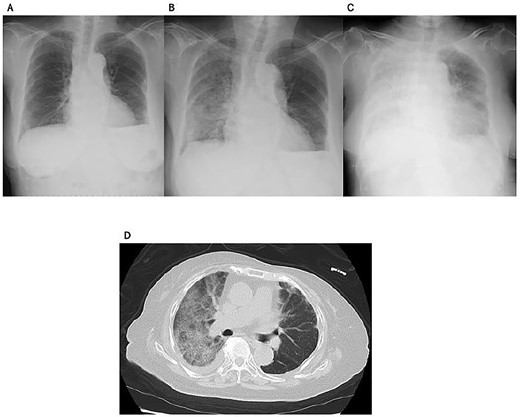
Imaging studies of case 4. Chest X-rays 1 week before (A), at (B), and 3 days after (C) the onset of ILD. Chest CT scan at the onset of ILD (D).
Discussion
In this retrospective study, we found that 18 of 224 (8.0%) cases experienced CDK4/6 inhibitor-induced ILD including definite and probable cases. The frequency, even when limited to definite cases (13/224, 5.8%), was higher than that reported in clinical trials (1.0–1.3%). Although we explored the risk factors associated with CDK4/6 inhibitor-induced ILD, no factor was determined. Nonetheless, to our knowledge, this is the first report to analyze ILD cases caused by multiple CDK4/6 inhibitors.
The incidence of ILD was higher in our study than that reported in clinical trials, which were largely around 1% (9,18). The cause of this difference may be related to ethnic background. In our study, all patients were Japanese, whereas the majority of patients in clinical trials were from Western countries. In a subgroup analysis of Japanese patients in the PALOMA-2 and -3 trials, adverse events such as neutropenia were slightly more common than in the overall population (19). In the FAERS, CDK4/6 inhibitor-induced ILD tends to be more common in Asian individuals (10). In the monarchE trial, which evaluated the efficacy of abemaciclib as adjuvant therapy, ILD occurred in 6.6% of patients from Asia, whereas it was 2.3% in all-cohorts (9,20,21).
The mortality rate of CDK4/6 inhibitor-related ILD in the clinical trial was very low. No fatal cases of ILD with palbociclib were reported in a pooled analysis of the clinical trials (8). For abemaciclib, 14 (0.5%) severe ILD cases were reported in the monarchE trial, and there was only 1 fatal case of an Asian patient. Two deaths (0.5%) were reported in the MONARCH-2 trial and one death (0.1%) was reported in the MONARCH-3 trial (7). In Japan, 3 deaths among 14 severe ILD cases were reported after marketing, and a safety bulletin was issued (11). In our study, there were two deaths (0.9% among all patients who received CDK 4/6 inhibitors) from ILD. Because there may be slightly higher incidence and mortality of CDK 4/6 inhibitor-related ILD, a larger real-world data analysis of ILD is warranted.
Other drugs used for advanced breast cancer with a relatively high incidence of ILD include everolimus and trastuzumab deruxtecan. The incidence of everolimus-related ILD was 18% in the BOLERO-2 study (22), and trastuzumab deruxtecan-related ILD was observed in 14% of patients in the Destiny-Breast01 study (23). In addition, the incidence of ILD related to epidermal growth factor receptor tyrosine kinase inhibitors and trastuzumab deruxtecan was reported to be higher in Asian populations than in Western populations (24,25). Collectively, clinicians should take special caution with regard to ILD whenever they treat Asian patients with these drugs including CDK4/6 inhibitors. Because there is no specific time range in which CDK4/6 inhibitor-related ILD is likely to occur according to our study (Fig. 1) or the Japanese Adverse Drug Event Report (JADER) database (26), caution should be taken throughout the treatment.
Other risk factors for CDK4/6 inhibitor-related ILD were reported in some studies. In the FAERS, 72% of CDK4/6 inhibitor-related ILD occurred in patients aged 65 years or older (10). Chen et al. (27) reported the characteristics of patients treated with abemaciclib and diagnosed with ILD from post-marketing data in Japan. In this report, advanced age, pre-existing interstitial pneumonia and poor performance status were related to fatal cases (27). In the JADER database, the frequency of interstitial pneumonia was not associated with age (26), while the monarchE trial reported a slightly higher frequency of ILD in patients with prior thoracic radiation therapy (20). In our study, older age or radiotherapy involving the thoracic wall were not identified as risk factors for ILD. In addition, other patient factors reported to predispose to pneumonia from other cancers/drugs, such as pre-existing ILD or abnormality on CT scan or smoking (12,13), were not extracted as risk factors in our study. Overall, the risk factors for CDK4/6 inhibitor-related ILD remain unclear, and we need to continue our efforts to elucidate them by exploring a broader spectrum of factors such as patient-specific immunity.
Discontinuation of the causative drug and steroid therapy is the mainstay of treatment for drug-induced interstitial pneumonia (28), and most cases in the previously reported case reports were treated with corticosteroids (Table 5). In the two cases of severe CDK4/6 inhibitor-related ILD in our current study, both were refractory to high-dose corticosteroid therapy (Figs 2 and 3), but one case was rescued using cyclophosphamide (Fig. 2). Cyclophosphamide is a highly potent immunosuppressant that has demonstrated efficacy in inducing and maintaining remission in autoimmune and inflammatory disease. In connective tissue disease-associated lung disease, cyclophosphamide was reported to improve the clinical condition of dyspnea (29). However, there is not enough evidence of the efficacy of cyclophosphamide for anticancer drug-induced ILD. Although other immunosuppressive treatments such as infliximab, tocilizumab, mycophenolate mofetil and intravenous immunoglobulins are described as treatment for patients with drug-induced ILD who are refractory to corticosteroids in the recommendations of an Italian multidisciplinary group (27), none of these have been established as a standard of care.
| Case reports . | CDK4/6 inhibitor . | Age . | CT appearance . | Combination endocrine therapy . | Time to onset of ILD . | Bronchoscopy . | Treatment . | Outcome . | CDK4/6 re-challenge . |
|---|---|---|---|---|---|---|---|---|---|
| Jazieh et al. (30) | Palbociclib | 74 | Bilateral GGO | Fulvestrant | 27 months | Non-specific lung injury | Methylprednisolone | Recovered | No |
| Jazieh et al. (30) | Abemaciclib | 60 | Patchy multifocal alveolar ground-glass densities within the upper lobes | Fulvestrant | Not known | Inflammatory cells | Methylprednisolone | Dead | No |
| Ofer et al. (31) | Palbociclib | 63 | Pulmonary embolism and GGO | Letrozole | Approximately 1.5 months | Subacute interstitial lung injury | High-dose glucocorticoids | Dead | No |
| Ahsan et al. (32) | Palbociclib | 52 | GGO | Unknown | Not known | Not done | Antibiotics | Not known | No |
| Gong et al. (33) | Palbociclib | 72 | GGO | Fulvestrant | 8 months | Not done | Discontinuation of CDK4/6 inhibitor | Recovered | No |
| Algwaiz et al. (34) | Ribociclib | 46 | Subsegmental pulmonary embolism with bilateral GGOs more pronounced on the right side | GnRH, fulvestrant | 3 months (with 4 weeks discontinuation due to other cause) | Non-specific lung injury | Methylprednisolone (1.5 mg/kg/day) | Recovered | No |
| Mathew et al. (35) | Palbociclib | 67 | Multiple new patchy and confluent GGOs in bilateral lung fields | Letrozole | 4 months | Not done | Methylprednisolone | Dead | No |
| Sarkisian et al. (36) | Palbociclib | 72 | GGO | Letrozole | 2 weeks | Atypical squamous cells suggestive of reactive pattern | Prednisolones | Recovered | No |
| Wilgus et al. (37) | Abemaciclib | 56 | Bilateral lower lobe airspace and ground glass infiltrates | Fulvestrant | Not known | Cell count was 80% lymphocyte-predominant and cultures were negative | Dexamethasone | Recovered | Ribociclib |
| Case reports . | CDK4/6 inhibitor . | Age . | CT appearance . | Combination endocrine therapy . | Time to onset of ILD . | Bronchoscopy . | Treatment . | Outcome . | CDK4/6 re-challenge . |
|---|---|---|---|---|---|---|---|---|---|
| Jazieh et al. (30) | Palbociclib | 74 | Bilateral GGO | Fulvestrant | 27 months | Non-specific lung injury | Methylprednisolone | Recovered | No |
| Jazieh et al. (30) | Abemaciclib | 60 | Patchy multifocal alveolar ground-glass densities within the upper lobes | Fulvestrant | Not known | Inflammatory cells | Methylprednisolone | Dead | No |
| Ofer et al. (31) | Palbociclib | 63 | Pulmonary embolism and GGO | Letrozole | Approximately 1.5 months | Subacute interstitial lung injury | High-dose glucocorticoids | Dead | No |
| Ahsan et al. (32) | Palbociclib | 52 | GGO | Unknown | Not known | Not done | Antibiotics | Not known | No |
| Gong et al. (33) | Palbociclib | 72 | GGO | Fulvestrant | 8 months | Not done | Discontinuation of CDK4/6 inhibitor | Recovered | No |
| Algwaiz et al. (34) | Ribociclib | 46 | Subsegmental pulmonary embolism with bilateral GGOs more pronounced on the right side | GnRH, fulvestrant | 3 months (with 4 weeks discontinuation due to other cause) | Non-specific lung injury | Methylprednisolone (1.5 mg/kg/day) | Recovered | No |
| Mathew et al. (35) | Palbociclib | 67 | Multiple new patchy and confluent GGOs in bilateral lung fields | Letrozole | 4 months | Not done | Methylprednisolone | Dead | No |
| Sarkisian et al. (36) | Palbociclib | 72 | GGO | Letrozole | 2 weeks | Atypical squamous cells suggestive of reactive pattern | Prednisolones | Recovered | No |
| Wilgus et al. (37) | Abemaciclib | 56 | Bilateral lower lobe airspace and ground glass infiltrates | Fulvestrant | Not known | Cell count was 80% lymphocyte-predominant and cultures were negative | Dexamethasone | Recovered | Ribociclib |
GGO, ground-glass opacity.
| Case reports . | CDK4/6 inhibitor . | Age . | CT appearance . | Combination endocrine therapy . | Time to onset of ILD . | Bronchoscopy . | Treatment . | Outcome . | CDK4/6 re-challenge . |
|---|---|---|---|---|---|---|---|---|---|
| Jazieh et al. (30) | Palbociclib | 74 | Bilateral GGO | Fulvestrant | 27 months | Non-specific lung injury | Methylprednisolone | Recovered | No |
| Jazieh et al. (30) | Abemaciclib | 60 | Patchy multifocal alveolar ground-glass densities within the upper lobes | Fulvestrant | Not known | Inflammatory cells | Methylprednisolone | Dead | No |
| Ofer et al. (31) | Palbociclib | 63 | Pulmonary embolism and GGO | Letrozole | Approximately 1.5 months | Subacute interstitial lung injury | High-dose glucocorticoids | Dead | No |
| Ahsan et al. (32) | Palbociclib | 52 | GGO | Unknown | Not known | Not done | Antibiotics | Not known | No |
| Gong et al. (33) | Palbociclib | 72 | GGO | Fulvestrant | 8 months | Not done | Discontinuation of CDK4/6 inhibitor | Recovered | No |
| Algwaiz et al. (34) | Ribociclib | 46 | Subsegmental pulmonary embolism with bilateral GGOs more pronounced on the right side | GnRH, fulvestrant | 3 months (with 4 weeks discontinuation due to other cause) | Non-specific lung injury | Methylprednisolone (1.5 mg/kg/day) | Recovered | No |
| Mathew et al. (35) | Palbociclib | 67 | Multiple new patchy and confluent GGOs in bilateral lung fields | Letrozole | 4 months | Not done | Methylprednisolone | Dead | No |
| Sarkisian et al. (36) | Palbociclib | 72 | GGO | Letrozole | 2 weeks | Atypical squamous cells suggestive of reactive pattern | Prednisolones | Recovered | No |
| Wilgus et al. (37) | Abemaciclib | 56 | Bilateral lower lobe airspace and ground glass infiltrates | Fulvestrant | Not known | Cell count was 80% lymphocyte-predominant and cultures were negative | Dexamethasone | Recovered | Ribociclib |
| Case reports . | CDK4/6 inhibitor . | Age . | CT appearance . | Combination endocrine therapy . | Time to onset of ILD . | Bronchoscopy . | Treatment . | Outcome . | CDK4/6 re-challenge . |
|---|---|---|---|---|---|---|---|---|---|
| Jazieh et al. (30) | Palbociclib | 74 | Bilateral GGO | Fulvestrant | 27 months | Non-specific lung injury | Methylprednisolone | Recovered | No |
| Jazieh et al. (30) | Abemaciclib | 60 | Patchy multifocal alveolar ground-glass densities within the upper lobes | Fulvestrant | Not known | Inflammatory cells | Methylprednisolone | Dead | No |
| Ofer et al. (31) | Palbociclib | 63 | Pulmonary embolism and GGO | Letrozole | Approximately 1.5 months | Subacute interstitial lung injury | High-dose glucocorticoids | Dead | No |
| Ahsan et al. (32) | Palbociclib | 52 | GGO | Unknown | Not known | Not done | Antibiotics | Not known | No |
| Gong et al. (33) | Palbociclib | 72 | GGO | Fulvestrant | 8 months | Not done | Discontinuation of CDK4/6 inhibitor | Recovered | No |
| Algwaiz et al. (34) | Ribociclib | 46 | Subsegmental pulmonary embolism with bilateral GGOs more pronounced on the right side | GnRH, fulvestrant | 3 months (with 4 weeks discontinuation due to other cause) | Non-specific lung injury | Methylprednisolone (1.5 mg/kg/day) | Recovered | No |
| Mathew et al. (35) | Palbociclib | 67 | Multiple new patchy and confluent GGOs in bilateral lung fields | Letrozole | 4 months | Not done | Methylprednisolone | Dead | No |
| Sarkisian et al. (36) | Palbociclib | 72 | GGO | Letrozole | 2 weeks | Atypical squamous cells suggestive of reactive pattern | Prednisolones | Recovered | No |
| Wilgus et al. (37) | Abemaciclib | 56 | Bilateral lower lobe airspace and ground glass infiltrates | Fulvestrant | Not known | Cell count was 80% lymphocyte-predominant and cultures were negative | Dexamethasone | Recovered | Ribociclib |
GGO, ground-glass opacity.
There were few reports about re-challenge with CDK 4/6 inhibitors after ILD. Only one report introduced a case in which the patient was re-challenged with ribociclib after abemaciclib-related ILD (Table 5), but the outcome after the re-challenge was not known. Although the guidelines adjunct with the study protocols of MONARCH-2, three studies permitted the restart of abemaciclib with a reduced dose after recovery to grade ≤ 1 (7), and the Japanese package insert of abemaciclib recommends permanent discontinuation in the event of ILDs with any grade (38). In our current study, no patients with abemacilib-related ILD restarted any CDK4/6 inhibitors. Conversely, three cases of grade 1 palbociclib-related ILD restarted the same drug, and none of them experienced recurrence of ILD. To evaluate the safety of re-challenge, more cases are needed for analysis.
The mechanism of CDK4/6 inhibitor-induced ILD is not clear. Pre-clinical studies suggest that CDK4/6 inhibition may increase inflammatory infiltration in the lung (39). Birnhuber et al. (29) reported that palbociclib decreased collagen deposition but induced augmented inflammatory cell recruitment in a bleomycin-induced lung injury mouse model. Conversely, another preclinical study showed that pharmacological inhibition of CDK4/6 and hydrogen sulfide significantly improved pancreas and lung histopathological changes in an acute pancreatitis mouse model (40). Understanding the actual status of drug-induced ILD and its mechanism is important for safer treatment and maximum therapeutic effect and should be continued to be studied.
There are some limitations of our study. First, it was a retrospective study and there was a small number of cases. There may be bias in the patient population, and we did not perform a multivariate analysis of the risk factors because of the small number of cases of ILD. Second, because a diagnosis of ILD was confirmed by only one radiologist who specializes in thoracic imaging, there may be bias in the definition. Third, we could not analyze biomarkers other than the clinical information. To solve these problems and identify accurate risk factors of ILD, prospective large-scale observational studies that include clinical information as well as other biomarker analyses are necessary. Despite these limitations, we believe that our study is important because it provides detailed clinical information on CDK4/6 inhibitor-induced ILD.
In conclusion, our study showed a higher incidence of CDK4/6 inhibitor-induced ILD in practical settings. Further studies are warranted to validate our findings and the potential ethnic background-related risk of CDK4/6 inhibitor-related ILD in Japanese patients and to identify the mechanism involved.
Data statement
The data can be shared with the journal for review if required.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
A waiver from the requirement to provide written informed consent was granted by the IRB of the National Cancer Center.
Abbreviations
CDK, cyclin dependent kinase; ANA, anastrozole; LET, letrozole; FUL, fulvestrant; ILD, interstitial lung disease; HP, hypersensitivity pneumonia; OP, organizing pneumonia; NSIP, non-specific interstitial pneumonia; AIP/ARDS, acute interstitial pneumonia/acute respiratory distress syndrome; PSL, prednisolone; mPSL, methylprednisolone.
Acknowledgements
We thank H.N.M., PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
Toru Mukohara received research funding from Daiichi-Sankyo, Sysmex, MSD, Pfizer, Sanofi and Chugai Pharmaceuticals. This research did not receive a specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest statement
The other authors declare that they have no conflicts of interest.


