-
PDF
- Split View
-
Views
-
Cite
Cite
Keiichiro Nakajo, Yusuke Yoda, Hiroki Yamashita, Kenji Takashima, Tatsuro Murano, Tomohiro Kadota, Kensuke Shinmura, Hiroaki Ikematsu, Tetsuo Akimoto, Tomonori Yano, Salvage endoscopic resection for cT1N0M0 local recurrence after chemoradiotherapy for esophageal squamous cell carcinoma: endoscopic submucosal dissection versus endoscopic mucosal resection, Japanese Journal of Clinical Oncology, Volume 52, Issue 9, September 2022, Pages 982–991, https://doi.org/10.1093/jjco/hyac090
Close - Share Icon Share
Abstract
Salvage endoscopic resection is recommended when the local recurrence at primary site after chemoradiotherapy for esophageal squamous cell carcinoma is localized and superficial. This retrospective study aimed to comparatively analyse the short-term outcomes and local control of salvage endoscopic submucosal dissection versus salvage endoscopic mucosal resection for local recurrence after chemoradiotherapy or radiotherapy.
A total of 96 patients who underwent initial salvage endoscopic resection for cT1N0M0 local recurrence after chemoradiotherapy or radiotherapy for esophageal squamous cell carcinoma between December 1998 and August 2019 patients were assigned to either the salvage endoscopic submucosal dissection (40 patients; 40 lesions) or salvage endoscopic mucosal resection (56 patients; 56 lesions) group. We evaluated the en bloc and R0 resection rates, severe adverse events and local failure rate after salvage endoscopic resection. Multivariate analysis was conducted to identify risk factors of local failure after salvage endoscopic resection.
The en bloc resection rate was significantly higher in the salvage endoscopic submucosal dissection group than in the salvage endoscopic mucosal resection group (95% versus 63%; P < 0.001). There were no differences in R0 resection rate between the two groups (73% versus 52%, P = 0.057). One patient (3%) in the salvage endoscopic submucosal dissection group had perforation. The 3-year cumulative local failure rate of salvage endoscopic mucosal resection was significantly higher than that of salvage endoscopic submucosal dissection (27% versus 5%, P = 0.032). In multivariate analysis, salvage endoscopic mucosal resection (hazard ratio: 2.7, P = 0.044) was the only independent risk factor of local failure after salvage endoscopic resection.
Salvage endoscopic submucosal dissection is the effective treatment for local recurrence based on the short-term outcomes and local efficacy.
Introduction
Definitive chemoradiotherapy (CRT) is considered one of the curative treatment options for esophageal cancer (1–3); however, local failure has been reported in 12–40% of the patients who received this treatment (4,5). Consequently, salvage treatment for local recurrence is required for these patients.
Salvage endoscopic resection (ER) may be effective if the local recurrence at the primary site is localized and superficial without lymph node or distant metastasis (6–12). Two types of salvage ER have been reported for local recurrence after CRT: salvage endoscopic submucosal dissection (ESD) and salvage endoscopic mucosal resection (EMR) (6–12). Salvage EMR has been reported to have therapeutic potential and could be a less invasive treatment option for local recurrence (6–8). However, piecemeal resection frequently occurs in salvage EMR because of radiation-induced fibrosis in the submucosal layer after CRT, especially in cases of large lesions (6–8). In contrast, salvage ESD has been recently reported to be technically feasible and an effective treatment option for local recurrence and beneficial for one-piece resection, regardless of lesion size (9–12). However, it is considered a technically challenging and time-consuming procedure (12). Thus, in patients with local recurrence after CRT for esophageal cancer, adaptation criteria for salvage EMR and ESD remain controversial.
In patients with local recurrence at the primary site and without lymph node and distant metastasis, local control at the primary site by salvage ER is important. Thus, assessing the risk factors of local failure, including residual lesion or local recurrence, after salvage ER may help formulate appropriate salvage treatment strategies for local recurrence after CRT for esophageal cancer.
This study aimed to comparatively analyse clinical outcomes including long-term local control of salvage ESD versus salvage EMR for local recurrence after CRT or radiotherapy (RT), and assess the risk factors associated with long-term local control based on local failure after salvage ER.
Methods
Patients and ethical considerations
Patients with local failure, including residual lesions and local recurrence, after CRT or RT for esophageal squamous cell carcinoma (ESCC), who subsequently underwent initial salvage endoscopic therapy at the National Cancer Center Hospital East, Kashiwa, Japan, between December 1998 and August 2019, were retrospectively enrolled. Of those treated, the patients who underwent salvage endoscopic therapy for residual lesion after CRT or RT were excluded. Of the patients who underwent salvage endoscopic therapy for local recurrence that defined as the failure lesions which developed after achieving CR after CRT or RT, patients who underwent salvage photodynamic therapy (PDT), and had follow-up periods of <6 months after salvage ER were excluded. The baseline staging of ESCC before CRT or RT was determined by the tumour-node-metastasis classification according to the International Union Against Cancer, seventh edition (13). All local recurrences were diagnosed using endoscopic examination and confirmed histologically by the presence of cancer cells in the biopsy specimens from the primary site. The definition of CR after CRT or RT was as follows: disappearance of the tumour lesion, no ulceration at the primary site and absence of cancer cells in the biopsy specimens (14).
Salvage ER method included ESD or EMR for local recurrence. Salvage ESD was introduced at our institute gradually since 2009. While salvage ESD was frequently performed during 2011–2019, most of the salvage ER performed between 1999 and 2010 were salvage EMR. The indication criteria for salvage ER were as follows: no lymph node or distant metastases detected on computed tomography (CT) after CRT or RT; absence of deep ulceration in the lesion; and tumour staging with endoscopic examination limited to the mucosa or slight submucosa (cT1a or cT1b-SM1). Endoscopic ultrasonography was additionally performed to determine the depth of invasion as necessary. Since 2011, when ESD was officially introduced at our institute, the choice of procedure between ESD and EMR was made at the discretion of the physician based on the degree of fibrosis in the submucosa, depth of invasion and size of the lesion according to the following criteria. (1) EMR: mild fibrotic lesions, sized 10 mm or less, that can be resected en bloc by EMR; (2) ESD: lesions that do not meet the first criteria.
This study was approved by the Institutional Review Board at National Cancer Center Hospital East (approval no. 2017-434) and conducted according to the Epidemiological Study Guideline issued by the Japan Ministry of Health, Labour and Welfare. Written informed consent for salvage ER was obtained from all patients.
Procedure of salvage EMR and ESD
Salvage EMR involved the strip biopsy method as modified by Momma et al. (15) Briefly, we initially identified the lesion margins with Lugol’s solution. Saline solution was then injected into the submucosal layer to lift the lesion. EMR was performed with a two-channel endoscope (2 T240; Olympus, Tokyo, Japan). Snare and forceps were introduced through each channel of the endoscope. We grasped the lesion with the forceps from within one channel, then removed it with a snare from another channel. The EMR with piecemeal resection was continued if an unstained area was detected at the margin of the mucosal defect.
ESD was performed as previously described before (12). Briefly, the border of the tumour was marked with spots with a dual-knife (KD-650Q; Olympus, Tokyo, Japan) around the periphery of the lesion. Subsequently, a mixture of 0.4% sodium hyaluronate solution (MucoUp, Johnson and Johnson Medical, Tokyo, Japan) diluted with epinephrine and a small amount of indigo carmine was injected into the submucosal layer around the tumour to lift the lesion, and a circular incision in the mucosa was made with the dual-knife. After a circular incision, the submucosal layer was dissected using the same knife.
Histopathological assessment after salvage ER
All resected specimens were cut into 2 mm slices and stained with haematoxylin and eosin. Invasion depth of the tumour, histologic type, vertical and horizontal resection margins and lymphovascular involvement were evaluated by experienced pathologists. Pathological evaluation was judged to be difficult when the esophageal epithelium was detached over a large surface and the subepithelial tissue was denatured due to a burning effect.
Follow-up after salvage ER
Endoscopic examination and CT were performed at 3, 6 and 12 months after salvage ER, and every 6 months thereafter. Local recurrence after salvage ER was defined as the presence of an exclusively local lesion without lymph node or distant metastasis. Local recurrence was confirmed histologically by biopsy of the salvage ER scar. Lymph node recurrence and distant metastasis after salvage ER were diagnosed using CT.
Assessment of short-term outcomes and local efficacy of salvage ER
For the assessment of short-term outcomes, en bloc resection rate, R0 resection rate, adverse event (perforation and bleeding related to the procedure) and procedure time were compared between the salvage ESD and salvage EMR groups. Furthermore, lesions were divided into two subgroups according to the tumour size: <10 mm and ≥ 10 mm. We determined the cut-off value to be a slightly smaller tumour size than the reported size as the indication standard of conventional EMR since the technical difficulty of snaring for salvage EMR increases due to fibrosis of the submucosa (16). The en bloc and R0 resection rates were analysed in the two groups. En bloc resection was defined as the resection of the lesion as a single piece. When en block resection was achieved and vertical and horizontal margins of the specimens were free of tumour cells, it was defined as R0 resection. Perforation was diagnosed endoscopically just after resection either by an obvious endoscopic view into the mediastinum or by the presence of mediastinal air on chest CT or subcutaneous emphysema on physical examination. Bleeding related to the procedure was defined as bleeding that required blood transfusion or endoscopic intervention. The procedure time of ESD was measured from the time of marking to the end of resection, and that of EMR was measured from the time of the injection to the end of resection.
To assess the long-term outcomes of salvage ER, the cumulative local failure, recurrence-free survival (RFS) and overall survival (OS) rates after ER were calculated in patients with follow-up periods of ≥6 months. In addition, the cumulative local failure rate was compared between the two groups for cases in which salvage ER was performed after 2011, when ESD was gradually introduced at our institute. For the assessment of the local efficacy, univariate and multivariate analyses were performed to identify the risk factors of local failure after salvage ER.
Statistical analyses
Comparisons between the groups were made using the chi-square test or Fisher’s exact test for categorical data and Student t-test for quantitative data. Quantitative data are expressed as medians with a range. The cumulative local failure, including local recurrence or residual lesions, was estimated using a cumulative incidence function that accounted for death from any cause as a competing risk and censored at the date of the last follow-up. The comparison between groups was performed with a Gray’s test, and hazard ratios were estimated using univariate and multivariate Fine & Gray models. The RFS was calculated from the date of salvage ER to the date of recurrence or death, or the last known date of follow-up. The OS was calculated from the date of salvage ER to the date of death or the last known date of follow-up. RFS and OS were censored at the date of the last known follow-up. The RFS and OS rates after salvage ER were calculated using the Kaplan–Meier method and compared between groups using the log-rank test. P < 0.05 was considered to indicate statistical significance. Factors with P < 0.05 in univariate analysis were included in the multivariate analysis. Calculations were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 2.13.0; The R Foundation for Statistical Computing, Vienna, Austria).
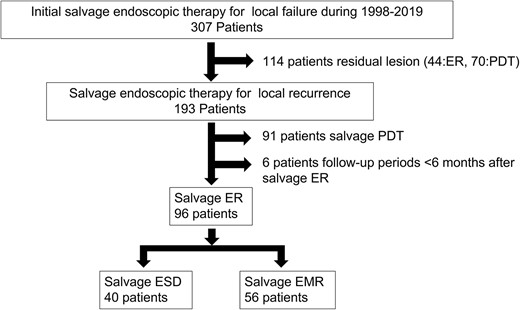
Study flowchart. During the study period, 307 patients with local failure after chemoradiotherapy (CRT) or radiotherapy (RT) for esophageal squamous cell carcinoma (ESCC), who subsequently underwent initial salvage endoscopic therapy at our institute, were enrolled. Of those treated, 114 patients who had residual lesion were excluded. Of the 193 patients who underwent salvage endoscopic therapy for local recurrence, 91 who underwent salvage photodynamic therapy (PDT), six with follow-up periods of <6 months after salvage endoscopic resection (ER) were excluded. Of the remaining 96 patients (96 lesions), the salvage endoscopic submucosal dissection (ESD) and salvage endoscopic mucosal resection (EMR) groups included 40 (40 lesions) and 56 (56 lesions), respectively.
Results
Patient and lesion characteristics
During the study period, 307 patients with local failure after CRT or RT for ESCC, who subsequently underwent initial salvage endoscopic therapy at our institute, were enrolled. Of those treated, 114 patients who had residual lesion were excluded. Of the 193 patients who underwent salvage endoscopic therapy for local recurrence, 91 who underwent salvage PDT, six with follow-up periods of <6 months after salvage ER were excluded. Of the remaining 96 patients (96 lesions), the salvage ESD and salvage EMR groups included 40 (40 lesions) and 56 (56 lesions), respectively (Fig. 1). The baseline characteristics of patients and lesions are summarized in Table 1. The median age in the salvage ESD group was significantly higher than that in the salvage EMR group (71 versus 67 years, P = 0.029). tumour size was significantly larger in the salvage ESD group than in the salvage EMR group (16 mm [range, 4–60] versus 8 mm [range, 2–32]; P < 0.001) (Table 2). All specimens in the salvage ESD group and all but three specimens in the salvage EMR group underwent pathological evaluation. In the case of four specimens in the salvage EMR group, accurate pathological evaluation was difficult due to a severe burning effect.
Short-term outcomes of salvage ER
Short-term outcomes of salvage ER are summarized in Table 3. The initial salvage ER could not be completed in four patients who were then switched to another treatment (two hybrid ESD, one PDT and one esophagectomy). The en bloc resection rate was significantly higher in the salvage ESD group than in the salvage EMR group (95% versus 63%; P < 0.001). Concerning the tumours ≥10 mm, the ESD group demonstrated significantly higher en bloc and R0 resection rates than the EMR group (94% versus 39%, P < 0.001; 67% versus 26%, P = 0.006, respectively). For tumours <10 mm, the en block and R0 resection rates did not differ significantly between the groups (100% versus 83%, P = 0.57; 100% versus 79%, P = 0.31, respectively). The median procedure time was significantly longer in the ESD group than in the EMR group (70 min [range, 27–120] versus 15 min [range, 7–120]; P < 0.001). While perforation was not observed in the salvage EMR group, one patient (3%) in the salvage ESD group had delayed perforation. The patient recovered with conservative medical treatments such as parenteral nutrition, antibiotics and a continual drainage of saliva at the perforation site through a nasal tube. The case that was detected delayed perforation after ESD is shown in Fig. 2. Bleeding requiring blood transfusion or endoscopic intervention was not observed in both groups.
Long-term outcomes and clinical course after salvage ER
The median follow-up periods following salvage ESD and salvage EMR were 46 months (range, 6–206) and 66.5 months (range, 6–213), respectively. Cumulative local failure rate in salvage ESD group was significantly lower than that in salvage EMR group (P = 0.032). The 3-year cumulative local failure rates of salvage ESD and salvage EMR were 5% and 27%, respectively (Fig. 3). The 3-year RFS and OS rates in the salvage ESD group were significantly higher than those in salvage EMR group (RFS: 86% versus 48%, P < 0.001; OS: 91% versus 72%, P = 0.0026; Fig. 3). In the 54 patients (38 ESD, 16 EMR) who underwent salvage ER since 2011, the cumulative local recurrence rate of EMR was significantly higher than that of ESD (P = 0.027; Fig. 3).
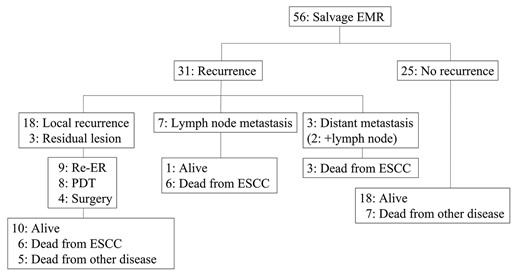
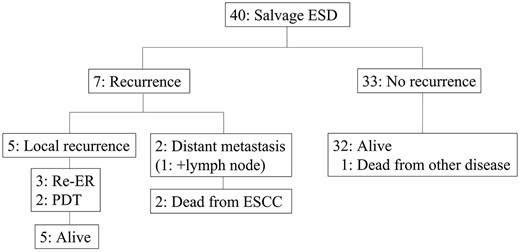
The clinical course after salvage EMR is presented in Fig. 4. Of the 56 patients who underwent salvage EMR, 31 developed recurrence after salvage EMR; 18 had local recurrence, three had residual lesion at the primary site, seven had lymph node metastasis, one had distant metastasis and two had both lymph node and distant metastasis. All 21 patients with local recurrence/residual lesion underwent additional salvage treatments: nine ER; eight PDT; and four surgery. Among the 21 patients who underwent additional salvage treatments, 10 patients were alive, and six patients died due to esophageal cancer after receiving salvage treatments. The clinical course after salvage ESD is presented in Fig. 5. Of the 40 patients who underwent salvage ESD, seven developed recurrence; five had local recurrence, one had distant metastasis and one had both lymph node and distant metastasis. All patients who underwent additional salvage treatments (three ER; two PDT) were alive. Though the remaining two patients with metastatic recurrence received chemotherapy. All patients died from the primary disease.
Risk factors of local failure after salvage ER
The results of univariate and multivariate analyses for risk factors of local failure after salvage ER are shown in Table 4. In the multivariate analysis, salvage EMR (hazard ratio: 2.7 [95% confidence interval: 1.0–7.1], P = 0.044) was found to be the only independent risk factor of local failure after salvage ER.
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| Patient characteristics | |||
| No. of patients | 40 | 56 | |
| Sex, n (%) | 0.088 | ||
| Male | 33 (83) | 53 (95) | |
| Female | 7 (17) | 3 (5) | |
| Age, years, median (range) | 71 (45–87) | 67 (44–87) | 0.029 |
| Years of salvage ER, n (%) | <0.001 | ||
| 1998–2010 | 2 (5) | 40 (71) | |
| 2011- | 38 (95) | 16 (29) | |
| cT-stage before CRT, n (%) | 0.63 | ||
| T1a | 1 (3) | 3 (5) | |
| T1b | 23 (62) | 25 (45) | |
| T2 | 3 (8) | 8 (14) | |
| T3 | 9 (24) | 17 (30) | |
| T4 | 1 (3) | 3 (6) | |
| unknown | 3 | 0 | |
| cStage (TNM UICC 7th) before CRT, n (%) | 0.30 | ||
| 0 | 4 (11) | 2 (3) | |
| I | 17 (45) | 21 (40) | |
| II | 7 (19) | 17 (29) | |
| III | 6 (16) | 14 (25) | |
| IV | 3 (9) | 2 (3) | |
| unknown | 3 | 0 | |
| Lesion characteristics | |||
| No. of lesions | 40 | 56 | |
| Location, n (%) | 0.28 | ||
| Upper (Ce, Ut) | 3 (8) | 10 (18) | |
| Middle (Mt) | 30 (75) | 34 (61) | |
| Lower (Lt, Ae) | 7 (17) | 12 (21) | |
| Lesion circumference, n (%) | <0.001 | ||
| <1/4 | 3412 (30) | 41 (75) | |
| ≥1/4 | 28 (70) | 14 (25) | |
| Macroscopic type, n (%) | 0.56 | ||
| Protruded or SMT | 7 (18) | 7 (13) | |
| Flat and depressed | 33 (82) | 49 (87) | |
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| Patient characteristics | |||
| No. of patients | 40 | 56 | |
| Sex, n (%) | 0.088 | ||
| Male | 33 (83) | 53 (95) | |
| Female | 7 (17) | 3 (5) | |
| Age, years, median (range) | 71 (45–87) | 67 (44–87) | 0.029 |
| Years of salvage ER, n (%) | <0.001 | ||
| 1998–2010 | 2 (5) | 40 (71) | |
| 2011- | 38 (95) | 16 (29) | |
| cT-stage before CRT, n (%) | 0.63 | ||
| T1a | 1 (3) | 3 (5) | |
| T1b | 23 (62) | 25 (45) | |
| T2 | 3 (8) | 8 (14) | |
| T3 | 9 (24) | 17 (30) | |
| T4 | 1 (3) | 3 (6) | |
| unknown | 3 | 0 | |
| cStage (TNM UICC 7th) before CRT, n (%) | 0.30 | ||
| 0 | 4 (11) | 2 (3) | |
| I | 17 (45) | 21 (40) | |
| II | 7 (19) | 17 (29) | |
| III | 6 (16) | 14 (25) | |
| IV | 3 (9) | 2 (3) | |
| unknown | 3 | 0 | |
| Lesion characteristics | |||
| No. of lesions | 40 | 56 | |
| Location, n (%) | 0.28 | ||
| Upper (Ce, Ut) | 3 (8) | 10 (18) | |
| Middle (Mt) | 30 (75) | 34 (61) | |
| Lower (Lt, Ae) | 7 (17) | 12 (21) | |
| Lesion circumference, n (%) | <0.001 | ||
| <1/4 | 3412 (30) | 41 (75) | |
| ≥1/4 | 28 (70) | 14 (25) | |
| Macroscopic type, n (%) | 0.56 | ||
| Protruded or SMT | 7 (18) | 7 (13) | |
| Flat and depressed | 33 (82) | 49 (87) | |
ER, Endoscopic resection; CRT, Chemoradiotherapy; UICC 7th, Union of International Cancer Control seventh edition
ESD, Endoscopic submucosal dissection; EMR, Endoscopic mucosal resection; Ce, Cervical; Ut, Upper thoracic; Mt, Middle thoracic; Lt, Lower thoracic; Ae, Abdominal oesophagus; SMT, Submucosal tumor
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| Patient characteristics | |||
| No. of patients | 40 | 56 | |
| Sex, n (%) | 0.088 | ||
| Male | 33 (83) | 53 (95) | |
| Female | 7 (17) | 3 (5) | |
| Age, years, median (range) | 71 (45–87) | 67 (44–87) | 0.029 |
| Years of salvage ER, n (%) | <0.001 | ||
| 1998–2010 | 2 (5) | 40 (71) | |
| 2011- | 38 (95) | 16 (29) | |
| cT-stage before CRT, n (%) | 0.63 | ||
| T1a | 1 (3) | 3 (5) | |
| T1b | 23 (62) | 25 (45) | |
| T2 | 3 (8) | 8 (14) | |
| T3 | 9 (24) | 17 (30) | |
| T4 | 1 (3) | 3 (6) | |
| unknown | 3 | 0 | |
| cStage (TNM UICC 7th) before CRT, n (%) | 0.30 | ||
| 0 | 4 (11) | 2 (3) | |
| I | 17 (45) | 21 (40) | |
| II | 7 (19) | 17 (29) | |
| III | 6 (16) | 14 (25) | |
| IV | 3 (9) | 2 (3) | |
| unknown | 3 | 0 | |
| Lesion characteristics | |||
| No. of lesions | 40 | 56 | |
| Location, n (%) | 0.28 | ||
| Upper (Ce, Ut) | 3 (8) | 10 (18) | |
| Middle (Mt) | 30 (75) | 34 (61) | |
| Lower (Lt, Ae) | 7 (17) | 12 (21) | |
| Lesion circumference, n (%) | <0.001 | ||
| <1/4 | 3412 (30) | 41 (75) | |
| ≥1/4 | 28 (70) | 14 (25) | |
| Macroscopic type, n (%) | 0.56 | ||
| Protruded or SMT | 7 (18) | 7 (13) | |
| Flat and depressed | 33 (82) | 49 (87) | |
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| Patient characteristics | |||
| No. of patients | 40 | 56 | |
| Sex, n (%) | 0.088 | ||
| Male | 33 (83) | 53 (95) | |
| Female | 7 (17) | 3 (5) | |
| Age, years, median (range) | 71 (45–87) | 67 (44–87) | 0.029 |
| Years of salvage ER, n (%) | <0.001 | ||
| 1998–2010 | 2 (5) | 40 (71) | |
| 2011- | 38 (95) | 16 (29) | |
| cT-stage before CRT, n (%) | 0.63 | ||
| T1a | 1 (3) | 3 (5) | |
| T1b | 23 (62) | 25 (45) | |
| T2 | 3 (8) | 8 (14) | |
| T3 | 9 (24) | 17 (30) | |
| T4 | 1 (3) | 3 (6) | |
| unknown | 3 | 0 | |
| cStage (TNM UICC 7th) before CRT, n (%) | 0.30 | ||
| 0 | 4 (11) | 2 (3) | |
| I | 17 (45) | 21 (40) | |
| II | 7 (19) | 17 (29) | |
| III | 6 (16) | 14 (25) | |
| IV | 3 (9) | 2 (3) | |
| unknown | 3 | 0 | |
| Lesion characteristics | |||
| No. of lesions | 40 | 56 | |
| Location, n (%) | 0.28 | ||
| Upper (Ce, Ut) | 3 (8) | 10 (18) | |
| Middle (Mt) | 30 (75) | 34 (61) | |
| Lower (Lt, Ae) | 7 (17) | 12 (21) | |
| Lesion circumference, n (%) | <0.001 | ||
| <1/4 | 3412 (30) | 41 (75) | |
| ≥1/4 | 28 (70) | 14 (25) | |
| Macroscopic type, n (%) | 0.56 | ||
| Protruded or SMT | 7 (18) | 7 (13) | |
| Flat and depressed | 33 (82) | 49 (87) | |
ER, Endoscopic resection; CRT, Chemoradiotherapy; UICC 7th, Union of International Cancer Control seventh edition
ESD, Endoscopic submucosal dissection; EMR, Endoscopic mucosal resection; Ce, Cervical; Ut, Upper thoracic; Mt, Middle thoracic; Lt, Lower thoracic; Ae, Abdominal oesophagus; SMT, Submucosal tumor
| Lesion characteristics . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Tumour size (mm), median (range) † | 16 (4–60) | 8 (2–32) | <0.001 |
| Pathological depth, n (%) | 0.068 | ||
| EP/LPM | 29 (72) | 27 (52) | |
| MM | 4 (10) | 4 (8) | |
| SM1‡ | 1 (3) | 9 (17) | |
| SM2§ | 6 (15) | 12 (23) | |
| Lymphatic invasion (+), n (%) | 2 (5) | 4 (8) | 0.70 |
| Venous invasion (+), n (%) | 2 (5) | 7 (13) | 0.29 |
| Horizontal margin (+), n (%) | 9 (23) | 7 (13) | 0.19 |
| Vertical margin (+), n (%) | 3 (8) | 4 (7) | 0.49 |
| Not evaluated | 0 | 4 |
| Lesion characteristics . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Tumour size (mm), median (range) † | 16 (4–60) | 8 (2–32) | <0.001 |
| Pathological depth, n (%) | 0.068 | ||
| EP/LPM | 29 (72) | 27 (52) | |
| MM | 4 (10) | 4 (8) | |
| SM1‡ | 1 (3) | 9 (17) | |
| SM2§ | 6 (15) | 12 (23) | |
| Lymphatic invasion (+), n (%) | 2 (5) | 4 (8) | 0.70 |
| Venous invasion (+), n (%) | 2 (5) | 7 (13) | 0.29 |
| Horizontal margin (+), n (%) | 9 (23) | 7 (13) | 0.19 |
| Vertical margin (+), n (%) | 3 (8) | 4 (7) | 0.49 |
| Not evaluated | 0 | 4 |
EP, epithelium; LPM, lamia propria mucosae; MM, muscularis mucosae; SM, submucosal layer
†Excluded four lesions with unknown tumour size in EMR group
‡SM1 ≤ 200 μm from MM, §SM2 > 200 μm from MM
| Lesion characteristics . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Tumour size (mm), median (range) † | 16 (4–60) | 8 (2–32) | <0.001 |
| Pathological depth, n (%) | 0.068 | ||
| EP/LPM | 29 (72) | 27 (52) | |
| MM | 4 (10) | 4 (8) | |
| SM1‡ | 1 (3) | 9 (17) | |
| SM2§ | 6 (15) | 12 (23) | |
| Lymphatic invasion (+), n (%) | 2 (5) | 4 (8) | 0.70 |
| Venous invasion (+), n (%) | 2 (5) | 7 (13) | 0.29 |
| Horizontal margin (+), n (%) | 9 (23) | 7 (13) | 0.19 |
| Vertical margin (+), n (%) | 3 (8) | 4 (7) | 0.49 |
| Not evaluated | 0 | 4 |
| Lesion characteristics . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Tumour size (mm), median (range) † | 16 (4–60) | 8 (2–32) | <0.001 |
| Pathological depth, n (%) | 0.068 | ||
| EP/LPM | 29 (72) | 27 (52) | |
| MM | 4 (10) | 4 (8) | |
| SM1‡ | 1 (3) | 9 (17) | |
| SM2§ | 6 (15) | 12 (23) | |
| Lymphatic invasion (+), n (%) | 2 (5) | 4 (8) | 0.70 |
| Venous invasion (+), n (%) | 2 (5) | 7 (13) | 0.29 |
| Horizontal margin (+), n (%) | 9 (23) | 7 (13) | 0.19 |
| Vertical margin (+), n (%) | 3 (8) | 4 (7) | 0.49 |
| Not evaluated | 0 | 4 |
EP, epithelium; LPM, lamia propria mucosae; MM, muscularis mucosae; SM, submucosal layer
†Excluded four lesions with unknown tumour size in EMR group
‡SM1 ≤ 200 μm from MM, §SM2 > 200 μm from MM
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Procedure uncompleted, n (%) | 1 (3) | 3 (5) | 0.64 |
| En block resection, n (%) | 38 (95) | 35 (63) | <0.001 |
| <10 mm† | 7/7 (100) | 25/30 (83) | 0.57 |
| ≥10 mm† | 31/33 (94) | 9/22 (39) | <0.001 |
| R0 resection, n (%) | 29 (73) | 29 (52) | 0.067 |
| <10 mm† | 7/7 (100) | 23/29 (79) | 0.31 |
| ≥10 mm† | 22/33 (67) | 6/23 (26) | 0.006 |
| Adverse events, n (%) | |||
| Bleeding | 0 | 0 | 1.0 |
| Perforation | 1 (3) | 0 | 1.0 |
| Procedure time median [range] (min) | 70 [27–120] | 15 [7–120] | < 0.001 |
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Procedure uncompleted, n (%) | 1 (3) | 3 (5) | 0.64 |
| En block resection, n (%) | 38 (95) | 35 (63) | <0.001 |
| <10 mm† | 7/7 (100) | 25/30 (83) | 0.57 |
| ≥10 mm† | 31/33 (94) | 9/22 (39) | <0.001 |
| R0 resection, n (%) | 29 (73) | 29 (52) | 0.067 |
| <10 mm† | 7/7 (100) | 23/29 (79) | 0.31 |
| ≥10 mm† | 22/33 (67) | 6/23 (26) | 0.006 |
| Adverse events, n (%) | |||
| Bleeding | 0 | 0 | 1.0 |
| Perforation | 1 (3) | 0 | 1.0 |
| Procedure time median [range] (min) | 70 [27–120] | 15 [7–120] | < 0.001 |
†Excluded four lesions with unknown tumour size in EMR group
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Procedure uncompleted, n (%) | 1 (3) | 3 (5) | 0.64 |
| En block resection, n (%) | 38 (95) | 35 (63) | <0.001 |
| <10 mm† | 7/7 (100) | 25/30 (83) | 0.57 |
| ≥10 mm† | 31/33 (94) | 9/22 (39) | <0.001 |
| R0 resection, n (%) | 29 (73) | 29 (52) | 0.067 |
| <10 mm† | 7/7 (100) | 23/29 (79) | 0.31 |
| ≥10 mm† | 22/33 (67) | 6/23 (26) | 0.006 |
| Adverse events, n (%) | |||
| Bleeding | 0 | 0 | 1.0 |
| Perforation | 1 (3) | 0 | 1.0 |
| Procedure time median [range] (min) | 70 [27–120] | 15 [7–120] | < 0.001 |
| . | Salvage ESD . | Salvage EMR . | P value . |
|---|---|---|---|
| No. of lesions | 40 | 56 | |
| Procedure uncompleted, n (%) | 1 (3) | 3 (5) | 0.64 |
| En block resection, n (%) | 38 (95) | 35 (63) | <0.001 |
| <10 mm† | 7/7 (100) | 25/30 (83) | 0.57 |
| ≥10 mm† | 31/33 (94) | 9/22 (39) | <0.001 |
| R0 resection, n (%) | 29 (73) | 29 (52) | 0.067 |
| <10 mm† | 7/7 (100) | 23/29 (79) | 0.31 |
| ≥10 mm† | 22/33 (67) | 6/23 (26) | 0.006 |
| Adverse events, n (%) | |||
| Bleeding | 0 | 0 | 1.0 |
| Perforation | 1 (3) | 0 | 1.0 |
| Procedure time median [range] (min) | 70 [27–120] | 15 [7–120] | < 0.001 |
†Excluded four lesions with unknown tumour size in EMR group
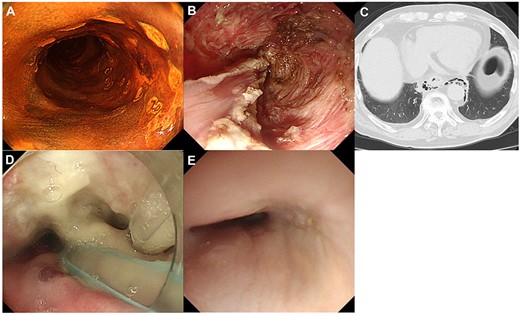
Clinical course of a patient who was detected delayed perforation after ESD. A: Local recurrent lesion after CRT, Type 0-IIc located in the right wall of the middle thoracic esophagus; B: During ESD, severe fibrosis in the submucosal layer was observed; C: After ESD, a delayed perforation was occurred. The presence of mediastinal emphysema was observed on computed tomography; D: This patient required a continual drainage of saliva at the perforation site through a nasal tube; E: The closure of the perforation was confirmed by endoscopic observation and esophagography.
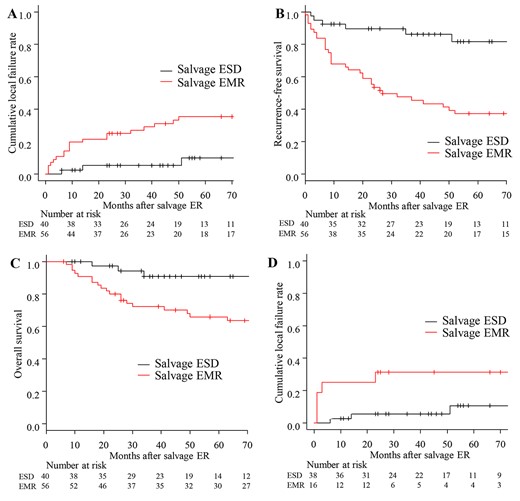
Survival curves after salvage endoscopic resection. A. Cumulative local failure. The 3-year cumulative local failure rate of salvage ESD and salvage EMR were 5% (95% CI: 1–16) and 27% (95% CI: 16–39), respectively. B. Recurrence-free survival (RFS). The 3-year RFS rate in the salvage ESD group was significantly higher than that in the salvage EMR group (86% (95% CI; 70–94) versus 48% (95% CI; 34–60), P < 0.001). C. Overall survival. The 3-year OS rate in salvage ESD group was significantly higher than that in the salvage EMR group (91% (95% CI; 74–97) versus 72% (95% CI; 58–82), P = 0.0026). D. Cumulative local failure rates of salvage endoscopic resection performed since 2011. In the 54 patients (38 ESD, 16 EMR) who underwent salvage ER since 2011, when ESD was introduced at our institute, the cumulative local failure rate of EMR was significantly higher than that of ESD (P = 0.027).
Discussion
In this study, we evaluated the optimal salvage ER method for treating patients with local recurrence after CRT or RT for ESCC by comparatively analysing the short-term outcomes and long-term local efficacy of salvage ESD versus salvage EMR. Additionally, we assessed the risk factors associated with local failure after salvage ER. Our findings clarified that salvage ESD was an effective treatment strategy, regardless of the tumour size at the point of appropriate local control.
Our data indicated that the en bloc resection rate was significantly higher in the salvage ESD group than in the salvage EMR group. In conventional ER for naïve cases, en block and R0 resections are required to prevent local recurrence (17). Salvage EMR was originally developed for small localized esophageal cancer lesions as an alternative to surgery because it showed similar efficacy and was less invasive than salvage esophagectomy (18–21). However, it has been reported that the en block and R0 resection rates were relatively low for salvage EMR, as well as conventional EMR, when utilized for large lesions, which is consistent with our results (6–8,22–27). In contrast, salvage ESD has been recently reported to be a technically feasible treatment option for local recurrence and beneficial for one-piece resection without any serious adverse events (9–12). Moreover, although local recurrence after CRT is often accompanied by severe fibrosis, which causes poor lifting after submucosal injection, ESD may allow the dissection of fibrous submucosa under direct visualization, compared with EMR (28). Given the results of this and the prior studies, salvage ESD can be recommended as a salvage ER for local recurrence.
It has been reported that local control is a surrogate marker for achieving a better survival outcome after salvage endoscopic treatments (29). When evaluating the local efficacy of salvage ER for local recurrence, it is necessary to evaluate the long-term outcome of local control after salvage ER. Local failure after CRT includes residual lesions and local recurrences. It has been reported that the risk of primary disease death due to lymph node or distant metastatic recurrence is significantly higher in cases of residual lesion compared with those of local recurrence (30,31). Therefore, since the purpose of this study was to evaluate the usefulness of salvage ER with respect to local control, patients with local recurrence, for whom local control was particularly useful, were included in the study. In this study, the 3- and 5-year cumulative local failure rates of salvage EMR were significantly higher than those of salvage ESD. It has been reported that the local recurrence rate after salvage EMR is relatively high, which is consistent with our results (18–20). Ego et al. (28) reported that the local recurrence rate of salvage EMR was 21% (9/43 cases) during a median follow-up period of 57 months. A study by Yano et al. (7), which was performed at our institution, reported a local recurrence rate of 29% (6/21 cases) in a 54-month median follow-up period following salvage EMR. In contrast, we previously reported a low local recurrence rate after salvage ESD; however, the median follow-up period was relatively short (12). Kimura et al. (32) reported that 13% of patients who underwent salvage ESD had local recurrence during a median follow-up period of 51 months. Our findings in this study suggest favourable long-term local control after salvage ESD with a median follow-up period of 46 months, consistent with the report by Kimura et al. Moreover, salvage EMR method was identified as the only risk factor of local recurrence after salvage ER in multivariate analyses. Considering long-term local control, salvage ESD may be recommended.
In this study, R0 and en bloc resections were not significant factors in the univariate analysis of risk factors for local failure after salvage ER. This result is different from the reports on Conventional ER. We believe that one of the reasons for this discrepancy may be the different characteristics between local recurrence after CRT and treatment naïve esophageal cancer. Local recurrence after conventional ER for treatment-naïve esophageal cancer is rare if the tumour is completely resected by ER with R0 resection. In contrast, local re-recurrence after salvage therapy for post-CRT local recurrence of esophageal cancer has been reported to frequently occur from the primary site, even when the salvage therapy is successful. This could be partly attributed to possible multifocal small residual lesions at the primary site that are not visible on post-CRT endoscopy. Therefore, even if an endoscopically detectable lesion can be completely resected by salvage ER, residual tumour cells within the primary site may subsequently become apparent and be recognized as local re-recurrence after salvage ER.
Univariate and multivariate analyses of risk factors of local failure after salvage ER
| . | . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|---|
| Subgroup . | . | P value . | Hazard ratio (95% CI) . | P value . |
| Variables associated with patient’s factors | ||||
| Age | <65 | 0.27 | ||
| ≥65 | ||||
| Sex | Male | 0.55 | ||
| Female | ||||
| Variables with associated with lesions and technical factors | ||||
| cT-stage before CRT | cT1 | 0.78 | ||
| cT2–4 | ||||
| cN-stage before CRT | cN0 | 0.93 | ||
| cN1–3 | ||||
| Location | Upper | 0.18 | ||
| Middle | ||||
| Lower | ||||
| Lesion circumference | <1/4 | 0.76 | ||
| ≥1/4 | ||||
| Tumour size (mm) † | <10 | 0.14 | ||
| ≥10 | ||||
| Macroscopic type | Protruded or SMT | 0.046 | 1 | |
| Flat and depressed | 5.2 (0.74–36) | 0.097 | ||
| Pathological tumour depth | T1a | 0.37 | ||
| T1b | ||||
| Method of salvage ER | ESD | 0.032 | 1 | |
| EMR | 2.7 (1.0–7.1) | 0.044 | ||
| En block resection | En block | 0.48 | ||
| Piecemeal | ||||
| R0 resection | R0 | 0.73 | ||
| R1 | ||||
| . | . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|---|
| Subgroup . | . | P value . | Hazard ratio (95% CI) . | P value . |
| Variables associated with patient’s factors | ||||
| Age | <65 | 0.27 | ||
| ≥65 | ||||
| Sex | Male | 0.55 | ||
| Female | ||||
| Variables with associated with lesions and technical factors | ||||
| cT-stage before CRT | cT1 | 0.78 | ||
| cT2–4 | ||||
| cN-stage before CRT | cN0 | 0.93 | ||
| cN1–3 | ||||
| Location | Upper | 0.18 | ||
| Middle | ||||
| Lower | ||||
| Lesion circumference | <1/4 | 0.76 | ||
| ≥1/4 | ||||
| Tumour size (mm) † | <10 | 0.14 | ||
| ≥10 | ||||
| Macroscopic type | Protruded or SMT | 0.046 | 1 | |
| Flat and depressed | 5.2 (0.74–36) | 0.097 | ||
| Pathological tumour depth | T1a | 0.37 | ||
| T1b | ||||
| Method of salvage ER | ESD | 0.032 | 1 | |
| EMR | 2.7 (1.0–7.1) | 0.044 | ||
| En block resection | En block | 0.48 | ||
| Piecemeal | ||||
| R0 resection | R0 | 0.73 | ||
| R1 | ||||
†Excluded four lesions with unknown tumour size in EMR group
Univariate and multivariate analyses of risk factors of local failure after salvage ER
| . | . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|---|
| Subgroup . | . | P value . | Hazard ratio (95% CI) . | P value . |
| Variables associated with patient’s factors | ||||
| Age | <65 | 0.27 | ||
| ≥65 | ||||
| Sex | Male | 0.55 | ||
| Female | ||||
| Variables with associated with lesions and technical factors | ||||
| cT-stage before CRT | cT1 | 0.78 | ||
| cT2–4 | ||||
| cN-stage before CRT | cN0 | 0.93 | ||
| cN1–3 | ||||
| Location | Upper | 0.18 | ||
| Middle | ||||
| Lower | ||||
| Lesion circumference | <1/4 | 0.76 | ||
| ≥1/4 | ||||
| Tumour size (mm) † | <10 | 0.14 | ||
| ≥10 | ||||
| Macroscopic type | Protruded or SMT | 0.046 | 1 | |
| Flat and depressed | 5.2 (0.74–36) | 0.097 | ||
| Pathological tumour depth | T1a | 0.37 | ||
| T1b | ||||
| Method of salvage ER | ESD | 0.032 | 1 | |
| EMR | 2.7 (1.0–7.1) | 0.044 | ||
| En block resection | En block | 0.48 | ||
| Piecemeal | ||||
| R0 resection | R0 | 0.73 | ||
| R1 | ||||
| . | . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|---|
| Subgroup . | . | P value . | Hazard ratio (95% CI) . | P value . |
| Variables associated with patient’s factors | ||||
| Age | <65 | 0.27 | ||
| ≥65 | ||||
| Sex | Male | 0.55 | ||
| Female | ||||
| Variables with associated with lesions and technical factors | ||||
| cT-stage before CRT | cT1 | 0.78 | ||
| cT2–4 | ||||
| cN-stage before CRT | cN0 | 0.93 | ||
| cN1–3 | ||||
| Location | Upper | 0.18 | ||
| Middle | ||||
| Lower | ||||
| Lesion circumference | <1/4 | 0.76 | ||
| ≥1/4 | ||||
| Tumour size (mm) † | <10 | 0.14 | ||
| ≥10 | ||||
| Macroscopic type | Protruded or SMT | 0.046 | 1 | |
| Flat and depressed | 5.2 (0.74–36) | 0.097 | ||
| Pathological tumour depth | T1a | 0.37 | ||
| T1b | ||||
| Method of salvage ER | ESD | 0.032 | 1 | |
| EMR | 2.7 (1.0–7.1) | 0.044 | ||
| En block resection | En block | 0.48 | ||
| Piecemeal | ||||
| R0 resection | R0 | 0.73 | ||
| R1 | ||||
†Excluded four lesions with unknown tumour size in EMR group
This study has some limitations. First, this was a single-centre retrospective study that involved a relatively small number of patients and local recurrence events. Second, the choice of salvage ER treatment strategy for local recurrence was at the discretion of the operators and supervisors of the procedure, thereby leading to a certain degree of selection bias. Third, although salvage EMR was often performed for local recurrence after CRT until 2010, the number of salvage ESD procedures has increased since 2011. Furthermore, the learning curve of salvage ER procedures may have influenced the outcomes. Fourth, the follow-up period of salvage ESD was relatively shorter than that of salvage EMR. In addition, a certain number of patients in both groups had a short follow-up period, which may have led to the calculation of a lower local recurrence rate.
Nonetheless, our study provides evidence that salvage ESD is the effective treatment method for local recurrence after CRT or RT regardless of the tumour size based on the short-term outcomes and local efficacy.
Acknowledgments
None.
Funding
All authors disclose no financial relationships relevant to this publication.
Conflict of interests
The authors declare no conflicts of interest for this article.


