-
PDF
- Split View
-
Views
-
Cite
Cite
Takashi Kobayashi, Ario Takeuchi, Hiroyuki Nishiyama, Masatoshi Eto, Current status and future perspectives of immunotherapy against urothelial and kidney cancer, Japanese Journal of Clinical Oncology, Volume 51, Issue 10, October 2021, Pages 1481–1492, https://doi.org/10.1093/jjco/hyab121
Close - Share Icon Share
Abstract
Much attention has been paid to immune checkpoint inhibitors to various cancer treatments. In urothelial cancer, pembrolizumab was initially approved for patients who either recurred or progressed following platinum-based chemotherapy. For the platinum-fit population, although the standard first-line treatment is still platinum-based systemic chemotherapy, avelumab has been recently approved as a maintenance therapy for patients who have not had disease progression with four to six cycles of first-line chemotherapy. In addition, adjuvant nivolumab has just prolonged disease-free survival (DFS) by ~10 months, compared with placebo in patients with muscle-invasive bladder urothelial cancer or upper tract urothelial cancer at high-risk of recurrence after radical surgical resection.
On the other hand, in kidney cancer, nivolumab was initially approved for advanced renal cell carcinoma patients after one or two prior anti-angiogenic therapies. Next, combinations of two immune checkpoint inhibitors (nivolumab + ipilimumab) and immune checkpoint inhibitor + tyrosine kinase inhibitors (pembrolizumab + axitinib and avelumab + axitinib) were approved for the first-line treatment for patients with advanced renal cell carcinoma. Recently, new generation tyrosine kinase inhibitors, such as cabozantinib and lenvatinib have been combined with immune checkpoint inhibitors. Both nivolumab + cabozantinib and pembrolizumab + lenvatinib have demonstrated superior progression-free survival and objective response rate, compared with sunitinib. So far, no prospective trials have demonstrated the duration of immune checkpoint inhibitor treatments. We are now doing the Japan Clinical Oncology Group 1905 trial, where patients with advanced renal cell carcinoma who have received an immune checkpoint inhibitor for 24 weeks are divided into two groups: those who continue immune checkpoint inhibitor treatment and those who discontinue immune checkpoint inhibitor treatment.
Introduction
Urothelial and kidney cancer have been historically considered to be sensitive to immunotherapy because of their standard treatments of intravesical instillation of bacillus Calmette-Guerin (BCG) (1) and administration of interferon-α (2). In addition, after the introduction of immune checkpoint inhibitors to urothelial (3) and kidney cancer (4), recent clinical trials have been drastically changing their standard treatments. Here, we summarize current status and future perspectives of immunotherapy (including various combination therapies) against urothelial and kidney cancer.
Urothelial cancer
Characteristics of urothelial cancer as a target of immune checkpoint inhibitor therapy
Urothelial cancer is known to be one of the malignancies with a high frequency of gene mutation (5). Molecular mechanisms for urothelial carcinogenesis are complicated (6,7), and it is often not caused by a single driver gene mutation (8). Recent accumulation of genomic analyses has revealed that UC can be categorized into several molecular subtypes, suggesting high intertumoral heterogeneity (9). Therefore, there has never been a highly effective molecular-targeting agent for UC with rare exceptions (10).
UC is also characterized by its immunogenicity as evident by the fact that intravesical instillation of BCG has been used for non-muscle-invasive bladder cancer (NMIBC) for decades (1) in addition to its high mutation burden (5,8). Thus, UC was one of the malignancies that had been expected to respond to immunotherapy. Indeed, the efficacy of anti-PD-1/PD-L1 treatment was reported ahead of many other cancers (11). Since then, the effectiveness of immune checkpoint inhibitors (ICIs) has been tested in UC patients in various treatment settings. Some of them have already been shown to improve treatment outcomes. Furthermore, it is expected that the effectiveness of ICI will be proved in other settings, leading to expanded indication in the future. This part of the article reviews current status and discusses future perspective of ICIs in the treatment of UC.
Current standard treatment for stage IV disease
Methotrexate, vinblastine, adriamycin plus cisplatin (MVAC) was shown to prolong survival compared with older chemotherapy combinations in 1990’s (12), followed by gemcitabine plus cisplatin (GC) shown to be less toxic than MVAC without compromising survival outcomes in 2000 (13). Those two regimens are approved in Japan as the current standard for patients with stage IV UC (14). Gemcitabine plus carboplatin (G-CBDCA) is often used for cisplatin-ineligible patients as an approved regimen, although it was reported to yield lower complete response rate and shorter overall survival (OS) (15). Better efficacy by modifications of dose density has been reported (16), although those modified regimens have not been approved in Japan. Additionally, those regimens are also frequently used as perioperative chemotherapy either at the neoadjuvant or adjuvant setting (14). Current standard chemotherapy is active in the majority of the patients; objective response (OR; partial response [PR] or complete remission [CR]) in 40 to 50% and disease control (stable disease, PR or CR) in 75 to 80%. However, most patients have disease progression within ~9 months, and the median OS was 14–15 months.
Immune checkpoint inhibitors for unresectable, chemoresistant UC
There had been no effective treatment for patients with UC that progressed against first-line systemic chemotherapy. This long-standing problem was overcome by KEYNOTE-045 study (ClinicalTrials.gov number, NCT02256436) in 2017 (3). In this phase 3 trial, 542 patients who either recurred or progressed following platinum-based chemotherapy were randomized to receive either pembrolizumab or chemotherapy (paclitaxel, docetaxel or vinflunine). OS of those who received pembrolizumab was significantly superior to that of those who received chemotherapy (10.3 vs 7.4 months; 95% confidence interval (CI) 8.0–11.8 vs 6.1–8.3; hazard ratio [HR] 0.73; 95% CI 0.59–0.91; P = 0.002). The OR rate was 21.1% for pembrolizumab group compared with 11.4% for chemotherapy group. Similar trends for the better oncological outcomes were observed in the Japanese sub-population (17) and after >2-year follow-up (18).
In terms of tolerability, treatment-related adverse event (AE) was reported in 60.9% of patients in the pembrolizumab group compared with 90.2% of those in the chemotherapy group. Another report of exploratory results demonstrated that the quality of life in patients administered pembrolizumab was significantly better than that in those administered chemotherapy (19) despite several criticisms were raised (20).
These results prompted US Food and Drug Administration (FDA) and Japan Pharmaceuticals and Medical Devices Agency (PMDA) to give pembrolizumab a full approval for treatment of post-platinum UC patients in both countries by the end of 2017. Since then, as the only life-prolonging treatment in UC refractory to the standard first-line chemotherapy, the use of second-line pembrolizumab has increased rapidly without clear guidance for validated prognostication. Several investigators reported relatively small-scale outcome studies from their initial experiences with prognostic markers related to performance status (PS), number or site of metastasis, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP) and time from previous chemotherapy (Table 1) (21–26).
Retrospective patient series of second-line pembrolizumab for urothelial cancer
| Author . | Number of patients . | PFS (months) . | OS (months) . | Risk factors . |
|---|---|---|---|---|
| Tamura et al. (21) | 41 | 2.5 | 11.9 | OS: ECOG PS (>1), Number of metastatic site (>1), NLR kinetics (+6.12% at 6 M) |
| Yasuoka et al. (22) | 40 | 4.1 | 10.0 | OS: ECOG PS (>1), liver metastasis (yes), CRP (>0.5 mg/dL) |
| Ogihara et al. (24) | 78 | N.S. | N.S. | CSM: NLR (≥3.35) Disease progression: ECOG PS (>0), liver metastasis (yes), NLR (≥3.35) |
| Furubayashi et al. (23) | 34 | 3.3 | 11.7 | OS: liver metastasis (yes), time from previous chemotherapy (≥3 M) |
| Yamamoto et al. (25) | 121 | N.S. | N.S. | OS: ECOG PS (>1), LN metastasis only (no), CRP (>0.56 mg/dL), NLR (>3.0) |
| Kijima et al. (26) | 97 | 7.5 | 13.1 | OS: ECOG PS (>1), liver meta (yes), CRP (>0.5 mg/dL, kinetics) |
| Kobayashi et al. (27) | Discovery: 463 Validation: 292 | N.S. | 10.2 12.5 | OS: ECOG PS (>1), Metastasis site (Liver/Other organs/LN only), Hb (<11), NLR (>3.0) |
| Author . | Number of patients . | PFS (months) . | OS (months) . | Risk factors . |
|---|---|---|---|---|
| Tamura et al. (21) | 41 | 2.5 | 11.9 | OS: ECOG PS (>1), Number of metastatic site (>1), NLR kinetics (+6.12% at 6 M) |
| Yasuoka et al. (22) | 40 | 4.1 | 10.0 | OS: ECOG PS (>1), liver metastasis (yes), CRP (>0.5 mg/dL) |
| Ogihara et al. (24) | 78 | N.S. | N.S. | CSM: NLR (≥3.35) Disease progression: ECOG PS (>0), liver metastasis (yes), NLR (≥3.35) |
| Furubayashi et al. (23) | 34 | 3.3 | 11.7 | OS: liver metastasis (yes), time from previous chemotherapy (≥3 M) |
| Yamamoto et al. (25) | 121 | N.S. | N.S. | OS: ECOG PS (>1), LN metastasis only (no), CRP (>0.56 mg/dL), NLR (>3.0) |
| Kijima et al. (26) | 97 | 7.5 | 13.1 | OS: ECOG PS (>1), liver meta (yes), CRP (>0.5 mg/dL, kinetics) |
| Kobayashi et al. (27) | Discovery: 463 Validation: 292 | N.S. | 10.2 12.5 | OS: ECOG PS (>1), Metastasis site (Liver/Other organs/LN only), Hb (<11), NLR (>3.0) |
PFS; progression-free survival, OS; overall survival, NLR; neutrophil-to-lymphocyte ratio, CRP; C-reactive protein, CSM; cancer-specific mortality, Hb; hemoglobin.
Retrospective patient series of second-line pembrolizumab for urothelial cancer
| Author . | Number of patients . | PFS (months) . | OS (months) . | Risk factors . |
|---|---|---|---|---|
| Tamura et al. (21) | 41 | 2.5 | 11.9 | OS: ECOG PS (>1), Number of metastatic site (>1), NLR kinetics (+6.12% at 6 M) |
| Yasuoka et al. (22) | 40 | 4.1 | 10.0 | OS: ECOG PS (>1), liver metastasis (yes), CRP (>0.5 mg/dL) |
| Ogihara et al. (24) | 78 | N.S. | N.S. | CSM: NLR (≥3.35) Disease progression: ECOG PS (>0), liver metastasis (yes), NLR (≥3.35) |
| Furubayashi et al. (23) | 34 | 3.3 | 11.7 | OS: liver metastasis (yes), time from previous chemotherapy (≥3 M) |
| Yamamoto et al. (25) | 121 | N.S. | N.S. | OS: ECOG PS (>1), LN metastasis only (no), CRP (>0.56 mg/dL), NLR (>3.0) |
| Kijima et al. (26) | 97 | 7.5 | 13.1 | OS: ECOG PS (>1), liver meta (yes), CRP (>0.5 mg/dL, kinetics) |
| Kobayashi et al. (27) | Discovery: 463 Validation: 292 | N.S. | 10.2 12.5 | OS: ECOG PS (>1), Metastasis site (Liver/Other organs/LN only), Hb (<11), NLR (>3.0) |
| Author . | Number of patients . | PFS (months) . | OS (months) . | Risk factors . |
|---|---|---|---|---|
| Tamura et al. (21) | 41 | 2.5 | 11.9 | OS: ECOG PS (>1), Number of metastatic site (>1), NLR kinetics (+6.12% at 6 M) |
| Yasuoka et al. (22) | 40 | 4.1 | 10.0 | OS: ECOG PS (>1), liver metastasis (yes), CRP (>0.5 mg/dL) |
| Ogihara et al. (24) | 78 | N.S. | N.S. | CSM: NLR (≥3.35) Disease progression: ECOG PS (>0), liver metastasis (yes), NLR (≥3.35) |
| Furubayashi et al. (23) | 34 | 3.3 | 11.7 | OS: liver metastasis (yes), time from previous chemotherapy (≥3 M) |
| Yamamoto et al. (25) | 121 | N.S. | N.S. | OS: ECOG PS (>1), LN metastasis only (no), CRP (>0.56 mg/dL), NLR (>3.0) |
| Kijima et al. (26) | 97 | 7.5 | 13.1 | OS: ECOG PS (>1), liver meta (yes), CRP (>0.5 mg/dL, kinetics) |
| Kobayashi et al. (27) | Discovery: 463 Validation: 292 | N.S. | 10.2 12.5 | OS: ECOG PS (>1), Metastasis site (Liver/Other organs/LN only), Hb (<11), NLR (>3.0) |
PFS; progression-free survival, OS; overall survival, NLR; neutrophil-to-lymphocyte ratio, CRP; C-reactive protein, CSM; cancer-specific mortality, Hb; hemoglobin.
More recently, a multicenter study by Japan Urological Oncology Group (JUOG) analyzed a total of 755 UC patients who received pembrolizumab for chemoresistant disease (27). The study proposed risk stratification based on four factors namely Eastern Cooperative Oncology Group (ECOG) PS (>1), metastasis sites (liver/other organs/lymph node only), hemoglobin (<11 g/dL) and NLR (>3.0), which was established by multivariate Cox proportional hazard analysis on 463 patients and externally validated using data on distinct 292 patients. At median follow up of 17.7 months, median (95% CI) OS after the initiation of pembrolizumab treatment was 2.3 (1.2–2.6) months for high-risk, 6.8 (5.8–8.9) months for intermediate and not reached (NR) (NR–19.1) for low-risk patients. C-index of the model for the validation cohort was 0.747. Importantly, the risk stratification was significantly associated with OR rate (ORR) as well. Because there have been no prognostic or predictive biomarkers for second-line pembrolizumab treatment, the model using conventional clinical parameters is clinically useful and also informative for the design of future clinical trials. These prognostic markers will be growingly important as novel agents including enfortumab vedotin and erdafitinib are introduced as an alternative or subsequent treatment.
Similarly, Sternberg et al. (28) reported results from SAUL (NCT02928406), a single-arm multicenter international open-label phase 3B safety study of atezolizumab in a real-world population, which specified a ‘difficult-to-treat’ group defined as having ECOG PS 2 or more or central nervous system metastasis. In another report, Sonpavde et al. (29) analyzed data from phase 1/2 trials for chemoresistant UC patients receiving PD-L1 inhibitors (avelumab or durvalumab). They reported that a five-factor prognostic model incorporating ECOG-PS, liver metastasis, platelet count, NLR ratio and lactate dehydrogenase yielded robust discrimination of survival of between low, intermediate and high-risk groups.
In terms of PS, Khaki et al. (30) reported real-world data of UC patients receiving ICI and suggested that those with an ECOG PS 3 were unlikely to benefit from ICIs although a meaningful number of patients with an ECOG PS 2 appeared to benefit, at least on the basis of the ORR. In this regard, another JUOG study showed similar OS outcome between those ECOG PS 2 and ≥ 3, which was better discriminated by NLR and liver metastasis (31), suggesting that even those with ECOG PS 3 may be benefitted from pembrolizumab if they do not have high NLR nor liver metastasis.
NLR is also robustly associated with the survival of UC patients treated with ICIs described above. However, it is not a specific prognosticator for this patient population but for UC patients in other treatment or disease stages, those with other malignant or non-malignant diseases, and even in a general population (27). It is noteworthy that several investigators reported better prognosis of patients achieving the reduction of NLR after pembrolizumab treatment (21,24,25). Additionally, a JUOG study showed better prognosis after pembrolizumab treatment of patients achieving the reduction of NLR after first-line chemotherapy (32). Biological and clinical significance of this unique biomarker should be further studied in the future.
First-line treatment for unresectable locally advanced or metastatic UC patients
Use of ICI as the first-line treatment was initially approved for CDDP-unfit population (33). In a phase 2 IMvigor 210 trial (NCT02108652), atezolizumab showed 23.5% of ORR including radiological CR in 6.7% of the patients (34). Another phase 2 KEYNOTE-052 trial (NCT02335424) reported 28.6% of ORR including radiological CR in 8.9% (35). Based on these findings both atezolizumab and pembrolizumab have been approved for this patient population in the USA but not in Japan as neither of the two studies enrolled Japanese patients.
Several other phase 3 trials explored ICIs as first-line treatment using different approaches (DANUBE [NCT02516241] (36), KEYNOTE-361 [NCT02853305] (37) and IMvigor 130 [NCT02807636] (38) (Table 2). Although atezolizumab in combination with chemotherapy showed superior progression-free survival (PFS) compared with chemotherapy alone without increasing AEs rate, difference in OS did NR the predefined threshold of statistical significance at least at the time of interim analysis (38), which means we have to wait until the final OS analysis to have definitive conclusion for OS benefit by additional atezolizumab to standard chemotherapy at the first-line treatment setting. The other two failed to show OS advantage over standard chemotherapy in intention-to-treat (ITT) population (36,37). There are several more trials that are still ongoing (CheckMate-901 [NCT03036098], NILE [NCT03682068], LEAP-011 [NCT03898180], and EV-302 [NCT04223856]). (Table 2 and ref. (39)) Until any of these trials shows survival advantage over the standard of care, first-line chemotherapy and subsequent, whatever salvage or maintenance, ICI will stay at the center of treatment strategy in this setting. Then the timing of switching from chemotherapy to ICI, particularly in those who have their disease controlled with chemotherapy, will become a very important clinical question. Usually accumulation of chemotherapy usually substantially worsens patient quality of life, and probably systemic immune status as well, which may affect the effectiveness of subsequent ICI (40). On the other hand, it takes courage to change an effective treatment to another as someone said ‘never mess with the winning team’.
Key phase-3 trials on first-line treatment regimen using immune checkpoint inhibitor for unresectable or metastatic urothelial cancer
| Name . | Trial ID . | Total sample size . | Treatment regimen . | Control regimen . | Primary endpoints . | Key secondary oncological endpoints . | Key results . |
|---|---|---|---|---|---|---|---|
| DANUBEa (36) | NCT 02516241 | 1032 | Durva. Durva. + Tremeli. | G-C or G-CBDCA | OSc | PFS, ORR, DOR |
|
| IMvigor130a (38) | NCT 02807636 | 1213 | Atezo. Atezo. + G-C or G-CBDCA | G-C or G-CBDCA | PFSd, OSe, safety | ORR, DOR |
|
| KEYNOTE-361a (37) | NCT 02853305 | 1010 | Pembro. Pembro. + G-C or G-CBDCA | G-C or G-CBDCA | PFS, OS | ORR, DOR, DCR |
|
| CheckMate-901a | NCT 03036098 | 1290b | Nivo. + Ipi. Nivo. + G-C | G-C or G-CBDCA | PFS, OS | Not yet reported | |
| NILEa | NCT 03682068 | 1434b | Durva. + G-C or G-CBDCA Durva. + Tremeli. + G-C or G-CBDCA | G-C or G-CBDCA | OS | PFS, ORR, DOR, DCR, PFS2 | Not yet reported |
| EV-302a | NCT 04223856 | 760b | Pembro. + EV | G-C or G-CBDCA | PFS, OS | ORR, DOR | Not yet reported |
| Name . | Trial ID . | Total sample size . | Treatment regimen . | Control regimen . | Primary endpoints . | Key secondary oncological endpoints . | Key results . |
|---|---|---|---|---|---|---|---|
| DANUBEa (36) | NCT 02516241 | 1032 | Durva. Durva. + Tremeli. | G-C or G-CBDCA | OSc | PFS, ORR, DOR |
|
| IMvigor130a (38) | NCT 02807636 | 1213 | Atezo. Atezo. + G-C or G-CBDCA | G-C or G-CBDCA | PFSd, OSe, safety | ORR, DOR |
|
| KEYNOTE-361a (37) | NCT 02853305 | 1010 | Pembro. Pembro. + G-C or G-CBDCA | G-C or G-CBDCA | PFS, OS | ORR, DOR, DCR |
|
| CheckMate-901a | NCT 03036098 | 1290b | Nivo. + Ipi. Nivo. + G-C | G-C or G-CBDCA | PFS, OS | Not yet reported | |
| NILEa | NCT 03682068 | 1434b | Durva. + G-C or G-CBDCA Durva. + Tremeli. + G-C or G-CBDCA | G-C or G-CBDCA | OS | PFS, ORR, DOR, DCR, PFS2 | Not yet reported |
| EV-302a | NCT 04223856 | 760b | Pembro. + EV | G-C or G-CBDCA | PFS, OS | ORR, DOR | Not yet reported |
aJapanese patients enrolled.
bEstimated enrollment.
cTwo OS comparisons; (1) between the Durva. vs CT groups in the population of patients with high PD-L1 expression and (2) between the Durva. + Treme. vs CT groups in the ITT population.
dPFS between the Atezo. + CT vs CT groups in the ITT population.
eOS between the Atezo. vs CT groups in the ITT population, which was to be formally tested only if OS was positive for the Atezo. + CT vs CT groups. Only the interim result is reported as of the end of 2020.
G-C, Gemcitabine + cisplatin; G-CBDCA, Gemcitabine + carboplatin; Durva., Durvalumab; Tremeli., Tremelimumab; Atezo., Atezolizumab; Pembro., Pembrolizumab; Nivo., Nivolumab; Ipi., Ipilimumab; EV, Enfortumab vedotin; PFS, Progression-free survival; OS, Overall survival; ORR, Objective response rate; DOR, Duration of response; DCR, Disease control rate; PFS2, Second profression-free survival; CT, Chemotherapy; ITT, Intention to treat.
Key phase-3 trials on first-line treatment regimen using immune checkpoint inhibitor for unresectable or metastatic urothelial cancer
| Name . | Trial ID . | Total sample size . | Treatment regimen . | Control regimen . | Primary endpoints . | Key secondary oncological endpoints . | Key results . |
|---|---|---|---|---|---|---|---|
| DANUBEa (36) | NCT 02516241 | 1032 | Durva. Durva. + Tremeli. | G-C or G-CBDCA | OSc | PFS, ORR, DOR |
|
| IMvigor130a (38) | NCT 02807636 | 1213 | Atezo. Atezo. + G-C or G-CBDCA | G-C or G-CBDCA | PFSd, OSe, safety | ORR, DOR |
|
| KEYNOTE-361a (37) | NCT 02853305 | 1010 | Pembro. Pembro. + G-C or G-CBDCA | G-C or G-CBDCA | PFS, OS | ORR, DOR, DCR |
|
| CheckMate-901a | NCT 03036098 | 1290b | Nivo. + Ipi. Nivo. + G-C | G-C or G-CBDCA | PFS, OS | Not yet reported | |
| NILEa | NCT 03682068 | 1434b | Durva. + G-C or G-CBDCA Durva. + Tremeli. + G-C or G-CBDCA | G-C or G-CBDCA | OS | PFS, ORR, DOR, DCR, PFS2 | Not yet reported |
| EV-302a | NCT 04223856 | 760b | Pembro. + EV | G-C or G-CBDCA | PFS, OS | ORR, DOR | Not yet reported |
| Name . | Trial ID . | Total sample size . | Treatment regimen . | Control regimen . | Primary endpoints . | Key secondary oncological endpoints . | Key results . |
|---|---|---|---|---|---|---|---|
| DANUBEa (36) | NCT 02516241 | 1032 | Durva. Durva. + Tremeli. | G-C or G-CBDCA | OSc | PFS, ORR, DOR |
|
| IMvigor130a (38) | NCT 02807636 | 1213 | Atezo. Atezo. + G-C or G-CBDCA | G-C or G-CBDCA | PFSd, OSe, safety | ORR, DOR |
|
| KEYNOTE-361a (37) | NCT 02853305 | 1010 | Pembro. Pembro. + G-C or G-CBDCA | G-C or G-CBDCA | PFS, OS | ORR, DOR, DCR |
|
| CheckMate-901a | NCT 03036098 | 1290b | Nivo. + Ipi. Nivo. + G-C | G-C or G-CBDCA | PFS, OS | Not yet reported | |
| NILEa | NCT 03682068 | 1434b | Durva. + G-C or G-CBDCA Durva. + Tremeli. + G-C or G-CBDCA | G-C or G-CBDCA | OS | PFS, ORR, DOR, DCR, PFS2 | Not yet reported |
| EV-302a | NCT 04223856 | 760b | Pembro. + EV | G-C or G-CBDCA | PFS, OS | ORR, DOR | Not yet reported |
aJapanese patients enrolled.
bEstimated enrollment.
cTwo OS comparisons; (1) between the Durva. vs CT groups in the population of patients with high PD-L1 expression and (2) between the Durva. + Treme. vs CT groups in the ITT population.
dPFS between the Atezo. + CT vs CT groups in the ITT population.
eOS between the Atezo. vs CT groups in the ITT population, which was to be formally tested only if OS was positive for the Atezo. + CT vs CT groups. Only the interim result is reported as of the end of 2020.
G-C, Gemcitabine + cisplatin; G-CBDCA, Gemcitabine + carboplatin; Durva., Durvalumab; Tremeli., Tremelimumab; Atezo., Atezolizumab; Pembro., Pembrolizumab; Nivo., Nivolumab; Ipi., Ipilimumab; EV, Enfortumab vedotin; PFS, Progression-free survival; OS, Overall survival; ORR, Objective response rate; DOR, Duration of response; DCR, Disease control rate; PFS2, Second profression-free survival; CT, Chemotherapy; ITT, Intention to treat.
Despite various approaches using ICIs are exploring as first-line treatment, it is unclear whether ICI can outperform chemotherapy in the ITT population. Subgroup analyses from several trials suggest that use of a predictive biomarker is critically important. According to the interim analysis of IMvigor 130 trial, atezolizumab plus chemotherapy did not achieve OS advantage over chemotherapy alone based on the predefined statistical significance in the ITT population. Subgroup analysis showed benefit from atezolizumab plus chemotherapy in those with high PD-L1 expression (38). In DANUBE (36), anti-PD-L1 durvalumab alone or durvalumab plus anti-CTLA-4 tremelimumab failed to show OS advantage over chemotherapy in the ITT population. Subgroup analysis showed OS benefit from durvalumab plus tremelimumab in the high PD-L1 group. Based on these results, the target of the primary endpoints may be changed from the ITT population to the PD-L1 high expression group in some ongoing trials. In that case, the introduction of companion diagnostics will be inevitable. However, standardization of the evaluation method for PD-L1 expression remains a critical issue (41).
As shown above, for the platinum-fit population, the standard first-line treatment is still platinum-based systemic chemotherapy. Even if OR or disease control is achieved initially, early chemotherapy resistance is often occurred, which limits PFS and OS after chemotherapy. In a phase 3 JAVELIN bladder 100 trial (NCT02603432), patients who did not have disease progression with first-line chemotherapy (4–6 cycles of GC or G-CBDCA) were randomized to receive best supportive care with or without maintenance avelumab (42). OS was significantly better for the avelumab group (21.4 vs 14.3 months; HR for death, 0.69 [0.56 to 0.86]; P = 0.001). As for maintenance ICI after first-line chemotherapy yielding disease control, pembrolizumab is currently being tested in a phase 2, randomized placebo-controlled trial (NCT02500121). In this setting, Abe et al. (43) reported that maintenance chemotherapy at two- to three-month interval yielded median OS of 37 months (95%CI: 24–60 months) from the initiation of induction chemotherapy, whereas median OS for propensity score-matched patients who did not receive maintenance chemotherapy was 19 months (95%CI: 11–30 months) (P = 0.0573). Because the patient in the control group of JAVELIN bladder 100 trial did not receive maintenance chemotherapy, it is still inclusive which of chemotherapy or immunotherapy is superior as the maintenance treatment. Novel predictive markers to discriminate patients who are benefited from chemotherapy or immunotherapy are strongly warranted in near future.
Perioperative therapy for stage II-III disease
Current consensus is that cisplatin-based neoadjuvant chemotherapy (NAC) improves OS after radical cystectomy (RC) in muscle-invasive bladder cancer (MIBC) (44). Advantage of NAC in pathological complete response (pCR) rate over immediate RC was also demonstrated in Japan; neoadjuvant M-VAC (methotrexate, doxorubicin, vinblastine and cisplatin) yielded higher pCR rate and favorable OS (45). As for adjuvant chemotherapy (AC), recent phase 3 trial (46) and large scale observational study with propensity score matching analysis (47) showed survival advantage of AC over differed treatment strategy. There has not been definitive data to support perioperative therapy in cisplatin-ineligible patients. Because it is considered that patients who achieved pCR are most benefited from NAC, pCR is widely accepted as an intermediate surrogate for survival. Recent reports showed that high-dose or dose-dense modification of M-VAC (48) or GC (49) yielded high pCR rates compared with conventional regimens. As for upper urinary tract cancer (UTUC), the POUT trial has shown advantage in DFS for AC (50) after long time lack of definitive evidence compared with bladder cancer. Although only relatively low-level evidence exists in NAC for UTUC (51), an increasing trend for the use of NAC was reported (52).
Use of ICI for perioperative treatment in MIBC has received an increasing attention in recent years. As extensively reviewed in recent literature (53–55), many phase 1 or 2 trials have been reported or are ongoing; they are testing mono-immunotherapy, combinatorial immunotherapy (e.g. anti-PD-L1 plus anti-CTLA-4) and chemo-immunotherapy combination (e.g. GC plus anti-PD-1). PURE-1 trial (NCT02736266) is an open-label single-arm phase 2 study testing the effect of three cycles of pembrolizumab (200 mg/body q3w) before RC on pCR rate (56). It was reported that pCR rate of 42% and pathological downstage (<pT2) rate of 54%. Similarly, ABACUS trial (NCT02662309) showed that two cycles (1200 mg/body, three times weekly) of preoperative atezolizumab achieved pCR rate of 31% and pathological downstage rate of 39% (57). In a phase 1b NABUCCO trial (NCT03387761), patients with MIBC received 3 mg/kg of ipilimumab (day 1), 3 mg/kg of ipilimumab plus 1 mg/kg nivolumab (day 22) and 3 mg/kg of nivolumab (day 43) followed by RC (58), resulting in pCR rate of 46% and pathological downstage rate of 58%. These proximity outcomes are comparable with those from dd-M-VAC (48) or dd-GC (49). Importantly, biomarker analyses suggested that higher response rate can be expected for those with higher PD-L1 expression. Currently, an international phase 3 study is ongoing; testing the efficacy of neoadjuvant GC plus perioperative nivolumab or neoadjuvant GC plus perioperative nivolumab and BMS-986205 (an oral IDO1 inhibitor) compared with neoadjuvant GC alone (NCT03661320). It will need several years to have primary outcomes including pCR rate and event-free survival. As for UTUC, several phase 2 trials on neoadjuvant or adjuvant immunotherapy are currently ongoing (51).
As for adjuvant therapy, CheckMate-274 (NCT02632409), a phase 3, randomized, double-blind, multicenter trial has revealed that adjuvant nivolumab prolonged DFS by ~10 months, which corresponds to a 30% reduction in the risk of disease recurrence or death, compared with placebo (median DFS 21.0 vs 10.9; HR, 0.70; 98.31% CI, 0.54–0.89; P < 0.001) in patients with muscle-invasive bladder UC or UTUC at high risk of recurrence after radical surgical resection (59). High risk of recurrence was determined as having adverse pathological findings; either ypT2–4a or ypN+ (ypT2–4 or ypN+ for UTUC) in patients treated with previous NAC or pT3–4a or pN+ (pT3–4 or pN+ for UTUC) in patients without previous NAC. On the other hand, IMVigor010 (NCT02450331), a phase 3, randomized, open-label, multicenter trial did not meet its primary endpoint of improved DFS in the atezolizumab group over observation (median DFS 19.4 vs 16.6 months; HR, 0.89; 95% CI, 0.74–1.08; P = 0.24) (60). Despite the almost identical eligibility criteria in terms of high risk for post-surgical recurrence, DFS for the patients in the control arms showed an impressive difference, whereas DFS for the patients in the treatment arms did not differ much. Although we cannot compare the outcomes between independently conducted clinical trials, careful scrutiny is needed for the conflicting results of two closely designed clinical trials.
Bladder-sparing approach for stage I-III disease
RC is the standard therapy for patients with BCG-unresponsive NMIBC or MIBC. However, quite a few patients are considered intolerable of highly invasive surgery or refuse due to postoperative impairment in quality of life. KEYNOTE-057, an open-label single-arm phase 2 trial (NCT02625961) demonstrated the efficacy of pembrolizumab (200 mg/body q3w) with CR rate of 38.8%, 80.2% of which had a CR duration of ≥6 months, an acceptable AE rate (grade 3/4, 12.6%), leading to FDA approval in January 2020 (61). Furthermore, an international phase 3 trial is currently ongoing; testing durvalumab plus BCG versus BCG alone in high-risk, BCG-naïve NMIBC (POTMAC, NCT03528694).
For MIBC, combinatorial radio-immunotherapy has attracted as a promising therapeutic approach (62). Combination of radiotherapy with immunotherapy has been reported to induce immunogenic cell death and an increase in immune markers thus leading to improved tumor control. Because there are some safety concerns for concomitant use of radiotherapy with immunotherapy, dose and timing of each modality including concomitant or sequential administration are still under optimization. As extensively reviewed in a recent literature, many early, phase 1/2 studies are currently ongoing. In Japan, an open-label, single arm phase 2 study (TSUKUBA-002, jRCT2031180060) (https://rctportal.niph.go.jp/en/detail?trial_id=jRCT2031180060) is testing atezolizumab (1200 mg/body q3w) plus radiotherapy (41.4 Gy/23 Fr to the lesser pelvis followed by additional 16.2 Gy/9 Fr to whole bladder) in patients who are not tolerable or refuse RC, with pCR as the primary endpoint.
A phase 3 trial testing pembrolizumab in combination with chemoradiotherapy (CRT) versus CRT alone in cT2-T4a MIBC (KEYNOTE-992, NCT04241185) is also ongoing. Cisplatin monotherapy (35 mg/m2 weekly), 5-fluorouracil (500 mg/m2 on days 1–5 and days 22–26) plus mitomycin C (12 mg/m2 on day 1) or gemcitabine monotherapy (27 mg/m2 twice weekly) are administered intravenously (IV) as radio-sensitizing agents during the CRT (63).
Molecular biomarkers as a predictor of ICI treatment
Treatment with ICI is characterized by the remarkable disparity between responders and non-responders. In KEYNOTE-045 trial, survival rate for pembrolizumab-treated group during the first 3 months was lower than that for the control group, suggesting the impact of initial non-responders (3,18). However, survival curves were then reversed and being separated over time thereafter, suggesting the presence of long-term responders (18). Long-term survivors in the control group are considered to be attributed to crossover use of pembrolizumab after the initial report (3). Therefore, it is particularly important to identify predictive marker for the response to ICI treatment.
Exploratory biomarker analysis from IMvigor010 trial (NCT02450331) has demonstrated that patients with muscle-invasive UC who had detectable circulating tumor DNA (ctDNA) were more likely to benefit from treatment with adjuvant atezolizumab (64). These findings suggest that liquid-base biomarker could help determine who may benefit most from ICI treatment particularly at adjuvant or maintenance settings. Indeed, another clinical trial IMvigor011 has been initiated to evaluate the efficacy and safety of adjuvant treatment with atezolizumab compared with placebo in patients with MIBC who are ctDNA positive and are at high risk for recurrence following cystectomy (NCT04660344).
There have been several clinical biomarkers associated with prognosis of the patients receiving ICI treatment including NLR, CRP, Hb, PS, metastasis site, etc. as described above. Several molecular biomarkers have been reported to act as predictive biomarkers (65). High PD-L1 expression assessed by immunohistochemistry was reportedly associated with favorable response to or survival after ICI treatment in most of clinical trials for the first-line (36,38) or perioperative (36,56) treatment settings. Because high PD-L1 expression is associated with unfavorable response to conventional chemotherapy, it may act as a predictive marker for the benefit of ICI treatment. However, there is no consensus in terms of the definition of ‘PD-L1 high’ cases including which antibody to use and how to count positive cells (41).
Another promising approach to predict clinical response to anti-PD-1/PD-L1 therapy is positron-emission tomography (PET) imaging using radioisotope-labeled antibody. Bensch et al. (66) reported the initial results from MPDL3280A-imaging-IST-UMCG study (NCT02453984) to assess the feasibility of PET imaging with zirconium-89-labeled atezolizumab (89Zr-atezolizumab) in patients with metastatic bladder cancer, non-small-cell lung cancer or triple-negative breast cancer. Uptake was generally high in tumors, particularly in bladder cancer. High heterogeneity within and among lesions, patients, and tumor types was observed in PET uptake, which was correlated with clinical responses to atezolizumab treatment. This approach is reminiscent of 177Lu-PSMA therapy in castration-resistant prostate cancer and seems highly promising. Further development is awaited.
High tumor mutation burden (TMB) (67,68) and microsatellite instability (MSI) (68,69) have been reported to be associated with better response to ICI treatment in UC. However, other studies demonstrated that TMB or MSI alone did not clearly discriminate responders from non-responders in UC and other cancers (70,71). High TMB or MSI is thought to enhance the effects of ICI through the induction of neoantigens. Recent studies suggested that true neo-antigen burden or the number of mutations actually targeted by T cells have a stronger relationship with the ICI response than TMB (65). Indeed, it was reported that the computationally predicted neoantigen burden based on single nucleotide variation data was associated with response to atezolizumab (68). Gene expression profiles provide molecular subtypes (8,9) and extent of infiltration of CD8+ (57) or M1 macrophage (72), which were also reported to be associated with ICI response (57,68,72). A recent report showed that association of these factors with TGFβ signaling pathway (68). The finding of the report is clinically important since TGFβ signaling can be a more direct, stronger predictor ICI response; moreover, it can be a therapeutic target to overcome resistance to ICI treatment. Further studies are warranted in the future.
Renal cell carcinoma
Characteristics of Renal cell carcinoma as a target of ICI therapy
ICIs have changed the approach to the treatment of advanced renal cell carcinoma (aRCC). For example, nivolumab improves the OS in patients with aRCC after anti-angiogenic therapy. In addition, nivolumab plus ipilimumab (73), pembrolizumab plus axitinib (74) and avelumab plus axitinib (75) combination therapies improve the OS and ORR when implemented as the first-line treatment for patients with aRCC. In contrast, questions have been raised regarding the efficacy of sunitinib, which served as the control agent in the randomized phase III trials of these first-line treatments. In addition, the search for biomarkers is important to more effectively select patients who may achieve maximum benefits. Unfortunately, little progress has been made in this area, but a few reports have been published. We believe that continued improvement in the emergence of anti-tumor immunity will lead to changes in the management of aRCC in the near future.
Nivolumab
Nivolumab, a monoclonal antibody that blocks PD-1, has been approved by the US FDA as a second-line treatment for patients with aRCC (4). The approval was based on the results of the Phase III CheckMate025 trial, which showed that in patients with aRCC, the OS was higher in the nivolumab group (n = 406) than in the everolimus group (n = 397) (25 months vs 19.6 months, respectively). The ORR was also higher in patients who received nivolumab for aRCC (4).
Combination of nivolumab and ipilimumab
The combination of ipilimumab, a monoclonal antibody that inhibits CTLA-4 and nivolumab has been approved by the US FDA as the first-line treatment for patients with intermediate- and poor-risk aRCC. The approval was based on the results of the Phase III CheckMate214 trial, which included 1096 patients divided into two groups, with 546 patients receiving ipilimumab and nivolumab (73). The survival rate at a median follow-up of 25.2 months was 75% in the ipilimumab plus nivolumab group and 60% in the sunitinib group. In addition, although the median OS for the ipilimumab plus nivolumab group was NR, the median OS for the sunitinib group was 26.0 months. The ORR was 42% in the ipilimumab plus nivolumab group and 27% in the sunitinib group. Median progression-free survival was 11.6 months and 8.4 months, respectively (73).
Combination of ICIs with molecular target agents
Some trials have been carried out to evaluate the different combinations of anti-angiogenic drugs (tyrosine kinase inhibitor; TKI) with ICIs in aRCC. For example, KEYNOTE-426 (74) was a randomized phase III trial in which 861 patients with previously untreated aRCC were randomly divided into two groups, one receiving pembrolizumab (200 mg) IV every 3 weeks plus axitinib (5 mg) orally twice daily (432 patients) and the other receiving sunitinib (50 mg) orally once daily (4 weeks on and 2 weeks off) (429 patients) (Table 3). The primary endpoint was OS and PFS in the ITT population, and the secondary endpoint was ORR. The median PFS was significantly longer in patients treated with the combination than in patients treated with sunitinib (15.1 months vs 11.1 months, HR 0.69, 95% CI = 0.57–0.84, P < 0.001). The most commonly reported G3 or G4 treatment-related AEs were diarrhea and hypertension in both groups. The incidence of hepatotoxicity was higher in the pembrolizumab-axitinib combination therapy group. However, there were no deaths related to hepatotoxicity. There were four treatment-related deaths in the combination group and seven deaths in the sunitinib group (74).
Phase III studies of immune checkpoint inhibitors in combination with vascular endothelial growth factor (VEGF)-targeted therapy for patients with untreated advanced RCC
| Trial name . | Ref No . | Therapy . | Population . | N . |
|---|---|---|---|---|
| KEYNOTE-426 | 74 | Pembrolizumab + axitinib | Clear cell component | 840 |
| JAVELIN Renal 101 | 75 | Avelumab + axitinib | Clear cell component | 583 |
| CLEAR/KEYNOTE-581(307) | 76 | Penbrolizumab + lenvatinib | Clear cell component | 1069 |
| CheckMate 9 ER | 77 | Nivolumab + cabozantinib | Clear cell component | 651 |
| Trial name . | Ref No . | Therapy . | Population . | N . |
|---|---|---|---|---|
| KEYNOTE-426 | 74 | Pembrolizumab + axitinib | Clear cell component | 840 |
| JAVELIN Renal 101 | 75 | Avelumab + axitinib | Clear cell component | 583 |
| CLEAR/KEYNOTE-581(307) | 76 | Penbrolizumab + lenvatinib | Clear cell component | 1069 |
| CheckMate 9 ER | 77 | Nivolumab + cabozantinib | Clear cell component | 651 |
Phase III studies of immune checkpoint inhibitors in combination with vascular endothelial growth factor (VEGF)-targeted therapy for patients with untreated advanced RCC
| Trial name . | Ref No . | Therapy . | Population . | N . |
|---|---|---|---|---|
| KEYNOTE-426 | 74 | Pembrolizumab + axitinib | Clear cell component | 840 |
| JAVELIN Renal 101 | 75 | Avelumab + axitinib | Clear cell component | 583 |
| CLEAR/KEYNOTE-581(307) | 76 | Penbrolizumab + lenvatinib | Clear cell component | 1069 |
| CheckMate 9 ER | 77 | Nivolumab + cabozantinib | Clear cell component | 651 |
| Trial name . | Ref No . | Therapy . | Population . | N . |
|---|---|---|---|---|
| KEYNOTE-426 | 74 | Pembrolizumab + axitinib | Clear cell component | 840 |
| JAVELIN Renal 101 | 75 | Avelumab + axitinib | Clear cell component | 583 |
| CLEAR/KEYNOTE-581(307) | 76 | Penbrolizumab + lenvatinib | Clear cell component | 1069 |
| CheckMate 9 ER | 77 | Nivolumab + cabozantinib | Clear cell component | 651 |
JAVELIN Renal 101 (75) was a randomized phase III trial evaluating the efficacy of avelumab (10 mg/kg, IV every 2 weeks) plus axitinib (5 mg orally twice daily) or sunitinib (50 mg orally once daily for 4 weeks on and 2 weeks off) in 886 patients with previously untreated aRCC (Table 3). OS and PFS were the two primary endpoints in patients with PD-L1 positive tumors. PFS, ORR, and safety in the overall population were secondary endpoints. A total of 560 patients (63.2%) had PD-L1-positive tumors. Among these patients, median PFS in the overall population was 13.8 months in the combination group and 7.2 months in the sunitinib group (HR 0.61, 95% CI = 0.47–0.79, P < 0.001). Median PFS was 13.8 months and 8.4 months in the overall population, respectively (HR 0.69, 95% CI = 0). In patients with PD-L1 positive tumors, ORR was 55.2% in the avelumab plus axitinib arm and 25.5% in the control arm. At the time of analysis, the OS data were immature. The most commonly reported G3 or G4 treatment-related AEs were hypertension, diarrhea, elevated alanine aminotransferase levels and palmar plantar erythrocytosis in the combination group, and hypertension, palmar plantar erythrocytosis and hematologic toxicity in the sunitinib group, with three and one treatment-related deaths, respectively (75).
Results from a Phase III clinical trial of CLEAR/KEYNOTE-581(307) were presented (Table 3) (76). The study reported that the combination of lenvatinib (20 mg orally once daily) plus pembrolizumab (200 mg every 3 weeks IV) was superior to sunitinib in terms of PFS in patients with untreated aRCC (23.9 months vs 9.2 months, HR = 0.39, 95% CI = 0.32–0.49, P < 0.001) (76). Moreover, results from a Phase III clinical trial of CheckMate9ER were presented (Table 3) (77). The study reported that the combination of cabozantinib and nivolumab was superior to sunitinib in terms of PFS in patients with untreated aRCC (16.6 months vs 8.3 months, HR = 0.51, 95% CI = 0.41–0.64, P = 0.0001). Long-term data on OS are still in its infancy and awaits further reports. These positive results support the possibility that TKIs enhances the response to ICIs (77).
How long should we use ICIs?
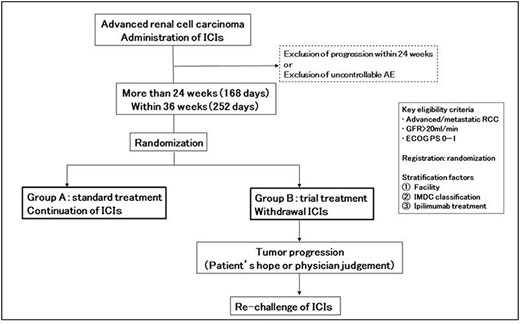
Treatment with ICIs differs from treatment with cytotoxic anticancer agents in several ways. First, in patients who obtain the treatment benefit, the tumors shrink significantly and the effect is sustained over time. Second, the types and timing of side effects are different from those of cytotoxic anticancer drugs. In particular, autoimmune disease-like side effects, which occur when immune cells react to normal tissues, are known to appear in multiple organs. Third, the effects persist after administration is discontinued; PD-1 pathway inhibitors have been proposed to induce immune memory, similar to vaccine therapy for infectious diseases, to activate and sustain immune responses against tumors (78,79). A patient with malignant melanoma was the first patient in whom the effect was sustained after discontinuation of the drug. In the same study, patients who discontinued ICIs had a sustained response to treatment, leading to the hypothesis that ICIs re-educate the immune system against the tumor, resulting in the acquisition of immune memory (80). A phase II trial is ongoing to prove this hypothesis (81). In this single-arm phase II trial, patients who achieved a reduction in tumor volume were withdrawn from treatment and given intermittent nivolumab therapy monitored by CT imaging (81). Four of five patients (80%) who discontinued treatment showed sustained anti-tumor responses and discontinued therapy. It is conceivable that such intermittent therapy could improve toxicity and tolerability without diminishing the therapeutic effect. If the withdrawal period could be extended, the cumulative toxicity and the cost of treatment could be reduced. Despite the limitations of this trial, this prospective study provides important possibility that an intermittent approach to immunotherapy is feasible in patients with aRCC. Regarding the possibility of a pause in ICI, we are now doing the Japan Clinical Oncology Group 1905 trial, where patients with renal cancer who have received an ICI for 24 weeks are randomized into two groups: those who continued ICI treatments and those who discontinued ICI treatments (Fig. 1). In the CheckMate 025 study, patients on nivolumab monotherapy after one or two prior TKI therapies had a relatively early response (6–8 weeks); however, some patients demonstrated early progression in spite of initial responses. Therefore, we have decided suspending ICI treatments only in patients who have continuous responses to the treatments for a certain period of time, rather than suspending treatments immediately after initial responses are obtained. Although there is no clear data to support when it is appropriate to stop ICI treatments, it seems more logical to stop them when tumors stopped shrinking and have reached a plateau rather than when tumors are continuously shrinking. The spider plot in the CheckMate 025 trial shows that the rate of tumor size reduction often reaches a plateau around 24 to 36 weeks after the start of nivolumab treatment (4). Tumors treated with pembrolizumab plus axitinib or avelumab plus axitinib also seem to reach a plateau around 4–6 months (74,75). Therefore, we have decided to enroll patients who have no exacerbations at 24 weeks after starting ICI therapies.
Biomarkers to predict prognosis and response to ICIs
Unfortunately, to date, no reliable biomarkers that can predict the efficacy of immunotherapy and define the specific subgroup of patients who respond best to anti-PD-1 agents have been revealed (82,83). Although PD-L1 expression is the most studied predictive biomarker for ICI outcomes, stratification of patient outcomes based on PD-L1 expression is currently met with skepticism due to limitations in sensitivity, specificity, reproducibility, and the unreliability of staining. The reasons for this are, first, that PD-L1 expression is highly heterogeneous both within tissue samples and between primary tumors and metastatic sites. Second, PD-L1 expression is dynamic and has been reported to be profoundly affected by treatments.
In a cohort of renal cell carcinoma (RCC) patients treated with nivolumab, whole exome sequencing was performed on tumor and normal tissue pairs. This study showed that there was an association between PBRM1 loss and survival (84). In other words, PBRM1 loss was identified as a biomarker of response to ICI treatment: patients with tumors with PBMR1 loss had higher ORR, longer PFS, and longer OS (85). Conversely, there was no difference in PFS, OS or ORR by PBRM1 status in patients treated with TKIs (85). Furthermore, TMB has recently been proposed as a predictive biomarker of response to ICI in patients with various cancer types. However, the percentage of TMB in RCC is 10–400 times lower than that in melanoma and NSCLC, even though the low TMB in RCC is characterized by a high absolute number of frameshift indel mutations and a high percentage of indels in total mutations (86). Frameshift indel mutation is known to be a major mechanism involved in the production of neoantigens through which T cell responses are induced (85). The association of frameshift indel mutations with response rates has been confirmed in patients with RCC treated with anti-PD-1 agents (87). Despite the limited sample size, the number of frameshift indel mutations was significantly associated with OS (87). In contrast, in patients treated with TKIs, there was no statistical difference in OS (88). Recently reported data on the clinical efficacy of pembrolizumab (an anti-PD-1 drug) in MMR-deficient patients support the hypothesis that MMR-deficient tumors are more likely to benefit from anti-PD-1 drugs than MMR-proficient tumors (88).
Conclusions and future vision
UC is a cancer for which immunotherapy is expected to be effective. ICI has been approved in chemoresistant UC as the novel standard treatment that prolongs prognosis. Approval of ICI is being expanded to other treatment settings for which no standard treatment currently exists, including maintenance therapy after effective first-line chemotherapy for metastatic urothelial carcinoma (mUC), first-line therapy for platinum-unfit mUC and salvage therapy for BCG-unresponsive NMIBC. Furthermore, there are a number of trials that explore the possibility for ICI to replace conventional standard of care, including cisplatin-based first-line chemotherapy for mUC and perioperative chemotherapy for MIBC prior to RC. There is an urgent need for predictive biomarkers for personalized clinical decision-making in the future. On the other hand, several problems need to be solved in order to optimize ICI treatment for patients with aRCC. It is yet unclear as to in which cases should we switch to different therapeutic agents, how long should ICI be used in the case of adjuvant therapy, and whether surgical resection of the primary tumor or metastases should be performed prior to ICI therapy. Furthermore, if ICI therapy is successful, then when should ICI therapy be discontinued? Further prospective trials are needed to answer these questions. It is expected that a number of prospective clinical trials will lead to the appropriate use of ICI in patients with recurrent renal cancer.
Funding
This work was supported in part by AMED under Grant Number JP20ck0106585.
Conflict of interest statement
Takashi Kobayashi received honoraria for lectures from Janssen Pharma, AstraZneca, Chugai, Bayer, MSD, Sanofi, Takeda, Astellas, Nippon Shinyaku, Nihon Kayaku, Merck, Pfizer and research funding from AstraZneca, Chugai. Ario Takeuchi has no conflicts of interest. Hiroyuki Nishiyama received honoraria for lectures from MSD, AstraZneca, and research funding from Chugai, Bayer, Ono, Takeda Astellas. Masatoshi Eto received honoraria for lectures from ONO, BMS, Pfizer, Novartis, Bayer, Takeda and research funding from ONO, Pfizer, Astellas, Takeda and Kissei.
References
Author notes
Takashi Kobayashi, Ario Takeuchi and Masatoshi Eto Contributed equally
- chemotherapy regimen
- immunologic adjuvants
- pharmaceutical adjuvants
- biological markers
- renal cell carcinoma
- carcinoma, transitional cell
- disease progression
- immunotherapy
- platinum
- urinary bladder
- protein-tyrosine kinase inhibitor
- sunitinib
- axitinib
- ipilimumab
- cabozantinib
- nivolumab
- progression-free survival
- pembrolizumab
- lenvatinib
- renal cancer
- immune checkpoint inhibitors
- avelumab


