-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroyoshi Suzuki, Toshitaka Shin, Satoshi Fukasawa, Katsuyoshi Hashine, Sumiko Kitani, Noriyuki Ohtake, Kazuhiro Shibayama, Namphuong Tran, Suneel Mundle, Karim Fizazi, Nobuaki Matsubara, Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naive prostate cancer: final subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, phase 3 study, Japanese Journal of Clinical Oncology, Volume 50, Issue 7, July 2020, Pages 810–820, https://doi.org/10.1093/jjco/hyaa030
Close - Share Icon Share
Abstract
LATITUDE was a randomized, double-blind, international and phase 3 study of abiraterone acetate plus prednisone in patients with high-risk metastatic hormone-naïve prostate cancer. In the first interim analysis of LATITUDE (clinical cutoff date: 31 October 2016), significant prolongation in overall survival and radiographic progression-free survival (co-primary endpoints) was observed when compared with placebo. The results of the Japanese subgroup analysis of LATITUDE first interim analysis were consistent with those of the overall population. In this study, overall survival and safety results from the final analysis of the Japanese subgroup of the LATITUDE study are presented (clinical cutoff date: 15 August 2018).
Abiraterone acetate (1000 mg/day) and prednisone (5 mg/day) were administered orally in the abiraterone acetate plus prednisone group, and matching placebos in the placebo group.
Of the 1199 patients included in LATITUDE, 70 constituted the Japanese subgroup (abiraterone acetate plus prednisone: n = 35, placebo: n = 35). Following a median (range) follow-up of 56.6 (2.5, 64.2) months, the median overall survival was not reached in both the treatment arms of the Japanese subgroup (hazard ratio: 0.61; 95% confidence interval: 0.27–1.42; nominal P = 0.2502). A total of 23 deaths (abiraterone acetate plus prednisone: 9 [25.7%], placebo group: 14 [40.0%]) were reported in Japanese subgroup. Grade 3/4 adverse events were reported in 24 (68.6%) and 9 (25.7%) patients in the abiraterone acetate plus prednisone and placebo groups, respectively.
In this Japanese subgroup analysis, addition of abiraterone acetate plus prednisone to androgen-deprivation therapy demonstrated favorable efficacy and safety outcomes in patients with newly diagnosed, high-risk metastatic hormone-naïve prostate cancer. Survival benefits observed in the Japanese subgroup first interim analysis were sustained long-term and were consistent with the overall population.
Introduction
Metastatic hormone-naïve prostate cancer (mHNPC) accounts for ~10% of all new prostate cancer cases in Japan, and castration using androgen-deprivation therapy (ADT) has remained the standard-of-care treatment for mHNPC (1–3). However, treatment with ADT alone has been associated with poor survival outcomes due to rapid progression to metastatic castration-resistant prostate cancer (mCRPC) (4–6). In patients with mHNPC, addition of docetaxel to ADT has demonstrated significant improvement in survival outcomes compared with ADT alone (7, 8). However, in Japan, the use of docetaxel is still unapproved for mHNPC and limited treatment options are currently available for this patient population.
Abiraterone acetate (AA) is a selective inhibitor of CYP17α hydroxylase enzyme that irreversibly inhibits intra- and extra-tumoral androgen biosynthesis. Addition of abiraterone acetate plus prednisone (AAP) to ADT has demonstrated a significant improvement in overall survival (OS) in patients with mCRPC in both chemotherapy-naïve and post-chemotherapy setting, in the global (4, 9) and Japanese populations (10–13). The addition of AAP to ADT was associated with lowering of prostate tissue androgens in men with localized prostate cancer (PC), which suggests its role in inhibiting the extra-gonadal androgen biosynthesis and preventing progression to castration resistance in mHNPC patients (14).
In the phase 3 LATITUDE study, combined treatment with AAP and ADT demonstrated a significant improvement in radiographic progression-free survival (rPFS) and OS, with manageable safety profile in patients with newly diagnosed, high-risk mHNPC in the first preplanned interim analysis (IA1) (clinical cut-off date: 31 October 2016) (15). Furthermore, treatment with AAP and ADT consistently improved overall patient-reported outcomes, including pain progression, PC symptoms, functional decline, fatigue and overall health-related quality-of-life in patients with newly diagnosed, high-risk mHNPC (16). Similarly, improved efficacy outcomes in terms of OS and rPFS, and an acceptable safety profile, were demonstrated in the Japanese subgroup analysis as well (17). In the current study, the updated long-term OS in the Japanese subgroup, from the final analysis of LATITUDE (5), is presented (clinical cutoff date: 15 August 2018).
Methods
The study protocol was approved by the local Institutional Review Board, and the study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study was consistent with International Conference on Harmonization and Good Clinical Practice guidelines, applicable regulatory requirements, and was compliant with the protocol. Written informed consent was obtained from all patients to participate in the study. The detailed methodology of this study has been published earlier (15, 17).
Patients
Men aged ≥18 years with newly diagnosed metastatic PC were eligible. Additionally, patients with high-risk mHNPC were enrolled only if they fulfilled ≥2 of the three following high-risk factors: (1) Gleason score of ≥8, (2) presence of ≥3 bone lesions by positive bone scans and (3) presence of measurable visceral metastasis on a computed tomography or an magnetic resonance imaging scan.
Patients with a medical condition that would contraindicate prednisone use or require >5 mg/day of systemic prednisone treatment or with pathological findings consistent with small cell carcinoma of the prostate, brain metastasis or with clinically significant cardiac, adrenal or liver disease were excluded.
Study design
The current subgroup analysis of a multicenter (235 sites in 34 countries), randomized (1:1), double-blind, placebo-controlled, phase 3 study (LATITUDE) (5) was performed in patients enrolled from Japan.
In the global LATITUDE study, a total of 1199 patients were randomly assigned to either the AAP group (n = 597) or the placebo group (n = 602). Before randomization (screening phase of up to 28 days), eligible patients were stratified by the presence of measurable visceral disease (yes vs no) and Eastern Cooperative Oncology Group performance status score (0/1 vs 2). The patients were subsequently randomized (1:1) to receive AA 1000 mg (4 × 250 mg tablets, orally once-daily, either ≥1 h pre-meal or ≥2 h post-meal), prednisone (5 mg, once-daily) (AAP group) and respective placebos (placebo group) (double-blind treatment phase 28 days/cycle). All patients without surgical castration received concurrent ADT (luteinizing hormone releasing hormone agonist) therapy. Patients who discontinued from the double-blind treatment phase were monitored for survival status, and for subsequent PC therapies (follow-up phase, up to 60 months or until lost to follow-up, withdrawal of consent, study termination, or death). An open-label extension phase was also planned to allow all patients to receive active AAP in the event of a positive study result at either the interim analyses or the final analysis. Following the Independent Data Monitoring Committee recommendation on 12 January 2017 to unblind the study at the time of the first IA, patients randomized to the placebo group were permitted to crossover to AAP treatment group (open-label extension phase).
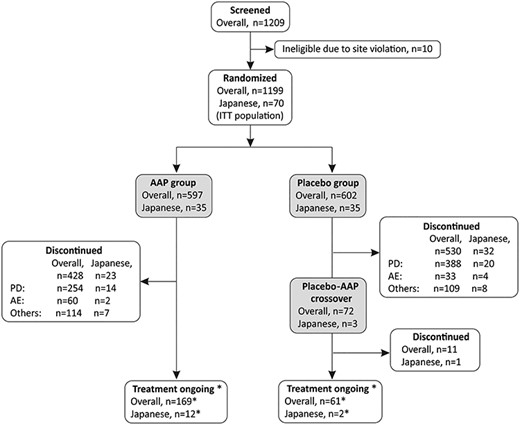
Patients disposition. Abbreviations: AAP: abiraterone acetate plus prednisone; AE: adverse event; ITT: intent-to-treat and PD: progressive disease. *The 12 patients in the AAP group and the 2 in the placebo-AAP crossover group who were ongoing were included in the final analysis as those who could continue receiving treatment but discontinued treatment after data collection for final analysis. They are therefore regarded as those discontinued treatment (consent withdrawal) in the final analysis of overall population.
Two dose reductions were permitted in the study for management of adverse events (AEs). At each dose reduction, one tablet of AA or matching placebo was reduced. Treatment continued until disease progression, withdrawal of consent, occurrence of unacceptable toxicity or death.
Efficacy endpoints
The co-primary efficacy endpoints were OS (time from randomization to death from any cause) and rPFS (time from randomization to the occurrence of radiographic progression or death from any cause), based on the prostate cancer clinical trials working group 2 (PCWG2) and response evaluation criteria in solid tumors, version 1.1 criteria.
Secondary efficacy endpoints were time to pain progression (defined as the time from randomization to first increase of 30% or more from baseline in brief pain inventory-short form [item 3]), time to prostate-specific antigen (PSA) progression (defined as the time interval from the date of randomization to the date of the PSA progression as defined in PCWG2 criteria), time to skeletal-related event (defined as the time from randomization to any one of the following skeletal-related event: clinical or pathological fracture, spinal cord compression, palliative radiation to bone or surgery to bone), time to chemotherapy initiation (defined as the time from randomization to the date of initiation of chemotherapy for PC) and time to subsequent PC therapy (defined as the time from randomization to the date of initiation of all subsequent therapy for PC, including hormonal therapy, chemotherapy, surgery and radiation therapies). Secondary PFS (defined as the time from randomization to second disease progression on subsequent treatment or death) was one of the exploratory endpoints.
Efficacy assessments
Survival status and subsequent chemotherapy for PC were assessed at regular follow-up intervals (of 4 months) up to 60 months or until death, lost to follow-up, withdrawal of consent or study termination.
Safety assessments
Safety was evaluated based on the AEs, clinical laboratory tests (hematology and serum chemistry) and vital sign measurements. The AEs, including laboratory AEs, were graded and summarized using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), Version 4.0.
Statistical methods
Statistical analyses for the overall population were described in the primary publication (5). A single final analysis of rPFS was planned after observing ~426 events and has been reported previously along with the IA of OS (15). For the co-primary endpoint of OS, the final analysis was planned when ~852 death events were observed. The overall level of significance for the study was 0.05, allocated between the two co-primary endpoints (rPFS: 0.001 and OS: 0.049). Efficacy endpoints were analyzed using the intent-to-treat population that included all the randomized patients who received ≥1 dose of study medication. The safety analysis set comprised all patients who received ≥1 dose of study medication. A non-stratified analysis was conducted for the co-primary and secondary efficacy endpoints in the Japanese subgroup. The OS was analyzed using Kaplan–Meier estimates. The hazard ratios (HR) and the associated 95% confidence interval (CI) were estimated using Cox-regression model.
| n (%) . | Japanese subgroup . | Overall population . | ||
|---|---|---|---|---|
| AAP (n = 35) . | Placebo (n = 35) . | AAP (n = 597) . | Placebo (n = 602) . | |
| Patients eligible for subsequent therapya | 21 | 33 | 394 | 517 |
| Patients with subsequent therapy for PC | 18 (85.7) | 30 (90.9) | 244 (61.9) | 355 (68.7) |
| Bicalutamide | 10 (47.6) | 23 (69.7) | 57 (14.5) | 97 (18.8) |
| Docetaxel | 10 (47.6) | 19 (57.6) | 144 (36.5) | 212 (41.0) |
| Enzalutamide | 7 (33.3) | 23 (69.7) | 57 (14.5) | 99 (19.1) |
| Cabazitaxel | 5 (23.8) | 9 (27.3) | 25 (6.3) | 50 (9.7) |
| Radium Ra 223 dichloride | 5 (23.8) | 6 (18.2) | 27 (6.9) | 44 (8.5) |
| Dexamethasone | 2 (9.5) | 6 (18.2) | 9 (2.3) | 16 (3.1) |
| Flutamide | 2 (9.5) | 7 (21.2) | 5 (1.3) | 21 (4.1) |
| Abiraterone | 1 (4.8) | 12 (36.4) | 16 (4.1) | 84 (16.2) |
| n (%) . | Japanese subgroup . | Overall population . | ||
|---|---|---|---|---|
| AAP (n = 35) . | Placebo (n = 35) . | AAP (n = 597) . | Placebo (n = 602) . | |
| Patients eligible for subsequent therapya | 21 | 33 | 394 | 517 |
| Patients with subsequent therapy for PC | 18 (85.7) | 30 (90.9) | 244 (61.9) | 355 (68.7) |
| Bicalutamide | 10 (47.6) | 23 (69.7) | 57 (14.5) | 97 (18.8) |
| Docetaxel | 10 (47.6) | 19 (57.6) | 144 (36.5) | 212 (41.0) |
| Enzalutamide | 7 (33.3) | 23 (69.7) | 57 (14.5) | 99 (19.1) |
| Cabazitaxel | 5 (23.8) | 9 (27.3) | 25 (6.3) | 50 (9.7) |
| Radium Ra 223 dichloride | 5 (23.8) | 6 (18.2) | 27 (6.9) | 44 (8.5) |
| Dexamethasone | 2 (9.5) | 6 (18.2) | 9 (2.3) | 16 (3.1) |
| Flutamide | 2 (9.5) | 7 (21.2) | 5 (1.3) | 21 (4.1) |
| Abiraterone | 1 (4.8) | 12 (36.4) | 16 (4.1) | 84 (16.2) |
Abbreviations: AAP: abiraterone acetate plus prednisone; n: number of patients and PC: prostate cancer.
Patients who discontinued from study treatment and were still alive to receive subsequent therapy by the given clinical cut-off date.
| n (%) . | Japanese subgroup . | Overall population . | ||
|---|---|---|---|---|
| AAP (n = 35) . | Placebo (n = 35) . | AAP (n = 597) . | Placebo (n = 602) . | |
| Patients eligible for subsequent therapya | 21 | 33 | 394 | 517 |
| Patients with subsequent therapy for PC | 18 (85.7) | 30 (90.9) | 244 (61.9) | 355 (68.7) |
| Bicalutamide | 10 (47.6) | 23 (69.7) | 57 (14.5) | 97 (18.8) |
| Docetaxel | 10 (47.6) | 19 (57.6) | 144 (36.5) | 212 (41.0) |
| Enzalutamide | 7 (33.3) | 23 (69.7) | 57 (14.5) | 99 (19.1) |
| Cabazitaxel | 5 (23.8) | 9 (27.3) | 25 (6.3) | 50 (9.7) |
| Radium Ra 223 dichloride | 5 (23.8) | 6 (18.2) | 27 (6.9) | 44 (8.5) |
| Dexamethasone | 2 (9.5) | 6 (18.2) | 9 (2.3) | 16 (3.1) |
| Flutamide | 2 (9.5) | 7 (21.2) | 5 (1.3) | 21 (4.1) |
| Abiraterone | 1 (4.8) | 12 (36.4) | 16 (4.1) | 84 (16.2) |
| n (%) . | Japanese subgroup . | Overall population . | ||
|---|---|---|---|---|
| AAP (n = 35) . | Placebo (n = 35) . | AAP (n = 597) . | Placebo (n = 602) . | |
| Patients eligible for subsequent therapya | 21 | 33 | 394 | 517 |
| Patients with subsequent therapy for PC | 18 (85.7) | 30 (90.9) | 244 (61.9) | 355 (68.7) |
| Bicalutamide | 10 (47.6) | 23 (69.7) | 57 (14.5) | 97 (18.8) |
| Docetaxel | 10 (47.6) | 19 (57.6) | 144 (36.5) | 212 (41.0) |
| Enzalutamide | 7 (33.3) | 23 (69.7) | 57 (14.5) | 99 (19.1) |
| Cabazitaxel | 5 (23.8) | 9 (27.3) | 25 (6.3) | 50 (9.7) |
| Radium Ra 223 dichloride | 5 (23.8) | 6 (18.2) | 27 (6.9) | 44 (8.5) |
| Dexamethasone | 2 (9.5) | 6 (18.2) | 9 (2.3) | 16 (3.1) |
| Flutamide | 2 (9.5) | 7 (21.2) | 5 (1.3) | 21 (4.1) |
| Abiraterone | 1 (4.8) | 12 (36.4) | 16 (4.1) | 84 (16.2) |
Abbreviations: AAP: abiraterone acetate plus prednisone; n: number of patients and PC: prostate cancer.
Patients who discontinued from study treatment and were still alive to receive subsequent therapy by the given clinical cut-off date.
Results
Patient disposition, characteristics and treatment exposure
Of the 1199 patients in the primary LATITUDE study, 70 patients were Japanese and were randomized to the AAP or placebo groups (n = 35 in each). Baseline and demographic characteristics of the Japanese subgroup were similar between the two groups as described previously (17); they were mostly consistent with the overall population (5).
At the time of final analysis, in the Japanese subgroup, 3/35 (8.6%) patients in the placebo group were crossed over to the AAP group (placebo-AAP group) while treatment was ongoing in 12/35 (34.3%) patients in the AAP group (Fig. 1). In this subgroup analysis, 12 patients from AAP group and the two patients from placebo-AAP group are included as those who were “treatment ongoing.” These patients could continue receiving treatment but discontinued treatment after data collection for final analysis. In the final analysis of the overall population, these patients were regarded as those who discontinued treatment (withdrew consent) (5). The most common reason for treatment discontinuation was disease progression (AAP group: 14/35 [40.0%], placebo group: 20/35 [57.1%]).
In the Japanese subgroup, 21/35 (60%) patients in the AAP group and 33/35 (94.3%) patients in the placebo group discontinued from study treatment but were still alive to receive subsequent therapy till the clinical cutoff date. Among them 18/21 (85.7%) in the AAP group and 30/33 (90.9%) in the placebo group received subsequent therapy for PC. The most commonly used subsequent PC therapy was bicalutamide and enzalutamide in the placebo group (23/33 [69.7% each]) and bicalutamide and docetaxel in the AAP group (10/21 [47.6% each]), with bicalutamide being the most commonly used subsequent therapy in both the groups (Table 1). In the corresponding overall population, 244/394 (61.9%) eligible patients in the AAP group and 355/517 (68.7%) eligible patients in the placebo group received subsequent PC therapy. The most commonly used subsequent PC therapy was docetaxel in both the AAP group (144/394 [36.5%]) and the placebo group (212/517 [41.0%]) (Table 1).
Median (range) treatment duration for the Japanese subgroup was 40.0 (1.4; 64.2) months in the AAP group, 18.4 (3.1; 44.1) months in the placebo group and 14.1 (1.1; 15.6) months in the placebo-AAP crossover group. The median (interquartile) treatment duration for the overall population in the final analysis was 25.8 (12.3; 49.0) months in the AAP group, 14.4 (7.3; 25.8) months in the placebo group and 11.9 (9.2; 12.9) months in the placebo-AAP crossover group (5).
Efficacy
At the time of final analysis (cutoff date: 15 August 2018), a total of 23 deaths (9/35 [25.7%] in the AAP group and 14/35 [40.0%] in the placebo group) were reported in the Japanese subgroup. P value was nominal. This is because subgroup analyses were not based on prespecified hypotheses. The median (range) follow-up time was 56.6 (2.5, 64.2) months, which is ~21 months of additional follow-up from the first IA that was conducted after a median follow-up time of around 35 months in the Japanese subgroup. Median OS was not reached in both AAP and placebo groups; however, the overall 5-year survival rate was 69.2% for the AAP group and 53.7% for the placebo group in the Japanese subgroup. The risk of death was 39% lower in the AAP group than in the placebo group in the Japanese subgroup (HR: 0.61; 95% CI: 0.27, 1.42; nominal P = 0.2502) (Fig. 2a), which was consistent with final analysis of the overall population (HR: 0.66; 95% CI: 0.56, 0.78; P < 0.0001) (Fig. 2b) (5). Furthermore, a favorable treatment effect of AAP on OS regardless of race and ethnicity is demonstrated in the forest plots (Fig. 2c). Previously reported results of a single final analysis of rPFS in the Japanese subgroup (17) were also consistent with the results in the overall population (15).
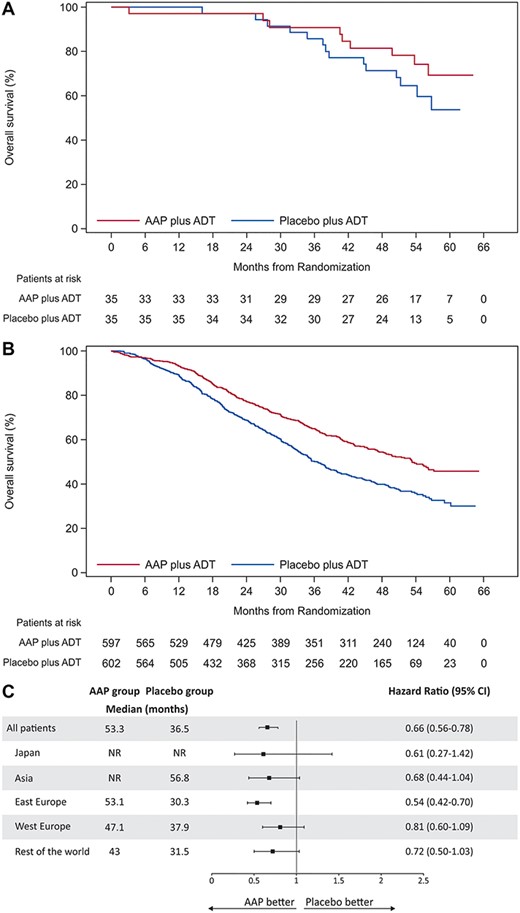
Overall survival (OS) (intent-to-treat population). (a) Kaplan–Meier curve of OS in Japanese subgroup. (b) Kaplan–Meier curve of OS in overall population. (c) Forest plots of treatment effect on OS within racial and ethnic subgroups. Abbreviations: ADT: androgen-deprivation therapy; CI: confidence interval and NR: not reached. Hazard ratio < 1 favors AAP treatment.
Treatment of Japanese patients with AAP showed improvement in the secondary endpoints of time to pain progression, PSA progression, chemotherapy initiation and subsequent PC therapy (all HR < 0.69), except skeletal-related event (HR: 1.65) (Table 2). This was consistent with the findings of the IA (all HR < 0.69; except skeletal-related event HR: 2.41) in the Japanese subgroup (17). However, in the interim (15) and final analysis (5) of overall population, improvement in all secondary endpoints was observed. Treatment with AAP improved the secondary PFS in the Japanese subgroup (HR: 0.32) (Fig. 3a, Table 2), which is consistent with the findings of the final analysis of overall population (Fig. 3b) (5).
Secondary and exploratory efficacy endpoints in the Japanese subgroup versus overall population (intent-to-treat population)
| . | Japanese subgroup . | Overall population . | . | ||||
|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Hazard ratioa (95% CI) . | AAP (n = 597) . | Placebo (n = 602) . | Hazard ratiob (95% CI) . | P value . |
| . | Median, months (95% CI) . | . | Median, months (95% CI) . | . | . | ||
| Time to secondary endpoints | |||||||
| Pain progression | NE (5.59, NE) | 10.15 (2.79, NE) | 0.68 (0.347, 1.327) | 47.4 (33.2, NE) | 16.6 (11.1, 24.0) | 0.72 (0.61–0.86) | 0.00024 |
| PSA progression | NE (20.34, NE) | 9.26 (6.51, 14.82) | 0.19 (0.097, 0.380) | 33.3 (29.4, 46.1) | 7.4 (7.2, 9.2) | 0.31 (0.27, 0.36) | <0.0001 |
| Skeletal-related event | 62.16 (29.50, NE) | NE (54.44, NE) | 1.65 (0.664, 4.109) | NE (NE, NE) | NE (NE, NE) | 0.75 (0.60–0.95) | 0.0181 |
| Chemotherapy initiation | NE (51.81, NE) | 38.90 (30.29, NE) | 0.46 (0.211, 0.983) | NE (62.6, NE) | 57.6 (38.2, NE) | 0.51 (0.41–0.63) | <0.0001 |
| Subsequent PC therapy | 54.87 (26.22, 61.93) | 18.50 (14.95, 22.97) | 0.31 (0.172, 0.573) | 54.9 (45.4, NE) | 21.2 (18.6, 23.5) | 0.45 (0.38–0.53) | <0.0001 |
| Exploratory endpoint | |||||||
| Secondary progression-free survival | NE (40.67, NE) | 30.42 (20.96, 43.96) | 0.32 (0.167, 0.620) | 53.3 (44.7, 58.1) | 30.1 (26.2, 33.4) | 0.58 (0.49, 0.68) | <0.0001 |
| . | Japanese subgroup . | Overall population . | . | ||||
|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Hazard ratioa (95% CI) . | AAP (n = 597) . | Placebo (n = 602) . | Hazard ratiob (95% CI) . | P value . |
| . | Median, months (95% CI) . | . | Median, months (95% CI) . | . | . | ||
| Time to secondary endpoints | |||||||
| Pain progression | NE (5.59, NE) | 10.15 (2.79, NE) | 0.68 (0.347, 1.327) | 47.4 (33.2, NE) | 16.6 (11.1, 24.0) | 0.72 (0.61–0.86) | 0.00024 |
| PSA progression | NE (20.34, NE) | 9.26 (6.51, 14.82) | 0.19 (0.097, 0.380) | 33.3 (29.4, 46.1) | 7.4 (7.2, 9.2) | 0.31 (0.27, 0.36) | <0.0001 |
| Skeletal-related event | 62.16 (29.50, NE) | NE (54.44, NE) | 1.65 (0.664, 4.109) | NE (NE, NE) | NE (NE, NE) | 0.75 (0.60–0.95) | 0.0181 |
| Chemotherapy initiation | NE (51.81, NE) | 38.90 (30.29, NE) | 0.46 (0.211, 0.983) | NE (62.6, NE) | 57.6 (38.2, NE) | 0.51 (0.41–0.63) | <0.0001 |
| Subsequent PC therapy | 54.87 (26.22, 61.93) | 18.50 (14.95, 22.97) | 0.31 (0.172, 0.573) | 54.9 (45.4, NE) | 21.2 (18.6, 23.5) | 0.45 (0.38–0.53) | <0.0001 |
| Exploratory endpoint | |||||||
| Secondary progression-free survival | NE (40.67, NE) | 30.42 (20.96, 43.96) | 0.32 (0.167, 0.620) | 53.3 (44.7, 58.1) | 30.1 (26.2, 33.4) | 0.58 (0.49, 0.68) | <0.0001 |
Abbreviations: CI: confidence interval; PSA: prostate specific antigen and NE: not estimable.
Hazard ratio (HR) is from unstratified proportional hazards model.
HR is from stratified proportional hazards model.
HR < 1 favors AAP.
Secondary and exploratory efficacy endpoints in the Japanese subgroup versus overall population (intent-to-treat population)
| . | Japanese subgroup . | Overall population . | . | ||||
|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Hazard ratioa (95% CI) . | AAP (n = 597) . | Placebo (n = 602) . | Hazard ratiob (95% CI) . | P value . |
| . | Median, months (95% CI) . | . | Median, months (95% CI) . | . | . | ||
| Time to secondary endpoints | |||||||
| Pain progression | NE (5.59, NE) | 10.15 (2.79, NE) | 0.68 (0.347, 1.327) | 47.4 (33.2, NE) | 16.6 (11.1, 24.0) | 0.72 (0.61–0.86) | 0.00024 |
| PSA progression | NE (20.34, NE) | 9.26 (6.51, 14.82) | 0.19 (0.097, 0.380) | 33.3 (29.4, 46.1) | 7.4 (7.2, 9.2) | 0.31 (0.27, 0.36) | <0.0001 |
| Skeletal-related event | 62.16 (29.50, NE) | NE (54.44, NE) | 1.65 (0.664, 4.109) | NE (NE, NE) | NE (NE, NE) | 0.75 (0.60–0.95) | 0.0181 |
| Chemotherapy initiation | NE (51.81, NE) | 38.90 (30.29, NE) | 0.46 (0.211, 0.983) | NE (62.6, NE) | 57.6 (38.2, NE) | 0.51 (0.41–0.63) | <0.0001 |
| Subsequent PC therapy | 54.87 (26.22, 61.93) | 18.50 (14.95, 22.97) | 0.31 (0.172, 0.573) | 54.9 (45.4, NE) | 21.2 (18.6, 23.5) | 0.45 (0.38–0.53) | <0.0001 |
| Exploratory endpoint | |||||||
| Secondary progression-free survival | NE (40.67, NE) | 30.42 (20.96, 43.96) | 0.32 (0.167, 0.620) | 53.3 (44.7, 58.1) | 30.1 (26.2, 33.4) | 0.58 (0.49, 0.68) | <0.0001 |
| . | Japanese subgroup . | Overall population . | . | ||||
|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Hazard ratioa (95% CI) . | AAP (n = 597) . | Placebo (n = 602) . | Hazard ratiob (95% CI) . | P value . |
| . | Median, months (95% CI) . | . | Median, months (95% CI) . | . | . | ||
| Time to secondary endpoints | |||||||
| Pain progression | NE (5.59, NE) | 10.15 (2.79, NE) | 0.68 (0.347, 1.327) | 47.4 (33.2, NE) | 16.6 (11.1, 24.0) | 0.72 (0.61–0.86) | 0.00024 |
| PSA progression | NE (20.34, NE) | 9.26 (6.51, 14.82) | 0.19 (0.097, 0.380) | 33.3 (29.4, 46.1) | 7.4 (7.2, 9.2) | 0.31 (0.27, 0.36) | <0.0001 |
| Skeletal-related event | 62.16 (29.50, NE) | NE (54.44, NE) | 1.65 (0.664, 4.109) | NE (NE, NE) | NE (NE, NE) | 0.75 (0.60–0.95) | 0.0181 |
| Chemotherapy initiation | NE (51.81, NE) | 38.90 (30.29, NE) | 0.46 (0.211, 0.983) | NE (62.6, NE) | 57.6 (38.2, NE) | 0.51 (0.41–0.63) | <0.0001 |
| Subsequent PC therapy | 54.87 (26.22, 61.93) | 18.50 (14.95, 22.97) | 0.31 (0.172, 0.573) | 54.9 (45.4, NE) | 21.2 (18.6, 23.5) | 0.45 (0.38–0.53) | <0.0001 |
| Exploratory endpoint | |||||||
| Secondary progression-free survival | NE (40.67, NE) | 30.42 (20.96, 43.96) | 0.32 (0.167, 0.620) | 53.3 (44.7, 58.1) | 30.1 (26.2, 33.4) | 0.58 (0.49, 0.68) | <0.0001 |
Abbreviations: CI: confidence interval; PSA: prostate specific antigen and NE: not estimable.
Hazard ratio (HR) is from unstratified proportional hazards model.
HR is from stratified proportional hazards model.
HR < 1 favors AAP.
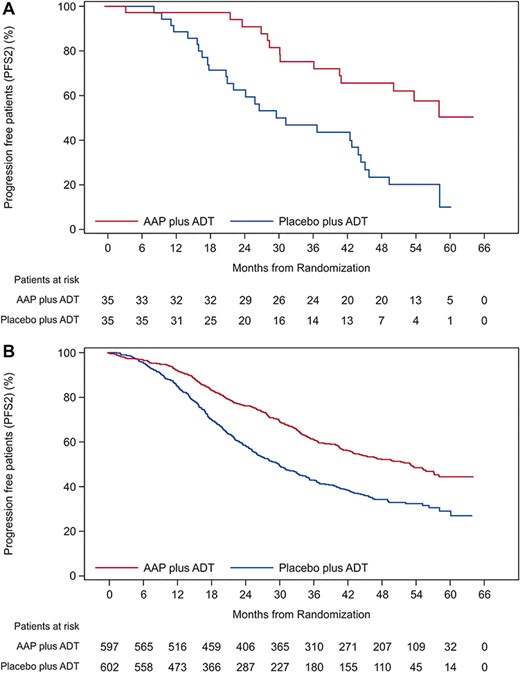
Secondary progression-free survival (PFS; intent-to-treat population). (a) Kaplan–Meier curve of secondary PFS in Japanese subgroup. (b) Kaplan–Meier curve of secondary PFS in overall population. Abbreviation: PFS2: secondary PFS.
Safety
The overall incidence of AEs was similar between the AAP group and the placebo groups in the Japanese subgroup (both 34/35 [97.1%]) as well as in the overall population (AAP group: 569/597 [95.3%], placebo group: 561/602 [93.2%]) (Supplementary Table SS1). Grade 3 or 4 events were reported in 24/35 (68.6%) patients in the AAP group and 9/35 (25.7%) patients in the placebo group. In the placebo-AAP crossover group, 1/3 (33.3%) serious AE of grade 3/4 was reported. The AEs that led to treatment discontinuation were 3/35 (8.6%) in the AAP group, 4/35 (11.4%) in the placebo group and 1/3 (33.3%) in the placebo-AAP crossover group.
AEs leading to death were reported in 2/35 (5.7%) in AAP group (Supplementary Table SS1).
Most common AEs (≥20% of patients in any group) in the Japanese subgroup (AAP vs placebo group) were hypertension (18/35 [51.4%] vs 8/35 [22.9%]), hypokalemia (15/35 [42.9%] vs “0”), nasopharyngitis (14/35 [40.0%] vs 11/35 [31.4%]), weight increased (12/35 [34.3%] in both the groups), hot flush (11/35 [31.4%] vs 12/35 [34.3%]), back pain (10/35 [28.6%] vs 7/35 [20.0%]), alanine aminotransferase (ALT) increased (9/35 [25.7%] vs 11/35 [31.4%]), aspartate aminotransferase (AST) increased (9/35 [25.7%] vs 10/35 [28.6%]) and hyperglycemia (8/35 [22.9%] vs 6/35 [17.1%]) (Table 3). In the overall population, the most common AEs that occurred in ≥20% of patients in any group (AAP vs placebo group) were hypertension (229/597 [38.4%] vs 133/602 [22.1%]), hypokalemia (143/597 [24.0%] vs 23/602 [3.8%]) and back pain (123/597 [20.6%] vs 128/602 [21.3%]).
Incidence of AEs of special interest (grade 3 or 4), mineralocorticoid-related AEs such as hypertension and hypokalemia were higher in the AAP group in comparison with the placebo group in the Japanese subgroup (Table 4). A similar trend was observed for hypertension and hypokalemia events in the final analysis of overall population. In the Japanese subgroup, incidence of grade 3 or 4 hepatotoxicity was higher in the AAP group (3/35 [8.6%]) compared with the placebo group (1/35 [2.9%]). A similar trend was observed in the final analysis of overall population (AAP group: 53/597 [8.9%]; placebo group: 21/602 [3.5%]) (Table 4).
Overall, AEs that led to deaths were reported in 2/35 (5.7%) patients in the AAP group and none in the placebo group and placebo-AAP crossover group (Supplementary Table SS1). Both the deaths in the AAP group (cerebral hemorrhage and cardiac arrest) were assessed to be treatment related. In the overall population, death due to AE was reported in 38/597 (6.4%) patients in the AAP group and 27/602 (4.5%) patients in the placebo group, with cardiac disorders being the most common cause of death (AAP group: 13/597 [2.2%] and placebo group: 6/602 [1.0%]).
Discussion
The LATITUDE is the first study to demonstrate improvement in OS as an androgen receptor-axis targeted therapy in patients with newly diagnosed, high-risk mHNPC, including Japanese patients. In the current Japanese subgroup analysis of LATITUDE, addition of AAP to ADT improved OS (co-primary endpoint) in comparison with the addition of placebo to ADT, which corroborates with the significant treatment benefit reported earlier in the IA of Japanese subgroup (17). Moreover, improvement in most of the secondary endpoints and the exploratory endpoint of secondary PFS was observed. The overall efficacy and safety findings of this study were also consistent with those reported in the final analysis of overall population (5) as well as with the interim analyses (15, 17).
In both the overall population as well as the Japanese subgroup analyses, an OS advantage was observed in the AAP group compared with placebo, despite a higher proportion of patients receiving subsequent therapy, including docetaxel, enzalutamide and AAP (5). Interestingly, more Japanese patients in the placebo group received AAP as subsequent therapy (Japanese subgroup: 36.4%, overall population: 16.2%). Moreover, the improvement in OS observed in the Japanese subgroup in our study is further highlighted when analyzed in the light of the fact that more patients in the Japanese subgroup compared with the overall population were eligible to receive subsequent therapy for PC in both the AAP and placebo groups (Japanese subgroup: AAP: 85.7%, placebo: 90.9%; overall population: AAP: 61.9%, placebo: 68.7%).
Summary of most common adverse events (AEs) in the Japanese subgroup (safety population)
| . | AAP (n = 35) . | Placebo (n = 35) . | ||||
|---|---|---|---|---|---|---|
| Any AE | 34 (97.1) | 34 (97.1) | ||||
| Grade 3–4 events | 24 (68.6) | 9 (25.7) | ||||
| Most common AEsa | ||||||
| AEs by gradeb, n (%) | Total | Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 |
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 |
| Nasopharyngitis | 14 (40.0) | 0 | 0 | 11 (31.4) | 0 | 0 |
| Weight increased | 12 (34.3) | 0 | 0 | 12 (34.3) | 2 (5.7) | 0 |
| Hot flush | 11 (31.4) | 0 | 0 | 12 (34.3) | 0 | 0 |
| Back pain | 10 (28.6) | 0 | 0 | 7 (20.0) | 0 | 0 |
| ALT increased | 9 (25.7) | 1 (2.9) | 0 | 11 (31.4) | 1 (2.9) | 0 |
| AST increased | 9 (25.7) | 1 (2.9) | 0 | 10 (28.6) | 1 (2.9) | 0 |
| Hyperglycemia | 8 (22.9) | 4 (11.4) | 0 | 6 (17.1) | 2 (5.7) | 0 |
| Rib fracture | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Insomnia | 5 (14.3) | 0 | 0 | 3 (8.6) | 0 | 0 |
| Influenza | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Constipation | 4 (11.4) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Dental caries | 4 (11.4) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 |
| Diarrhea | 4 (11.4) | 1 (2.9) | 0 | 3 (8.6) | 0 | 0 |
| Vomiting | 4 (11.4) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Haematuria | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Bone pain | 2 (5.7) | 1 (2.9) | 0 | 4 (11.4) | 0 | 0 |
| Malaise | 2 (5.7) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Oedema peripheral | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Rash | 1 (2.9) | 0 | 0 | 4 (11.4) | 0 | 0 |
| Gynaecomastia | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Anemia | 0 | 0 | 0 | 5 (14.3) | 0 | 0 |
| . | AAP (n = 35) . | Placebo (n = 35) . | ||||
|---|---|---|---|---|---|---|
| Any AE | 34 (97.1) | 34 (97.1) | ||||
| Grade 3–4 events | 24 (68.6) | 9 (25.7) | ||||
| Most common AEsa | ||||||
| AEs by gradeb, n (%) | Total | Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 |
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 |
| Nasopharyngitis | 14 (40.0) | 0 | 0 | 11 (31.4) | 0 | 0 |
| Weight increased | 12 (34.3) | 0 | 0 | 12 (34.3) | 2 (5.7) | 0 |
| Hot flush | 11 (31.4) | 0 | 0 | 12 (34.3) | 0 | 0 |
| Back pain | 10 (28.6) | 0 | 0 | 7 (20.0) | 0 | 0 |
| ALT increased | 9 (25.7) | 1 (2.9) | 0 | 11 (31.4) | 1 (2.9) | 0 |
| AST increased | 9 (25.7) | 1 (2.9) | 0 | 10 (28.6) | 1 (2.9) | 0 |
| Hyperglycemia | 8 (22.9) | 4 (11.4) | 0 | 6 (17.1) | 2 (5.7) | 0 |
| Rib fracture | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Insomnia | 5 (14.3) | 0 | 0 | 3 (8.6) | 0 | 0 |
| Influenza | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Constipation | 4 (11.4) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Dental caries | 4 (11.4) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 |
| Diarrhea | 4 (11.4) | 1 (2.9) | 0 | 3 (8.6) | 0 | 0 |
| Vomiting | 4 (11.4) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Haematuria | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Bone pain | 2 (5.7) | 1 (2.9) | 0 | 4 (11.4) | 0 | 0 |
| Malaise | 2 (5.7) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Oedema peripheral | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Rash | 1 (2.9) | 0 | 0 | 4 (11.4) | 0 | 0 |
| Gynaecomastia | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Anemia | 0 | 0 | 0 | 5 (14.3) | 0 | 0 |
Abbreviations: AE: adverse event; AST: aspartate aminotransferase and ALT: alanine aminotransferase.
Note: Table does not include grade 5 events.
AEs reported by common terminology criteria for AEs toxicity grades.
Most common AEs in ≥10% of patients in any treatment group are listed.
Summary of most common adverse events (AEs) in the Japanese subgroup (safety population)
| . | AAP (n = 35) . | Placebo (n = 35) . | ||||
|---|---|---|---|---|---|---|
| Any AE | 34 (97.1) | 34 (97.1) | ||||
| Grade 3–4 events | 24 (68.6) | 9 (25.7) | ||||
| Most common AEsa | ||||||
| AEs by gradeb, n (%) | Total | Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 |
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 |
| Nasopharyngitis | 14 (40.0) | 0 | 0 | 11 (31.4) | 0 | 0 |
| Weight increased | 12 (34.3) | 0 | 0 | 12 (34.3) | 2 (5.7) | 0 |
| Hot flush | 11 (31.4) | 0 | 0 | 12 (34.3) | 0 | 0 |
| Back pain | 10 (28.6) | 0 | 0 | 7 (20.0) | 0 | 0 |
| ALT increased | 9 (25.7) | 1 (2.9) | 0 | 11 (31.4) | 1 (2.9) | 0 |
| AST increased | 9 (25.7) | 1 (2.9) | 0 | 10 (28.6) | 1 (2.9) | 0 |
| Hyperglycemia | 8 (22.9) | 4 (11.4) | 0 | 6 (17.1) | 2 (5.7) | 0 |
| Rib fracture | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Insomnia | 5 (14.3) | 0 | 0 | 3 (8.6) | 0 | 0 |
| Influenza | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Constipation | 4 (11.4) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Dental caries | 4 (11.4) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 |
| Diarrhea | 4 (11.4) | 1 (2.9) | 0 | 3 (8.6) | 0 | 0 |
| Vomiting | 4 (11.4) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Haematuria | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Bone pain | 2 (5.7) | 1 (2.9) | 0 | 4 (11.4) | 0 | 0 |
| Malaise | 2 (5.7) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Oedema peripheral | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Rash | 1 (2.9) | 0 | 0 | 4 (11.4) | 0 | 0 |
| Gynaecomastia | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Anemia | 0 | 0 | 0 | 5 (14.3) | 0 | 0 |
| . | AAP (n = 35) . | Placebo (n = 35) . | ||||
|---|---|---|---|---|---|---|
| Any AE | 34 (97.1) | 34 (97.1) | ||||
| Grade 3–4 events | 24 (68.6) | 9 (25.7) | ||||
| Most common AEsa | ||||||
| AEs by gradeb, n (%) | Total | Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 |
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 |
| Nasopharyngitis | 14 (40.0) | 0 | 0 | 11 (31.4) | 0 | 0 |
| Weight increased | 12 (34.3) | 0 | 0 | 12 (34.3) | 2 (5.7) | 0 |
| Hot flush | 11 (31.4) | 0 | 0 | 12 (34.3) | 0 | 0 |
| Back pain | 10 (28.6) | 0 | 0 | 7 (20.0) | 0 | 0 |
| ALT increased | 9 (25.7) | 1 (2.9) | 0 | 11 (31.4) | 1 (2.9) | 0 |
| AST increased | 9 (25.7) | 1 (2.9) | 0 | 10 (28.6) | 1 (2.9) | 0 |
| Hyperglycemia | 8 (22.9) | 4 (11.4) | 0 | 6 (17.1) | 2 (5.7) | 0 |
| Rib fracture | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Insomnia | 5 (14.3) | 0 | 0 | 3 (8.6) | 0 | 0 |
| Influenza | 5 (14.3) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Constipation | 4 (11.4) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Dental caries | 4 (11.4) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 |
| Diarrhea | 4 (11.4) | 1 (2.9) | 0 | 3 (8.6) | 0 | 0 |
| Vomiting | 4 (11.4) | 0 | 0 | 1 (2.9) | 0 | 0 |
| Haematuria | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 4 (11.4) | 0 | 0 | 0 | 0 | 0 |
| Bone pain | 2 (5.7) | 1 (2.9) | 0 | 4 (11.4) | 0 | 0 |
| Malaise | 2 (5.7) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Oedema peripheral | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Rash | 1 (2.9) | 0 | 0 | 4 (11.4) | 0 | 0 |
| Gynaecomastia | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 |
| Anemia | 0 | 0 | 0 | 5 (14.3) | 0 | 0 |
Abbreviations: AE: adverse event; AST: aspartate aminotransferase and ALT: alanine aminotransferase.
Note: Table does not include grade 5 events.
AEs reported by common terminology criteria for AEs toxicity grades.
Most common AEs in ≥10% of patients in any treatment group are listed.
Grade 3 or 4 AEs of special interest in the Japanese subgroup versus the overall population (safety population)
| n (%) . | Japanese subgroup . | Overall population . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Placebo-AAP (n = 3) . | AAP (n = 597) . | Placebo (n = 602) . | Placebo-AAP (n = 72) . | ||||||||||||
| . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . |
| Mineralocorticoid-related AEs | ||||||||||||||||||
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 | 0 | 0 | 0 | 229 (38.4) | 125 (20.9) | 0 | 133 (22.1) | 59 (9.8) | 1 (0.2) | 4 (5.6) | 3 (4.2) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 143 (24.0) | 65 (10.9) | 5 (0.8) | 23 (3.8) | 9 (1.5) | 1 (0.2) | 9 (12.5) | 2 (2.8) | 0 |
| Hepatotoxicity | 13 (37.1) | 3 (8.6) | 0 | 13 (37.1) | 1 (2.9) | 0 | 1 (33.3) | 1 (33.3) | 0 | 146 (24.5) | 49 (8.2) | 4 (0.7) | 109 (18.1) | 21 (3.5) | 0 | 7 (9.7) | 3 (4.2) | 0 |
| Cataract | 3 (8.6) | 2 (5.7) | 0 | 1 (2.9) | 1 (2.9) | 0 | 0 | 0 | 0 | 15 (2.5) | 8 (1.3) | 0 | 4 (0.7) | 1 (0.2) | 0 | 0 | 0 | 0 |
| Osteoporosis including osteoporosis-related fractures | 8 (22.9) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 | 0 | 0 | 0 | 43 (7.2) | 9 (1.5) | 0 | 27 (4.5) | 13 (2.2) | 1 (0.2) | 1 (1.4) | 0 | 0 |
| Fluid retention/oedema Cardiac | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 | 0 | 0 | 0 | 81 (13.6) | 5 (0.8) | 0 | 71 (11.8) | 6 (1.0) | 0 | 3 (4.2) | 0 | 0 |
| Any | 4 (11.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95 (15.9) | 18 (3.0) | 5 (0.8) | 52 (8.6) | 6 (1.0) | 0 | 1 (1.4) | 0 | 0 |
| Arrhythmia | 3 (8.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 (8.2) | 8 (1.3) | 2 (0.3) | 24 (4.0) | 2 (0.3) | 0 | 0 | 0 | 0 |
| n (%) . | Japanese subgroup . | Overall population . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Placebo-AAP (n = 3) . | AAP (n = 597) . | Placebo (n = 602) . | Placebo-AAP (n = 72) . | ||||||||||||
| . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . |
| Mineralocorticoid-related AEs | ||||||||||||||||||
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 | 0 | 0 | 0 | 229 (38.4) | 125 (20.9) | 0 | 133 (22.1) | 59 (9.8) | 1 (0.2) | 4 (5.6) | 3 (4.2) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 143 (24.0) | 65 (10.9) | 5 (0.8) | 23 (3.8) | 9 (1.5) | 1 (0.2) | 9 (12.5) | 2 (2.8) | 0 |
| Hepatotoxicity | 13 (37.1) | 3 (8.6) | 0 | 13 (37.1) | 1 (2.9) | 0 | 1 (33.3) | 1 (33.3) | 0 | 146 (24.5) | 49 (8.2) | 4 (0.7) | 109 (18.1) | 21 (3.5) | 0 | 7 (9.7) | 3 (4.2) | 0 |
| Cataract | 3 (8.6) | 2 (5.7) | 0 | 1 (2.9) | 1 (2.9) | 0 | 0 | 0 | 0 | 15 (2.5) | 8 (1.3) | 0 | 4 (0.7) | 1 (0.2) | 0 | 0 | 0 | 0 |
| Osteoporosis including osteoporosis-related fractures | 8 (22.9) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 | 0 | 0 | 0 | 43 (7.2) | 9 (1.5) | 0 | 27 (4.5) | 13 (2.2) | 1 (0.2) | 1 (1.4) | 0 | 0 |
| Fluid retention/oedema Cardiac | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 | 0 | 0 | 0 | 81 (13.6) | 5 (0.8) | 0 | 71 (11.8) | 6 (1.0) | 0 | 3 (4.2) | 0 | 0 |
| Any | 4 (11.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95 (15.9) | 18 (3.0) | 5 (0.8) | 52 (8.6) | 6 (1.0) | 0 | 1 (1.4) | 0 | 0 |
| Arrhythmia | 3 (8.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 (8.2) | 8 (1.3) | 2 (0.3) | 24 (4.0) | 2 (0.3) | 0 | 0 | 0 | 0 |
Grade 3 or 4 AEs of special interest in the Japanese subgroup versus the overall population (safety population)
| n (%) . | Japanese subgroup . | Overall population . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Placebo-AAP (n = 3) . | AAP (n = 597) . | Placebo (n = 602) . | Placebo-AAP (n = 72) . | ||||||||||||
| . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . |
| Mineralocorticoid-related AEs | ||||||||||||||||||
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 | 0 | 0 | 0 | 229 (38.4) | 125 (20.9) | 0 | 133 (22.1) | 59 (9.8) | 1 (0.2) | 4 (5.6) | 3 (4.2) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 143 (24.0) | 65 (10.9) | 5 (0.8) | 23 (3.8) | 9 (1.5) | 1 (0.2) | 9 (12.5) | 2 (2.8) | 0 |
| Hepatotoxicity | 13 (37.1) | 3 (8.6) | 0 | 13 (37.1) | 1 (2.9) | 0 | 1 (33.3) | 1 (33.3) | 0 | 146 (24.5) | 49 (8.2) | 4 (0.7) | 109 (18.1) | 21 (3.5) | 0 | 7 (9.7) | 3 (4.2) | 0 |
| Cataract | 3 (8.6) | 2 (5.7) | 0 | 1 (2.9) | 1 (2.9) | 0 | 0 | 0 | 0 | 15 (2.5) | 8 (1.3) | 0 | 4 (0.7) | 1 (0.2) | 0 | 0 | 0 | 0 |
| Osteoporosis including osteoporosis-related fractures | 8 (22.9) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 | 0 | 0 | 0 | 43 (7.2) | 9 (1.5) | 0 | 27 (4.5) | 13 (2.2) | 1 (0.2) | 1 (1.4) | 0 | 0 |
| Fluid retention/oedema Cardiac | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 | 0 | 0 | 0 | 81 (13.6) | 5 (0.8) | 0 | 71 (11.8) | 6 (1.0) | 0 | 3 (4.2) | 0 | 0 |
| Any | 4 (11.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95 (15.9) | 18 (3.0) | 5 (0.8) | 52 (8.6) | 6 (1.0) | 0 | 1 (1.4) | 0 | 0 |
| Arrhythmia | 3 (8.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 (8.2) | 8 (1.3) | 2 (0.3) | 24 (4.0) | 2 (0.3) | 0 | 0 | 0 | 0 |
| n (%) . | Japanese subgroup . | Overall population . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AAP (n = 35) . | Placebo (n = 35) . | Placebo-AAP (n = 3) . | AAP (n = 597) . | Placebo (n = 602) . | Placebo-AAP (n = 72) . | ||||||||||||
| . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . | All grades . | Grade 3 . | Grade 4 . |
| Mineralocorticoid-related AEs | ||||||||||||||||||
| Hypertension | 18 (51.4) | 12 (34.3) | 0 | 8 (22.9) | 2 (5.7) | 0 | 0 | 0 | 0 | 229 (38.4) | 125 (20.9) | 0 | 133 (22.1) | 59 (9.8) | 1 (0.2) | 4 (5.6) | 3 (4.2) | 0 |
| Hypokalemia | 15 (42.9) | 4 (11.4) | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 143 (24.0) | 65 (10.9) | 5 (0.8) | 23 (3.8) | 9 (1.5) | 1 (0.2) | 9 (12.5) | 2 (2.8) | 0 |
| Hepatotoxicity | 13 (37.1) | 3 (8.6) | 0 | 13 (37.1) | 1 (2.9) | 0 | 1 (33.3) | 1 (33.3) | 0 | 146 (24.5) | 49 (8.2) | 4 (0.7) | 109 (18.1) | 21 (3.5) | 0 | 7 (9.7) | 3 (4.2) | 0 |
| Cataract | 3 (8.6) | 2 (5.7) | 0 | 1 (2.9) | 1 (2.9) | 0 | 0 | 0 | 0 | 15 (2.5) | 8 (1.3) | 0 | 4 (0.7) | 1 (0.2) | 0 | 0 | 0 | 0 |
| Osteoporosis including osteoporosis-related fractures | 8 (22.9) | 1 (2.9) | 0 | 2 (5.7) | 0 | 0 | 0 | 0 | 0 | 43 (7.2) | 9 (1.5) | 0 | 27 (4.5) | 13 (2.2) | 1 (0.2) | 1 (1.4) | 0 | 0 |
| Fluid retention/oedema Cardiac | 1 (2.9) | 0 | 0 | 5 (14.3) | 0 | 0 | 0 | 0 | 0 | 81 (13.6) | 5 (0.8) | 0 | 71 (11.8) | 6 (1.0) | 0 | 3 (4.2) | 0 | 0 |
| Any | 4 (11.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95 (15.9) | 18 (3.0) | 5 (0.8) | 52 (8.6) | 6 (1.0) | 0 | 1 (1.4) | 0 | 0 |
| Arrhythmia | 3 (8.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 (8.2) | 8 (1.3) | 2 (0.3) | 24 (4.0) | 2 (0.3) | 0 | 0 | 0 | 0 |
In the current Japanese subgroup study, the relative risk of death was 39% lower (HR: 0.61) in the AAP group. Although, the number of patients analyzed in the Japanese subgroup was small, these results are consistent with the results reported in the final analysis of overall global population (HR: 0.66) (5). Most of the results for secondary endpoints, including time to pain progression, PSA progression, initiation of chemotherapy and subsequent PC therapy in the Japanese subgroup showed consistency with the results in the overall population, supporting the addition of AAP to ADT. In this final analysis of the Japanese subgroup, the treatment effect of AAP, in terms of the primary endpoint (OS), most of secondary endpoints and the exploratory endpoint of secondary PFS were consistent with those of the overall population. Skeletal-related events were observed in 10 (28.6%) patients in the IA of Japanese subgroup (unpublished observation); however, no additional events were reported at the time of final analysis. This suggests that long-term administration of AAP did not increase the incidence of skeletal-related events. The observed higher risk for time to next skeletal-related event in the AAP group in comparison with the placebo group (HR: 1.65) in this study when compared with the final analysis of the overall population (HR: 0.75) can be explained by the fewer events observed in the Japanese subgroup (12 [34.3%]).
Western guidelines do not list combined androgen blockade (CAB) as a treatment option for advanced PC; however, Japanese guidelines do recommend CAB as a treatment option in mHNPC. In a recent community-based multi-institutional database study across Japan, PFS benefit was observed in all patients who underwent CAB therapy, except in the subgroup of patients with high-risk features (as assessed by the Japan Cancer of the Prostate Risk Assessment score). The results of the present study showed that combining AAP with ADT could be a promising treatment option for Japanese patients with high-risk mHNPC (18).
In this final analysis of the Japanese subgroup, the overall safety findings in the AAP group were consistent with that of the overall population (5) as well as with the interim analyses (15, 17). Mineralocorticoid-related AEs (hypertension and hypokalemia) and hepatotoxicity are well-known AEs reported with AA treatment. Prednisone/prednisolone was administrated to reduce the mineralocorticoid-related AEs. Consistent with the previous studies conducted in patients with mCRPC in the global population (4, 9, 19, 20) and in the Japanese population (11–13), hypertension was commonly observed in this study. The incidence of hypertension was higher in global LATITUDE study (5) compared with other global studies, COU-AA-301 and COU-AA-302 (4, 9), and a similar trend was observed for the LATITUDE Japanese subpopulation study when compared with other studies conducted in the Japanese population (11, 13). This difference in the incidence rates of hypertension could partly be explained by the lower daily dose of prednisone used in LATITUDE (5 mg/day) (5) compared with the other studies that used 10 mg/day (4, 9, 11, 13). The incidence of hepatotoxicity in this subgroup analysis was comparable with that of the IA of Japanese subgroup (17) and previous studies conducted in Japanese patients with mCRPC (10–13). Incidence of grade 3 or 4 AEs of cardiac disorders in this Japanese subgroup analysis was similar to that of the IA of Japanese subgroup (17), and a previous study was conducted in patients with chemotherapy-naïve mCRPC (21).
Conclusion
In this Japanese subgroup analysis of the LATITUDE study, the addition of AAP to ADT demonstrated favorable survival benefit in patients with newly diagnosed, high-risk mHNPC. The results for the primary and majority of secondary endpoints in the AAP group were favorable compared with the placebo group in the Japanese subgroup and were consistent with the final analysis of the overall population. The safety profile of the Japanese subgroup was similar to the final analysis of overall population, and no new safety signals were identified in this subgroup during this long-term analysis. Taken together, the results from this study support AAP as a potential standard therapy to improve the prognosis of Japanese patients with newly diagnosed, high-risk mHNPC.
Acknowledgments
Varkha Agrawal PhD and Akshada Deshpande PhD (both from SIRO Clinpharm Pvt. Ltd, India) provided writing assistance, and Yu Umebayashi (Janssen Pharmaceutical K.K., Japan) provided additional editorial support for this manuscript. Authors thank the study participants and their families without whom this study would not have been accomplished, and the investigational site staff for their contribution to this study.
Funding
This study was funded by Janssen Research & Development and Janssen Pharmaceutical K.K.
Conflict of interest statement
Dr Suzuki has received research grant from Astellas Pharma Inc., Bayer, Chugai, Daiichi-Sankyo, Kissei, Nippon Kayaku, Nihon Shinyaku, Pfizer, Sanofi, Taiho Pharmaceutical Co. Ltd and Takeda Pharmaceutical Co.; honoraria from Astellas Pharma Inc., AstraZeneca, Bayer, Daiichi-Sankyo, Fuji Film, Janssen Pharmaceutical K.K., MSD, Ono, Pfizer, Sanofi and Takeda Pharmaceutical Co. Dr Suzuki is a consultant and in advisory boards for Bayer, Daiichi-Sankyo, Janssen Pharmaceutical K.K., MSD, Nihon, Medi-Physics and Takeda Pharmaceutical Co. Dr Matsubara has received lecturer fees from Janssen Pharmaceutical K.K., Sanofi, AstraZeneca, MSD, Novartis Pharma K.K., Bayer and Hoffmann-La Roche Ltd; grants from Janssen Pharmaceutical K.K., Sanofi, Bayer, Hoffmann-La Roche Ltd, MSD, Eisai Co. Ltd, Taiho Pharmaceutical Co. Ltd, Eli Lilly Japan K.K. and AstraZeneca. Dr Hashine received grants from Parexel International Inc. and personal fees from Takeda Pharmaceutical Co. Dr Karim Fizazi has received personal fees from Amgen, Astellas Pharma Inc., AstraZeneca, Bayer, Clovis, Curevac, Essa, Genentech, Janssen Pharmaceutical K.K., Merck Sharp & Dohme, Orion and Sanofi.
The following authors declare a conflict of interest on the basis that they are full-time employees of Janssen Pharmaceutical K.K. of Johnson & Johnson: Sumiko Kitani, Noriyuki Ohtake and Kazuhiro Shibayama. Drs Namphuong Tran and Suneel Mundle are the employees of Janssen Research and Development, USA. All remaining authors have declared no conflicts of interest. The material presented in this article reflects authors own personal views and should not be interpreted as being representative of the views of their employers or institutions.
Author contributions
Drs Hiroyoshi Suzuki, Toshitaka Shin, Satoshi Fukasawa, Katsuyoshi Hashine, Karim Fizazi and Nobuaki Matsubara are investigators, and Kazuhiro Shibayama was the project statistician. Drs Namphuong Tran, Suneel Mundle and Karim Fizazi had primary roles in the study design; Kazuhiro Shibayama had the primary role in data analysis as the project statistician. All authors contributed to the data interpretation for the results. All authors met International Committee of Medical Journal Editors criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the study data and made the final decision about where to publish these data and approved submission to this journal.
References
Author notes
Contributed equally to this work.


