-
PDF
- Split View
-
Views
-
Cite
Cite
Razvan Galalae, Jean-Michel Hannoun-Lévi, Accelerated partial breast irradiation by brachytherapy: present evidence and future developments, Japanese Journal of Clinical Oncology, Volume 50, Issue 7, July 2020, Pages 743–752, https://doi.org/10.1093/jjco/hyaa064
Close - Share Icon Share
Abstract
Accelerated partial breast irradiation (APBI) delivers a short course of adjuvant RT after breast conserving surgery to only a limited part of the breast where the tumor was located. This procedure requires expertise, good communication, and close collaboration between specialized surgeons and attending radiation oncologists with adequate intraoperative tumor bed clip marking. However, APBI offers several intrinsic benefits when compared with whole breast irradiation (WBIR) including reduced treatment time (1 versus 4–6 weeks) and better sparing of surrounding healthy tissues. The present publication reviews the APBI level 1-evidence provided with various radiation techniques supplemented by long-term experience obtained from large multi-institutional phase II studies. Additionally, it offers an outlook on recent research with ultra-short or single-fraction APBI courses and new brachytherapy sources. Mature data from three randomized controlled trials (RCTs) clearly prove the noninferiority of APBI with ‘only two techniques—1/MIBT (multicatheter interstitial brachytherapy) (two trials) and 2/intensity modulated radiotherapy (one trial)’—in terms of equivalent local control/overall survival to the previous standard ‘conventionally fractionated WBIR’. However, MIBT-APBI techniques were superior in both toxicity and patient-reported outcomes (PROs) versus WBIR at long-term follow-up. Currently, in RCT-setting, alternative APBI techniques such as intraoperative electrons, 50-kV x-rays and three-dimensional conformal external beam radiotherapy (3D-CRT) failed to demonstrate noninferiority to conventionally fractionated WBIR. However, 3D-CRT-APBI compared noninferior to hypo-fractionated WBIR in preventing ipsilateral breast tumor recurrence (randomized RAPID-trial) but was associated with a higher rate of late radiation toxicity. Ultimately, MIBT remains the only APBI modality with noninferior survival/superior toxicity/PROs at 10-years and therefore should be prioritized over alternative methods in patients with breast cancer considered at low-risk for local recurrence according to recent international guidelines.
Background and scientific-historical development
Breast-conserving therapy concept
The composite therapy concept consisting of breast-conserving surgery (BCS) followed by whole-breast irradiation (WBIR) is appropriate and established therapy for UICC (Union International Cancer Centre) Stage I and II breast cancer, based on several mature randomized trials (1–8). The Protocol B-06 conducted from 1976 to 1984 as a phase III trial by the National Surgical Adjuvant Breast and Bowel Project (NSABP) (1,2) and the Milan randomized trial (1973–80) (3,4) demonstrated equivalence between the established radical mastectomy and—at that time—novel breast-conserving concept. In 1843, by NSABP analyzed women, the partial breast removal with resection margins free of tumor resulted in equivalent 5-year disease-free, distant-disease–free and overall survival rates versus total breast removal. Disease-free survival after segmentectomy plus postoperative radiation was, in effect, superior to total mastectomy (P = 0.04) (1). The 20-year update confirmed nonsuperiority in overall survival between breast-conserving therapy and mastectomy (2). Istituto Nazionale Tumori in Milan randomized 701 patients with breast cancer less than 2 cm in diameter and with no palpable axillary lymph nodes to Halsted radical mastectomy or to quadrantectomy with axillary dissection and postoperative radiotherapy to the ipsilateral breast with no difference between the two groups in disease-free (P = 0.54) or overall survival (P = 0.88) at 5 years (3). After a median follow-up of 20 years, the overall (P = 1.0) and breast-cancer–specific survival rates (P = 0.8) were also not statistically different in the two groups (4) and in concordance with the B-06 trial by NSABP. The phase III randomized EORTC 10801 trial (5) compared in 868 patients breast-conserving therapy (BCT) with modified radical mastectomy (MRM) in women with tumor size ≤5 cm and positive or negative axillary node disease. After a median follow-up of 22.1 years, the results did not significantly differ in terms of overall survival or time to distant metastases. In addition, further three randomized clinical trials demonstrated survival equivalence of BCT and MRM in early-stage breast cancer at 6 and 10 years, respectively (6–8). Thus, BCT is considered standard of care in stage I–II breast cancer. Historically, however, the ‘partial breast concept’ was introduced by Sir Geoffrey Keynes in 1922 when he first used ‘interstitial radium needles’ for both palliative and curative treatment intent in breast cancer achieving in ‘disease apparently confined to the breast’—for those times incredible—83.5% 3-year survival rates with or without partial removal of the tumor-bearing breast (9). This first attempt was based on the anatomic investigations of J.H. Gray at St. Bartholomew’s Hospital London which contradicted the theory—prevailing at that time—of centrifugal permeation of breast cancer and introduced the rationale of conservative treatment of breast cancer (10).
Significance of whole-breast irradiation as essential part of BCT
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) detected in a meta-analysis of 10801 women in 17 randomized trials of radiotherapy versus no radiotherapy after breast-preserving surgery (11) the absolute recurrence reduction produced by radiotherapy and the absolute recurrence risk remaining even with radiotherapy. In 7287 women with pN0 disease, the application of postoperative radiotherapy halved the 10-year risk of any first recurrence from 31 to 15.6% with an absolute reduction of 15.4% (P < 0.00001) and reduced the 15-year risk of breast cancer death by approximately a sixth from 20.5 to 17.2% with an absolute reduction of 3.3% (P = 0.005). However, the 10-year risk of a locoregional or distant recurrence varied significantly with age at entry (P < 0.00001). It was 14.2% in women of age 60–69 years, 15% (50–59 years), 20.8% (40–49 years) and 36% (<40 years), respectively. Only in older women (70+ years), the risk of any relapse dropped <10% (absolute 8.8%) indicating that age at therapy entry is an important criterion for defining risk strata in breast cancer. This publication revealed also tumor grade as another statistically significant variable for the 10-year risk of any recurrence (P < 0.00001): low (11%), intermediate (16.4%) and high grade (28.6%). Further predictors for higher risk of disease relapse were tumor size T2 versus T1 (P = 0.02) and ER-negative versus positive (P < 0.00001). The findings of this meta-analysis also introduced the ‘one-in-four rule’, suggesting that one death from breast cancer could be avoided for every four prevented local recurrences.
Concept of partial breast-volume dose escalation (boost)
The ‘modern partial breast concept’ of Veronesi (3,4) was transferred from surgery to radiation oncology by the randomized ‘Boost Versus No Boost’ EORTC 22881-10882 trial (12) which investigated in 5318 women the long-term impact of a boost radiotherapy (B-RT) dose of 16 Gy in addition to 50 Gy whole-breast irradiation (WBIR) on local control, fibrosis and overall survival for patients with stage I and II breast cancer who underwent breast-conserving therapy. Patients staged pT1–2, pN0–1 and cM0 breast cancer were stated eligible and entered the EORTC 22881-10882 study, while women with multifocal tumors, carcinoma in situ, ECOG status higher than 2, higher age than 70 years and R2 resections were considered ineligible. Technically the boost dose of 16 Gy was applied with electrons or tangential megavoltage photon beam fields in eight fractions of 2 Gy or using brachytherapy and an iridium-192 source with a dose rate of 0.5 Gy per hour. At a median follow-up of 10.8 years, the cumulative incidence of local recurrence (LR) was 10.2% for no boost versus 6.2% for the boost group (P < 0.0001). Although, the results demonstrated an absolute 10-year risk reduction for LR per age group, which was the largest in patients ≤40 years of age: 23.9–13.5% (P = 0.0014), the absolute recurrence risk remaining even with WBIR plus boost radiotherapy was highest in younger study participants: 13.5% in age group ≤40 years, 8.7% in 41–50 years, 4.9% in 51–60 years and 3.8% in patients older than 60 years. Grade 4 fibrosis was statistically significantly increased in the boost trial arm, with a 10-year rate of 4.4 versus 1.6% in the no boost group (P < 0.0001), fibrosis being scored in this trial by the treating physician and using four grades: 1 = none, 2 = minor, 3 = moderate and 4 = severe. The 10-year cumulative incidence rate of moderate to severe fibrosis (grade 3 and 4) was also higher in the boost group: 28.1 versus 13.2% (P < 0.0001). In addition to the boost RT, however, the study identified further independent predictors affecting for inferior cosmetic results: tumor location in breast lower quadrants, higher excision volume, infection and/or hematoma in the treated breast and clinical T2 stage. The authors stated that, especially in older patients, the benefit of combined WBIR plus boost RT with a higher radiation dose in terms of local control should be weighed against the increase of therapy-related fibrosis leading to an impaired cosmetic outcome. However, both effects had a relatively modest negative impact and affected only a limited subset of patients. Surprisingly, survival at 10 years did not differ, with 82% in both study arms. According to the authors, reasons for survival not being influenced might include that salvage mastectomy at the time of local recurrence was systematically performed as a life-saving intervention and was less often required in patients receiving the boost RT. In conclusion, the remaining 10-year risk of local recurrence after BCS followed by postoperative whole-breast irradiation with an additional boost dose of 16 Gy is significantly higher in younger age (<50 years) and higher tumor grade (Grade 3). These data are detailed in Table 1.
Remaining 10-year risk of local recurrence after breast-preserving surgery followed by postoperative whole-breast irradiation (WBIR) with or without boost RT
| Trial (radiotherapy) . | MFU (year)a . | Local recurrence rate (%) . | P value . | Local recurrence rate (%) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age ≤ 40 . | Age 41–50 . | Age 51–60 . | Grade 3 . | Grade 2 . | Grade 1 . | ||||
| EORTC 22881-10882 (WBI plus boost) | 10 | 13.5 | 8.7 | 4.9 | 0.0014 | 8.6 any age (9.9 < 60 years) | <5 | 0.01 | |
| EBCTCG meta-analysis (WBI) | 10 | 36b | 20.8b | 15b | 0.00001 | 28.6b | 16.4b | 11b | <0.00001 |
| Trial (radiotherapy) . | MFU (year)a . | Local recurrence rate (%) . | P value . | Local recurrence rate (%) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age ≤ 40 . | Age 41–50 . | Age 51–60 . | Grade 3 . | Grade 2 . | Grade 1 . | ||||
| EORTC 22881-10882 (WBI plus boost) | 10 | 13.5 | 8.7 | 4.9 | 0.0014 | 8.6 any age (9.9 < 60 years) | <5 | 0.01 | |
| EBCTCG meta-analysis (WBI) | 10 | 36b | 20.8b | 15b | 0.00001 | 28.6b | 16.4b | 11b | <0.00001 |
aMedian follow-up.
bAny first recurrence: locoregional or distant.
Remaining 10-year risk of local recurrence after breast-preserving surgery followed by postoperative whole-breast irradiation (WBIR) with or without boost RT
| Trial (radiotherapy) . | MFU (year)a . | Local recurrence rate (%) . | P value . | Local recurrence rate (%) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age ≤ 40 . | Age 41–50 . | Age 51–60 . | Grade 3 . | Grade 2 . | Grade 1 . | ||||
| EORTC 22881-10882 (WBI plus boost) | 10 | 13.5 | 8.7 | 4.9 | 0.0014 | 8.6 any age (9.9 < 60 years) | <5 | 0.01 | |
| EBCTCG meta-analysis (WBI) | 10 | 36b | 20.8b | 15b | 0.00001 | 28.6b | 16.4b | 11b | <0.00001 |
| Trial (radiotherapy) . | MFU (year)a . | Local recurrence rate (%) . | P value . | Local recurrence rate (%) . | P value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age ≤ 40 . | Age 41–50 . | Age 51–60 . | Grade 3 . | Grade 2 . | Grade 1 . | ||||
| EORTC 22881-10882 (WBI plus boost) | 10 | 13.5 | 8.7 | 4.9 | 0.0014 | 8.6 any age (9.9 < 60 years) | <5 | 0.01 | |
| EBCTCG meta-analysis (WBI) | 10 | 36b | 20.8b | 15b | 0.00001 | 28.6b | 16.4b | 11b | <0.00001 |
aMedian follow-up.
bAny first recurrence: locoregional or distant.
Further development of partial breast-volume concept
The evidence for the necessity of postoperative WBIR after BCS was indeed proved (11). However, is it always necessary to irradiate postoperatively the entire breast? Based on the rationale that up to 80% of the ipsilateral breast cancer recurrences occur in or in close vicinity of the tumor bed, the irradiation of the whole breast might be replaced by a more focused area of potential high-risk of recurrence avoiding much of the surrounding healthy tissues including the skin, thoracic wall, lung, heart, uninvolved ipsilateral breast and contralateral breast (13–16). This could help to reduce toxicity and improve patient reported outcomes. A series of early prospective studies evaluated the feasibility of multicatheter interstitial brachytherapy (MIBT) as partial breast irradiation following BCS and demonstrated excellent cosmesis but high local recurrence rates in cohorts of patients with unknown or positive margins. This initial experience was very significant in defining suitable inclusion criteria for partial breast irradiation after BCS by identification of risk factors for recurrence such as young age, positive margin status, larger tumors, high nuclear grade, extensive ductal carcinoma in situ, invasive lobular carcinoma, involved nodes and lymphovascular invasion (LVSI) (17–20).
Historically, the excellent prospective phase I/II experiences of Ochsner Clinic (21) and William Beamont Hospital (22) in USA pioneered the era of modern MIBT as post-BCS partial breast irradiation modality with low local recurrence rates and introduced fractionations schemes which were the base for later performed large nonrandomized/randomized trials. Vicini et al. published also a matched-pair analysis with 199 patients treated with LDR/HDR accelerated partial breast irradiation (APBI) after BCS who compared equivalent (P = 0.65) to 199 WBIR patients with 1% local tumor control in both cohorts and excellent cosmesis. According to the authors, match criteria were tumor size, lymph-node status, patient age, margins of excision, estrogen receptor status and use of adjuvant tamoxifen therapy. There was also no difference in regional failures (P = 0.54), distant metastases (P = 0.17), disease-free survival (P = 0.3), overall survival (P = 0.23) and cause-specific survival (P = 0.34). However, the contralateral breast failure rate was superior in the APBI cohort with 1 versus 4% in WBIR (P = 0.03) (23).
Nonrandomized multi-institutional APBI studies based on multicatheter interstitial brachytherapy
Based on the first pilot studies by King et al. (21) and Vicini et al. (22,23) a series of important multi-institutional prospective trials were subsequently performed. Kuske et al. (25), Arthur et al. (26) and Rabinovitch et al. (27) reported long-term results of the multi-institutional phase II trial of the Radiation Therapy Oncology Group (RTOG) 95-17 including 99 patients treated with LDR (45 Gy) or HDR (10 × 3.4 Gy, twice-daily fractionation with 6 hours interval) over 3.5–5 days. The accrual took place between 1997 and 2000 in 11 US-institutions. The treated volume included the BCS cavity with 2 cm margin peripherally but 1 cm antero-dorsal margins. The inclusion criteria corresponded to the historical single-institution studies with primary tumor ≤3 cm, involved axillary lymph nodes ≤3/no extracapsular extension and clear surgical margins (SM). The 5-year actuarial local failure rates were 4% in the entire cohort, 3% in HDR-treated patients and 6% in the LDR group with a follow-up (FU) of 7 years. The late toxicities were also higher with 18 versus 4% in the LDR-treated patients. Cosmesis was good with a good-to-excellent rate of 68%. Similarly, a European group (German-Austrian Trial) performed a multi-institutional phase II trial between 2000 and 2005 in 274 patients with early breast cancer (28). The inclusion criteria were tumor size ≤3 cm, clear SM by at least 2 mm, no lymph node metastases, age > 35 years, positive hormone receptors and histologic tumor grades 1 or 2. One hundred seventy five patients were treated with pulse dose rate (PDR) brachytherapy and 50 Gy and 99 patients with HDR by 8 × 4 Gy twice-daily fractionation (with 6 hours interval) over 4 days. The 5-year local recurrence rate was 2% after a median FU of 63 months. The late toxicity was excellent with grade ≥ 3 between 0.4 and 2.2%. The long-term cosmesis was excellent with a 90% good-to-excellent rate as well. This group identified age < 50 years as a predictor for local recurrence with an actuarial 5-year local recurrence rate free survival of 92.5 versus 98.9% (P = 0.030); however, lobular histology was not associated with compromised local control and compared equal to all other treated histologies (29). These data are summarized in Tables 2 and 3.
Initial experience of prospective phase I/II trials in multicatheter interstitial brachytherapy APBI (low dose rate—LDR or high dose rate—HDR)
| Institutions . | Ptsa . | MFU (months) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Ochsner Clinic King (19) | 50 | 75 | T ≤ 4 cm, ≤3 involved axillary lymph nodes, SM clearc | LDR/HDR 45/32 Gy (8 × 4 Gy)b, both over 4 days | 8 (locoregional) | 75 |
| WBH Vicini (20) | 60 | 20 | T ≤ 3 cm, ≥40 years, ≤3 involved axillary lymph nodes, SM >2 mm, no DCIS/lobular | LDR (I-125) 50 Gy | 0 | NRa |
| WBH Vicini (21) | 199 | 65 | See Vicini (20) | LDR (I-125)/HDR 50/32 Gy (8 × 4 Gy)b—34 Gy (10 × 3.4 Gy)b, HDR over 4 days | 1 | 99 |
| WBH Baglan (22) | 37 | 31 | See Vicini (20) | HDR 32 Gy (8 × 4 Gy)b, over 4 days | 2.6 | 100 |
| Institutions . | Ptsa . | MFU (months) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Ochsner Clinic King (19) | 50 | 75 | T ≤ 4 cm, ≤3 involved axillary lymph nodes, SM clearc | LDR/HDR 45/32 Gy (8 × 4 Gy)b, both over 4 days | 8 (locoregional) | 75 |
| WBH Vicini (20) | 60 | 20 | T ≤ 3 cm, ≥40 years, ≤3 involved axillary lymph nodes, SM >2 mm, no DCIS/lobular | LDR (I-125) 50 Gy | 0 | NRa |
| WBH Vicini (21) | 199 | 65 | See Vicini (20) | LDR (I-125)/HDR 50/32 Gy (8 × 4 Gy)b—34 Gy (10 × 3.4 Gy)b, HDR over 4 days | 1 | 99 |
| WBH Baglan (22) | 37 | 31 | See Vicini (20) | HDR 32 Gy (8 × 4 Gy)b, over 4 days | 2.6 | 100 |
aNot reported.
bTwice-daily fractionation.
cSurgical margins, WBH = William Beaumont Hospital.
Initial experience of prospective phase I/II trials in multicatheter interstitial brachytherapy APBI (low dose rate—LDR or high dose rate—HDR)
| Institutions . | Ptsa . | MFU (months) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Ochsner Clinic King (19) | 50 | 75 | T ≤ 4 cm, ≤3 involved axillary lymph nodes, SM clearc | LDR/HDR 45/32 Gy (8 × 4 Gy)b, both over 4 days | 8 (locoregional) | 75 |
| WBH Vicini (20) | 60 | 20 | T ≤ 3 cm, ≥40 years, ≤3 involved axillary lymph nodes, SM >2 mm, no DCIS/lobular | LDR (I-125) 50 Gy | 0 | NRa |
| WBH Vicini (21) | 199 | 65 | See Vicini (20) | LDR (I-125)/HDR 50/32 Gy (8 × 4 Gy)b—34 Gy (10 × 3.4 Gy)b, HDR over 4 days | 1 | 99 |
| WBH Baglan (22) | 37 | 31 | See Vicini (20) | HDR 32 Gy (8 × 4 Gy)b, over 4 days | 2.6 | 100 |
| Institutions . | Ptsa . | MFU (months) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Ochsner Clinic King (19) | 50 | 75 | T ≤ 4 cm, ≤3 involved axillary lymph nodes, SM clearc | LDR/HDR 45/32 Gy (8 × 4 Gy)b, both over 4 days | 8 (locoregional) | 75 |
| WBH Vicini (20) | 60 | 20 | T ≤ 3 cm, ≥40 years, ≤3 involved axillary lymph nodes, SM >2 mm, no DCIS/lobular | LDR (I-125) 50 Gy | 0 | NRa |
| WBH Vicini (21) | 199 | 65 | See Vicini (20) | LDR (I-125)/HDR 50/32 Gy (8 × 4 Gy)b—34 Gy (10 × 3.4 Gy)b, HDR over 4 days | 1 | 99 |
| WBH Baglan (22) | 37 | 31 | See Vicini (20) | HDR 32 Gy (8 × 4 Gy)b, over 4 days | 2.6 | 100 |
aNot reported.
bTwice-daily fractionation.
cSurgical margins, WBH = William Beaumont Hospital.
Mature prospective nonrandomized, multi-institutional phase II trials in multicatheter interstitial brachytherapy APBI (low dose rate—LDR or high dose rate—HDR or pulse dose rate—PDR)
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| RTOG 95–17 Kuske (23) Arthur (24) Rabinovitch (25) | 99 | 7 | T ≤ 3 cm, ≤3 involved axillary lymph nodes/no extracapsular extension, SM clearc | LDR/HDR 45/34 Gy (10 × 3.4 Gy)b, over 3.5–5 days | 4 entire cohort (3 HDR/6 LDR) | 68 |
| German-Austrian Strnad (26) Ott (27) | 274 | 5.1 | T ≤ 3 cm, >35 years, pN0, SM clear >2 mmc, ER+, grade 1 or 2 | PDR/HDR 50/32 Gy (8 × 4 Gy)b, both over 4 days | 2 (young age < 50 years, but not lobular histology predictor for inferior local control) | 90 |
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| RTOG 95–17 Kuske (23) Arthur (24) Rabinovitch (25) | 99 | 7 | T ≤ 3 cm, ≤3 involved axillary lymph nodes/no extracapsular extension, SM clearc | LDR/HDR 45/34 Gy (10 × 3.4 Gy)b, over 3.5–5 days | 4 entire cohort (3 HDR/6 LDR) | 68 |
| German-Austrian Strnad (26) Ott (27) | 274 | 5.1 | T ≤ 3 cm, >35 years, pN0, SM clear >2 mmc, ER+, grade 1 or 2 | PDR/HDR 50/32 Gy (8 × 4 Gy)b, both over 4 days | 2 (young age < 50 years, but not lobular histology predictor for inferior local control) | 90 |
aNot reported.
bTwice-daily fractionation.
cSurgical margins.
Mature prospective nonrandomized, multi-institutional phase II trials in multicatheter interstitial brachytherapy APBI (low dose rate—LDR or high dose rate—HDR or pulse dose rate—PDR)
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| RTOG 95–17 Kuske (23) Arthur (24) Rabinovitch (25) | 99 | 7 | T ≤ 3 cm, ≤3 involved axillary lymph nodes/no extracapsular extension, SM clearc | LDR/HDR 45/34 Gy (10 × 3.4 Gy)b, over 3.5–5 days | 4 entire cohort (3 HDR/6 LDR) | 68 |
| German-Austrian Strnad (26) Ott (27) | 274 | 5.1 | T ≤ 3 cm, >35 years, pN0, SM clear >2 mmc, ER+, grade 1 or 2 | PDR/HDR 50/32 Gy (8 × 4 Gy)b, both over 4 days | 2 (young age < 50 years, but not lobular histology predictor for inferior local control) | 90 |
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| RTOG 95–17 Kuske (23) Arthur (24) Rabinovitch (25) | 99 | 7 | T ≤ 3 cm, ≤3 involved axillary lymph nodes/no extracapsular extension, SM clearc | LDR/HDR 45/34 Gy (10 × 3.4 Gy)b, over 3.5–5 days | 4 entire cohort (3 HDR/6 LDR) | 68 |
| German-Austrian Strnad (26) Ott (27) | 274 | 5.1 | T ≤ 3 cm, >35 years, pN0, SM clear >2 mmc, ER+, grade 1 or 2 | PDR/HDR 50/32 Gy (8 × 4 Gy)b, both over 4 days | 2 (young age < 50 years, but not lobular histology predictor for inferior local control) | 90 |
aNot reported.
bTwice-daily fractionation.
cSurgical margins.
Level 1 evidence: randomized multi-institutional APBI studies based on multicatheter interstitial brachytherapy
The first randomized APBI trial performed was published in 2007 by Polgár et al. (30) at a median FU of 66 months and performed in a single institution between 1998 and 2004 in 258 selected patients with T1 N0–1mi, Grade 1–2, nonlobular breast cancer without EIC (extensive intraductal component) and clear SM (30). The patients were randomized either to WBIR study arm of 50 Gy in 25 fractions (n = 130) or partial breast irradiation study arm (n = 128). The latter was performed using two different techniques either by HDR multicatheter interstitial brachytherapy with 7 × 5.2 Gy (n = 88) or by electron beam radiotherapy with 50 Gy in 25 fractions (n = 40). The actuarial 5-year local recurrence rate was not statistically significantly different (P = 0.5) between the study arms with 3.4% for WBIR group and 4.7% for partial breast irradiation study group, respectively. There was no differentiation between the partial breast irradiation techniques in reporting. The same researcher group reported excellent 10-year outcomes with actuarial local recurrence rates of 5.1% in WBIR versus 5.9% in APBI (P = 0.77) and identical overall survival (82 versus 80%), cancer-specific survival (92 versus 94%) and disease-fee survival (84 versus 85%), respectively. The 10-year rate of excellent-good cosmetic results were in favor of the APBI group with 81 versus 63% in the control group (P < 0.01) (31). After completion of the Hungarian trial, the European GEC-ESTRO (Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology) society conducted a randomized multi-institutional trial (16 European institutions in 7 countries) between 2004 and 2009 in a total of 1184 patients with favorable early breast cancer. Inclusion criteria were age ≥ 40 years, T1–2 (<3 cm), pN0-mi, clear surgical margins of at least 2 mm, no lymphovascular invasion and unifocal/unicentric tumors. Patients were randomized either to WBIR with 50 Gy and a 10 Gy boost in 30 fractions or APBI by HDR multicatheter interstitial brachytherapy with 32 Gy (8 × 4 Gy) or 30.1 Gy (7 × 4.3 Gy) twice daily or PDR multicatheter interstitial brachytherapy with 50 Gy (0.60–0.80Gy per pulse). The 5-year rates of local control were excellent with 0.92% in WBIR versus 1.44% in APBI group (P = 0.42). In addition, disease-free survival and overall-survival rates were not statistically different as well (32). The GEC-ESTRO group published patient-reported outcomes measured with the validated European Organisation for Research and Treatment of Cancer (EORTC) core instrument QLQ-C30 and the breast cancer module QLQ-BR23 showing a statistically significant difference in breast symptom scores (P < 0.0001) in favor of the APBI patient group (33). The long-term toxicity and cosmesis results were published separately and demonstrated very favorable outcomes 93% APBI versus 92% WBIR (P = 0.62) (34). The APBI by IMBT level 1 evidence is summarized in Table 4. In addition, Fig. 1 depicts applicators and the dose distribution as an example for multicatheter interstitial brachytherapy.
Level 1 evidence by prospective randomized phase III trials in multicatheter interstitial brachytherapy APBI (low dose rate—LDR or high dose rate—HDR)
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Hungarian Trial (single institution) Polgár (28) | 130 vs. 128 (88/40) | 5.2 | T1 N0–1mi, nonlobular, no EIC, SM cleard | WBIR vs. HDR/electrons 50 Gy in 25 f vs. 36.4 Gy (7 × 5.2 Gy)c, over 4 days/50 Gy in 25 f | 3.4 in WBI vs. 4.7 in APBI (P = 0.5) | 77.6% APBI (HDR 81.2, 70% electrons) vs. 62.9% control group (52.2% telecobalt WBIR, 65.6% 6–9 MV photons (P = 0.009) |
| Hungarian Trial (single institution) Polgár (29) | 258 | 10.2 | See Polgár (28) | See Polgár (28) | 5.1 in WBI vs. 5.9 in APBI (P = 0.77) | 81% APBI vs. 63% WBIR (P < 0.01) |
| GEC-ESTRO (multi-institutional) Strnad (30) Polgár (32) | 1184 | 5 | Age ≥ 40 years, T1–2 (<3 cm), pN0-mi, SM clear >2 mmd, no LVSIb, unifocal/−centric | WBIR vs. APBI 50 + 10 boost in 30 f vs. 32 Gy (8 × 4 Gy)c, over 4 days | 0.92 in WBI vs. 1.44 in APBI (P = 0.42) | 93% APBI vs. 92% WBIR (P = 0.62) |
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Hungarian Trial (single institution) Polgár (28) | 130 vs. 128 (88/40) | 5.2 | T1 N0–1mi, nonlobular, no EIC, SM cleard | WBIR vs. HDR/electrons 50 Gy in 25 f vs. 36.4 Gy (7 × 5.2 Gy)c, over 4 days/50 Gy in 25 f | 3.4 in WBI vs. 4.7 in APBI (P = 0.5) | 77.6% APBI (HDR 81.2, 70% electrons) vs. 62.9% control group (52.2% telecobalt WBIR, 65.6% 6–9 MV photons (P = 0.009) |
| Hungarian Trial (single institution) Polgár (29) | 258 | 10.2 | See Polgár (28) | See Polgár (28) | 5.1 in WBI vs. 5.9 in APBI (P = 0.77) | 81% APBI vs. 63% WBIR (P < 0.01) |
| GEC-ESTRO (multi-institutional) Strnad (30) Polgár (32) | 1184 | 5 | Age ≥ 40 years, T1–2 (<3 cm), pN0-mi, SM clear >2 mmd, no LVSIb, unifocal/−centric | WBIR vs. APBI 50 + 10 boost in 30 f vs. 32 Gy (8 × 4 Gy)c, over 4 days | 0.92 in WBI vs. 1.44 in APBI (P = 0.42) | 93% APBI vs. 92% WBIR (P = 0.62) |
aNot reported.
bLymphovascular invasion (LVSI).
cTwice-daily fractionation.
dSurgical margins.
Level 1 evidence by prospective randomized phase III trials in multicatheter interstitial brachytherapy APBI (low dose rate—LDR or high dose rate—HDR)
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Hungarian Trial (single institution) Polgár (28) | 130 vs. 128 (88/40) | 5.2 | T1 N0–1mi, nonlobular, no EIC, SM cleard | WBIR vs. HDR/electrons 50 Gy in 25 f vs. 36.4 Gy (7 × 5.2 Gy)c, over 4 days/50 Gy in 25 f | 3.4 in WBI vs. 4.7 in APBI (P = 0.5) | 77.6% APBI (HDR 81.2, 70% electrons) vs. 62.9% control group (52.2% telecobalt WBIR, 65.6% 6–9 MV photons (P = 0.009) |
| Hungarian Trial (single institution) Polgár (29) | 258 | 10.2 | See Polgár (28) | See Polgár (28) | 5.1 in WBI vs. 5.9 in APBI (P = 0.77) | 81% APBI vs. 63% WBIR (P < 0.01) |
| GEC-ESTRO (multi-institutional) Strnad (30) Polgár (32) | 1184 | 5 | Age ≥ 40 years, T1–2 (<3 cm), pN0-mi, SM clear >2 mmd, no LVSIb, unifocal/−centric | WBIR vs. APBI 50 + 10 boost in 30 f vs. 32 Gy (8 × 4 Gy)c, over 4 days | 0.92 in WBI vs. 1.44 in APBI (P = 0.42) | 93% APBI vs. 92% WBIR (P = 0.62) |
| Trial . | Ptsa . | MFU (years) . | Selection criteria . | MBT type/fractionation . | LR rate (%) . | Excel/good cosmesis (%) . |
|---|---|---|---|---|---|---|
| Hungarian Trial (single institution) Polgár (28) | 130 vs. 128 (88/40) | 5.2 | T1 N0–1mi, nonlobular, no EIC, SM cleard | WBIR vs. HDR/electrons 50 Gy in 25 f vs. 36.4 Gy (7 × 5.2 Gy)c, over 4 days/50 Gy in 25 f | 3.4 in WBI vs. 4.7 in APBI (P = 0.5) | 77.6% APBI (HDR 81.2, 70% electrons) vs. 62.9% control group (52.2% telecobalt WBIR, 65.6% 6–9 MV photons (P = 0.009) |
| Hungarian Trial (single institution) Polgár (29) | 258 | 10.2 | See Polgár (28) | See Polgár (28) | 5.1 in WBI vs. 5.9 in APBI (P = 0.77) | 81% APBI vs. 63% WBIR (P < 0.01) |
| GEC-ESTRO (multi-institutional) Strnad (30) Polgár (32) | 1184 | 5 | Age ≥ 40 years, T1–2 (<3 cm), pN0-mi, SM clear >2 mmd, no LVSIb, unifocal/−centric | WBIR vs. APBI 50 + 10 boost in 30 f vs. 32 Gy (8 × 4 Gy)c, over 4 days | 0.92 in WBI vs. 1.44 in APBI (P = 0.42) | 93% APBI vs. 92% WBIR (P = 0.62) |
aNot reported.
bLymphovascular invasion (LVSI).
cTwice-daily fractionation.
dSurgical margins.
| Expert group/organization (year) . | Age . | Histology/LVSIb . | Tumor size . | N status . | Estrogen receptor status . | Surgical margins . |
|---|---|---|---|---|---|---|
| ABS Shah (53) 2018 | ≥45 years | All invasive histologies and ductal carcinoma in situ/LVSI negativeb | ≤3 cm | Node negative | Positive/negative | Negative surgical margins |
| RCR (57) 2016 | ≥50 years | Nonlobular invasive breast cancer, grade 1–2, HER2 negative | ≤3 cm | Node negative | Positive | Negative surgical margins ≥2 mm |
| ASTRO Correa (55) 2017 (updated from 2009) | ≥50 years (suitable) | All invasive histologies and low-risk ductal carcinoma in situc | pTis or pT1–2 (≤3 cm), clinically unifocal | Node negative | Positive | Negative surgical margins ≥2 mm |
| GEC-ESTRO Polgár (35) 2010 Strnad (56) 2018 | ≥50 years | Nonlobular invasive breast cancer; no EICa/LVSIb negative | pT1–2 (≤3 cm), unifocal/unicentric | Node negative (pN0) | Positive | Negative surgical margins ≥2 mm |
| Expert group/organization (year) . | Age . | Histology/LVSIb . | Tumor size . | N status . | Estrogen receptor status . | Surgical margins . |
|---|---|---|---|---|---|---|
| ABS Shah (53) 2018 | ≥45 years | All invasive histologies and ductal carcinoma in situ/LVSI negativeb | ≤3 cm | Node negative | Positive/negative | Negative surgical margins |
| RCR (57) 2016 | ≥50 years | Nonlobular invasive breast cancer, grade 1–2, HER2 negative | ≤3 cm | Node negative | Positive | Negative surgical margins ≥2 mm |
| ASTRO Correa (55) 2017 (updated from 2009) | ≥50 years (suitable) | All invasive histologies and low-risk ductal carcinoma in situc | pTis or pT1–2 (≤3 cm), clinically unifocal | Node negative | Positive | Negative surgical margins ≥2 mm |
| GEC-ESTRO Polgár (35) 2010 Strnad (56) 2018 | ≥50 years | Nonlobular invasive breast cancer; no EICa/LVSIb negative | pT1–2 (≤3 cm), unifocal/unicentric | Node negative (pN0) | Positive | Negative surgical margins ≥2 mm |
aExtensive intraductal component (EIC).
bLymphovascular invasion (LVSI).
cScreen-detected and low-to-intermediate nuclear grade and size ≤2.5 cm and surgical margins negative at ≥3 mm.
| Expert group/organization (year) . | Age . | Histology/LVSIb . | Tumor size . | N status . | Estrogen receptor status . | Surgical margins . |
|---|---|---|---|---|---|---|
| ABS Shah (53) 2018 | ≥45 years | All invasive histologies and ductal carcinoma in situ/LVSI negativeb | ≤3 cm | Node negative | Positive/negative | Negative surgical margins |
| RCR (57) 2016 | ≥50 years | Nonlobular invasive breast cancer, grade 1–2, HER2 negative | ≤3 cm | Node negative | Positive | Negative surgical margins ≥2 mm |
| ASTRO Correa (55) 2017 (updated from 2009) | ≥50 years (suitable) | All invasive histologies and low-risk ductal carcinoma in situc | pTis or pT1–2 (≤3 cm), clinically unifocal | Node negative | Positive | Negative surgical margins ≥2 mm |
| GEC-ESTRO Polgár (35) 2010 Strnad (56) 2018 | ≥50 years | Nonlobular invasive breast cancer; no EICa/LVSIb negative | pT1–2 (≤3 cm), unifocal/unicentric | Node negative (pN0) | Positive | Negative surgical margins ≥2 mm |
| Expert group/organization (year) . | Age . | Histology/LVSIb . | Tumor size . | N status . | Estrogen receptor status . | Surgical margins . |
|---|---|---|---|---|---|---|
| ABS Shah (53) 2018 | ≥45 years | All invasive histologies and ductal carcinoma in situ/LVSI negativeb | ≤3 cm | Node negative | Positive/negative | Negative surgical margins |
| RCR (57) 2016 | ≥50 years | Nonlobular invasive breast cancer, grade 1–2, HER2 negative | ≤3 cm | Node negative | Positive | Negative surgical margins ≥2 mm |
| ASTRO Correa (55) 2017 (updated from 2009) | ≥50 years (suitable) | All invasive histologies and low-risk ductal carcinoma in situc | pTis or pT1–2 (≤3 cm), clinically unifocal | Node negative | Positive | Negative surgical margins ≥2 mm |
| GEC-ESTRO Polgár (35) 2010 Strnad (56) 2018 | ≥50 years | Nonlobular invasive breast cancer; no EICa/LVSIb negative | pT1–2 (≤3 cm), unifocal/unicentric | Node negative (pN0) | Positive | Negative surgical margins ≥2 mm |
aExtensive intraductal component (EIC).
bLymphovascular invasion (LVSI).
cScreen-detected and low-to-intermediate nuclear grade and size ≤2.5 cm and surgical margins negative at ≥3 mm.
Multi-institutional APBI studies based on balloon-based brachytherapy applicators
The Mammo Site® (Hologic Inc., Marlbourough, MA) intracavitary breast brachytherapy applicator was developed as a single-channel balloon-based alternative to multicatheter interstitial brachytherapy (MIBT) and was approved by the FDA in 2002 in order to simplify APBI administration and make it more independent on center expertise in MIBT. The deflated balloon applicator is intraoperatively introduced into the BCS cavity or as a second procedure postoperatively. A large registry trial was conducted in 1449 patients and demonstrated an actuarial 5-year local failure rate of 3.8% at a FU of 63 months. The treatment schedule was 34 Gy in 10 fractions (35). The rate of good-to-excellent cosmesis was 90.6% at 84 months. However, the rates of fat necrosis and infections were relatively high with 2.5 and 9.6%. Wallace et al. published results of a prospective phase I/II study in 45 patients treated with balloon brachytherapy with 28 Gy in 4 fractions of 7 Gy. At a median FU of 11.4 months, a relatively high toxicity with fat necrosis in four cases (2 symptomatic) and rib fractures in 4% (2 cases) was observed reflecting the inferior dose distribution of balloon-based applicator brachytherapy (BAB) according the surrounding organs at risk (OARs) in comparison with multicatheter interstitial brachytherapy (36).
These intrinsic technical limitations of the balloon applicators with only one delivery channel may be diminished by the further development of balloon-based applicators with multicatheter design such as the SAVI® (Strut Assisted Volume Implant), which was FDA-approved in 2006. The SAVI applicators include a bundle of flexible, tiny catheters that can be expanded uniformly to conform better to the size and shape of tumor cavity and may compare superior according skin sparing to balloon-based single lumen applicators (37). A large multi-institutional phase IV registry study used the Contura® brachytherapy applicator—another multi-lumen balloon breast brachytherapy catheter—in order to apply APBI with 34 Gy in 10 fractions twice daily and registered 342 patients in 23 institutions. At median FU of 36 months, the 3-year local recurrence-free survival was 97.8%, and the good-to-excellent cosmesis rate was 88%. However, also in this cohort, the infection rate was high with 8.5% and symptomatic seroma rate was 4.4%. This study could also document a positive effect on cosmesis and toxicity at high-volume centers with similar 3-year local recurrence-free survival of 98.1% but lower infection/seroma rates (2.9/1.9%) (38). The summarized results of single- or multi-lumen balloon-based APBI, however, document a higher toxicity rate in comparison with multicatheter interstitial brachytherapy techniques but with advantages of multi-lumen over single-lumen balloon catheters.
Images of balloon-based breast applicators may be found on https://www.hologic.com/hologic-products/breast-skeletal/breast-brachytherapy for Mammo Site and Contura applicators and https://www.ciannamedical.com/savi/ for SAVI applicator, respectively.
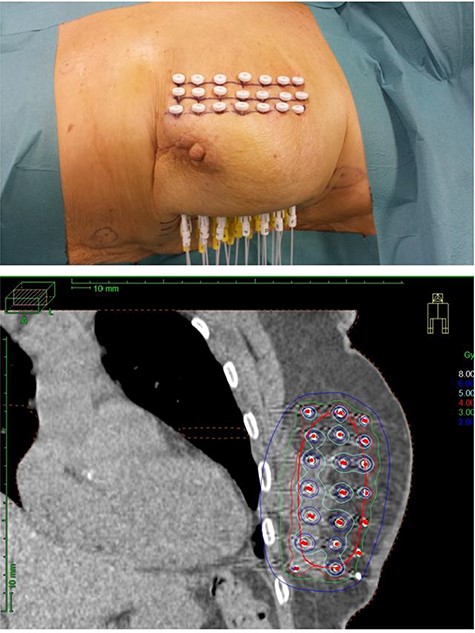
Multicatheter interstitial brachytherapy: implanted applicators (upper picture) and dose distribution (lower picture).
Latest/future developments: very-accelerated partial breast irradiation (vAPBI) and single-fraction APBI studies
Hannoun-Lévi et al. (39) have published 5-year results in 26 elderly patients (≥70 years, mean age 77 years) enrolled in a prospective phase II trial in Nice/France using a single fraction of 16 Gy of a vAPBI after breast conserving surgery. The 5-year rates of local recurrence-free survival, metastasis-free survival, cancer-specific survival and overall survival were 100%, 95.5%, 100% and 88.5%, respectively. Late toxicity was only grade 1 and 2, and the cosmesis good-to-excellent rate was 100% (19% good and 81% excellent). This was an update of the very promising 3-year interim outcomes in 48 patients with no grade 3 or higher toxicity observed and a 3-year local recurrence-free survival of 100% (40). Latorre et al. (41) could confirm the good feasibility of vAPBI using a single dose of 18 Gy HDR interstitial multicatheter brachytherapy in 20 patients with low-risk invasive breast carcinoma and ductal carcinoma in situ (≤3 cm, pN0 and M0) aged 50 years or older (median age 63.5 years). The 2-year local control was also 100% and the good-to-excellent cosmesis rate was 80% at 24 months of follow-up. There was no grade 3 or higher toxicity observed. Nose et al. (42) published the results of a prospective multi-institutional Japanese trial utilizing a short schedule of APBI by interstitial multicatheter HDR brachytherapy with 36 Gy in six fractions in 46 women with low-risk breast cancer (positive hormone receptors and tumors ≤3 cm, pN0M0). At median follow-up of 26 months, the 2-year local recurrence-free survival was 100%. The grade 3 toxicity was very limited with 4% fibrosis and 2% pain. The 2-year good-to-excellent cosmesis rate was 81%. Wilkinson et al. (43) from the William Beaumont and 21st century Oncology joint group reported 6-year outcomes of a 2-day schedule of APBI balloon-based brachytherapy with 28 Gy in four fractions of 7 Gy twice daily in the setting of a prospective phase I/II trial. The 6-year actuarial local recurrence-free survival, disease-free survival, cause-specific survival and overall survival were 100%, 96%, 100% and 93%, respectively. Five patients developed a fat necrosis and two experienced rib fractured which were managed conservatively.
Khan et al. (44) reported early results of a 4-fraction schedule of 28 Gy APBI using Contura multi-lumen balloon (MLB) applicator in 30 eligible women with age ≥ 50 years, unifocal invasive or in situ tumors ≤3.0 cm after breast conserving surgery with negative margins, and with negative lymph nodes and positive hormone receptors, respectively. This short-course APBI was dosimetrically feasible and resulted in an acceptable and MLB applicator typical toxicity with each two cases of fat necrosis and symptomatic seromas.
In summary, short courses and even single-fraction schedules of vAPBI appear to be feasible and associated with acceptable toxicity, cosmesis and excellent local control rates. However, longer-term outcomes are still sparse for vAPBI, and further trials, possibly multi-institutional studies, are required.
New brachytherapy sources
In all forms of APBI by brachytherapy, the predominantly used source is Ir-192 in form of PDR (28,29) or HDR brachytherapy (30–32,34). In minority also LDR with I-125 has been used (22,23). However, a new source for interstitial brachytherapy is available—the diffusing alpha-emitters radiation therapy (DaRT) brachytherapy source which was pre-clinically tested in a series of experimental tumors and could demonstrate a release of tumor antigens after in situ tumor destruction with strong stimulation of anti-tumor immune response (45,46). Mice with DA3 metastatic breast adenocarcinoma were treated with DaRT brachytherapy combined with myeloid-derived suppressor cell (MDSC) inhibitor (sildenafil), regulatory T cell (Tregs) inhibitor (low dose cyclophosphamide) and the immunostimulant, CpG (unmethylated cytosine-guanine dinucleotides). The combination of all therapies induced rejection of primary tumors and elimination of lung metastases (45). Popovtzer et al. (47) published the first-in-human utilization of DaRT-based brachytherapy in the setting of a multicenter prospective trial in 28 patients with 31 lesions of locally advanced and recurrent squamous cancers (SCC) of the skin and head and neck; 42% of the patients had a recurrent, previously irradiated tumor. A complete response (CR) to the Ra-224 DaRT treatment was observed in 22 patients (78.6%), and 6 patients (21.4%) developed a partial response with >30% tumor reduction. The 1-year overall survival rate was 75% in all patients, and 93% in complete responders, respectively. The 1-year local progression-free survival was 44% overall, and 60% (95% CI, 28.61–81.35%) for complete responders, respectively. The feasibility of DaRT implantation and the toxicity of the treatment were excellent with only mild local pain and erythema followed by grade 2 skin ulcerations, which resolved in the majority of patients within 3–5 weeks. One report also documented clinical evidence of abscopal effects (AE) in a patient with cutaneous squamous cell carcinoma and multiple synchronous lesions of the skin (confirmed by biopsy) who was treated by DaRT brachytherapy. The locally treated lesion was in complete remission after 1 year, and spontaneous regression of the untreated distant synchronous skin lesions could be observed (48). These experiments and the preliminary clinical data in humans suggest that DaRT-based brachytherapy as monotherapy induces a systemic antitumor immune response following tumor ablation by the highly destructive alpha radiation, which can be boosted by immunomodulatory strategies. This new kind of alpha-based radiation brachytherapy sources may serve in future clinical studies as an induction therapy promoting long-lasting abscopal effects especially in patients with oligometastatic disease or at high risk for distant metastases in various tumor entities including breast cancer. In this context, alpha radiation-based brachytherapy may transform the tumor into an in situ vaccine, which immunotherapy modulates into a larger immune response (49).
Discussion
Level 1 evidence has been achieved for the equivalence of whole-breast irradiation (WBIR) and APBI by multicatheter interstitial brachytherapy (MIBT APBI) in terms of local control (0.9% WBIR versus 1.4% MIBT APBI; P = 0.42) and overall survival (95.6% WBIR versus 97.3% APBI; P = 0.11) at 5 years (30–32). However, MIBT APBI compared statistically significant superior in terms of skin toxicity, breast pain and patient-reported outcomes to WBIR (33,34). Only APBI by intensity-modulated radiotherapy (IMRT) was proved, next to MIBT APBI, noninferior to conventionally fractionated WBIR in the setting of a prospective randomized controlled (PRC) trial reaching level 1 evidence as well. In the Florence study, 520 patients were randomized either to WBIR (n = 260) with 50 Gy in 25 fractions followed by a boost of 10 Gy in five fractions or to APBI by IMRT with 30 Gy in five daily fractions. The 5-year local recurrence rate was 1.5% in both groups (P = 0.86), and the 5-year overall survival rate was 96.6% in the WBIR cohort and 99.4% in the APBI arm (0.057), respectively. Acute toxicity (P = 0.0001), late toxicity (P = 0.004) and cosmesis rates (P = 0.045) were significantly better in APBI arm versus WBIR cohort (50). Other APBI techniques such as intraoperative electron radiation (IOERT) in ELIOT trial and 50-kV x-rays in TARGIT trial were tested in phase III studies but compared inferior to WBIR with 5-year local recurrence rates of 4.4 versus 0.4% (P = 0.0001) and 3.3 versus 1.3% (P = 0,042) (51). In addition, Olivotto et al. published inferior interim 3-year results of APBI by three-dimensional conformal external beam radiotherapy (3D-CRT) to WBIR with increased late toxicity (P < 0.001) and inferior cosmesis (P = 0.0022) as rated by patients (52). Lastly, an additional randomized phase 3 trial (NSABP B-39/RTOG 0413) did not meet the criteria to detect treatment equivalence between conventionally fractionated WBIR and non-MIBT partial breast irradiation at 10 years in terms of ipsilateral breast-tumor recurrence (IBTR) (53) and in the randomized RAPID trial APBI by 3D conformal radiotherapy compared noninferior to hypo-fractionated whole breast irradiation (WBIR) in preventing IBTR (3.0 versus 2.8%) (54). In the RAPID trial, however, late radiation toxicity (grade ≥ 2) was more common in patients treated with APBI (32 versus 13% and P < 0.0001) (54). The NSABP B-39/RTOG 0413 Trial assigned 4216 women with early-stage breast cancer between 2005 and 2013 either to the whole-breast irradiation with 50 Gy in 25 fractions (n = 2109) or to APBI in 10 fractions over 5 days (n = 2107), which was delivered with 34 Gy by intracavitary brachytherapy, MammoSite or other single-entry intracavitary device or 3-dimensional conformal APBI and 38.5 Gy and detected 3 versus 4% IBRT rates (HR 1.22, 90% CI 0.94–1.58) (53).
Based on the achieved level 1 evidence, several modern guidelines were released by expert groups/societies with a clear recommendation in favor of multicatheter interstitial brachytherapy and intensity-modulated radiotherapy APBI for appropriate candidates considered at low risk for ipsilateral breast tumor recurrence (35,55–60). Those recommendations are detailed in Table 5.
The Breast Cancer Working Group of Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO) generated practical/technical guidelines for multi-catheter image-guided brachytherapy in the conservative management of breast cancer patients used for either APBI or as a boost procedure (58). Shaitelman et al. (60), could also document a statistically significant increase in the use of APBI—mainly by brachytherapy—reflecting the wide acceptance of this technique.
Increasing evidence supports future developments toward further exploration of vAPBI/single-fraction APBI (39–44) and utilization of alpha radiation based brachytherapy in combination with immunotherapy (45–49).
Conclusion
Mature data from three randomized trials clearly prove the noninferiority of APBI with ‘only two techniques—1/multicatheter interstitial brachytherapy (two trials) and 2/intensity modulated radiotherapy (one trial)’—in terms of equivalent local control and overall survival to the previous standard ‘conventionally fractionated whole-breast irradiation’. However, ‘multicatheter interstitial brachytherapy’ APBI techniques were superior in both toxicity and patient-reported outcomes versus WBIR at long-term follow-up. Currently, alternative APBI techniques such as intraoperative electron radiation, 50-kV x-rays and three-dimensional conformal external beam radiotherapy (3D CRT) failed to demonstrate noninferiority to conventionally fractionated whole-breast irradiation in the setting of a randomized controlled trial (RCT). In one randomized trial (RAPID), however, APBI by 3D CRT compared noninferior to hypo-fractionated WBIR in preventing ipsilateral breast-tumor recurrence (IBTR) but was associated with a higher rate of late radiation toxicity. Ultimately, multicatheter interstitial brachytherapy remains the only APBI modality with available 10-year RCT data in terms of noninferior survival and superior toxicity/patient-reported outcomes and therefore should be prioritized over alternative treatment methods in suitable patients with breast cancer considered at low-risk for local recurrence according to recent international guidelines.
Conflict of interest statement
None declared.
References
- radiation therapy
- brachytherapy
- immunologic adjuvants
- breast neoplasms
- phase 2 clinical trials
- follow-up
- intraoperative care
- breast
- guidelines
- neoplasms
- breast cancer
- interstitial brachytherapy
- breast conserving surgery
- toxic effect
- patient self-report
- partial breast irradiation
- whole breast irradiation


