-
PDF
- Split View
-
Views
-
Cite
Cite
Masahiro Ashizawa, Yoshinobu Kanda, Preservation of fertility in patients with hematological malignancies, Japanese Journal of Clinical Oncology, Volume 50, Issue 7, July 2020, Pages 729–742, https://doi.org/10.1093/jjco/hyaa043
Close - Share Icon Share
Abstract
Oncofertility is the medical field that bridges oncology and reproduction that seeks to give healthcare providers and patients the opportunity to optimize residual fertility. The treatment for hematological malignancies carries gonadal toxicity, so that the preservation of fertility should be considered in all patients in childhood, adolescence and young adulthood. Most patients who receive only chemotherapy remain fertile, whereas those who receive regimens consisting of high-dose alkylating agents or total body irradiation can develop permanent infertility. In postpubertal patients, there are established methods for preserving fertility, such as the cryopreservation of sperm, oocytes and embryos. Although ideally performed before the initiation of gonadotoxic treatment, these procedures for fertility preservation can be performed any time prior to the loss of gonadal function. In contrast, a standard option is not available in prepubertal patients, and the preservation of fertility must be sought through experimental methods. Future advances in reproductive medicine may overcome this limitation. Gonadal tissue cryopreservation might be performed in the hope that sperm or mature oocytes could later be extracted from cryopreserved tissue. Healthcare providers, including hematologists, reproductive endocrinologists, nurses, clinical psychotherapists and embryologists, need to optimize the patient’s fertility through shared decision-making while always remaining aware of the rapidly progressing developments in reproductive medicine.
Necessity and difficulty of fertility preservation
Treatment for hematological malignancies has improved due to the development of chemotherapy and the spread of hematopoietic stem cell transplantation (HSCT). As a consequence, the number of long-term cancer survivors is increasing. Both cure of the disease and the improvement of quality of life (QOL) are important issues in patients with hematological malignancies. In Japan, the incidences of leukemia, malignant lymphoma and multiple myeloma in patients under 40 years of age who can expect to have a baby in the future are about 1700, 1300 and 40 cases per year, respectively (1–3). Treatment outcomes for childhood and adolescent and young adult (AYA) patients are better, especially in pediatric patients; the 10-year overall survival rate for leukemia and malignant lymphoma is around 80%.
The risk of gonadal toxicity and residual fertility after treatment for hematological malignancy depends mainly on the type of the disease, the dose of chemotherapeutic drugs and radiation and the age of the patient at the initiation of treatment. Alkylating agents and radiation carry strong gonadal toxicity, resulting in prolonged gonadal dysfunction. Infertility following treatment of hematological malignancies is an important issue in childhood and AYA patients with good survival. However, previously, only 40% of hematologists informed all of their reproductive-age patients about the risk of chemotherapy-related infertility before the initiation of treatment (4). This hesitance in providing an explanation was caused by a lack of resources and information on fertility preservation. Furthermore, hematological malignancy-specific issues that are not found with solid tumors make fertility preservation even more difficult. First, urgent treatment is often required for hematological malignancies, especially for those such as acute leukemia, so that patients have no time for fertility-preserving procedures. Second, invasive procedures such as oocyte retrieval and harvest of ovarian tissue are not feasible due to the risk of infection and bleeding induced by neutropenia and thrombocytopenia. Third, a diagnosis of cancer causes considerable distress in patients, who consider lifesaving as the only goal and often don’t prioritize QOL after treatment.
Oncofertility is an interdisciplinary subfield that bridges oncology and reproductive medicine (5). It encompasses gonadal toxicities induced by treatment, procedures for fertility preservation and decision-making in patients. The first guidelines were published by the American Society of Clinical Oncology (ASCO) in 2006 (6–8), and various other guidelines have been published since then (9–16), making it possible for cancer therapists to provide patients with appropriate information regarding fertility preservation. Based on these guidelines, healthcare providers must discuss the likelihood of infertility, the timing and methods for fertility preservation, prognosis, distress induced by infertility, costs and legal issues, with all reproductive-age patients.
Testicular toxicities induced by chemotherapeutic drugs and radiation
Spermatogonial stem cells have the ability to self-replicate and differentiate into sperm. Dividing and differentiating spermatogonia are more sensitive to chemotherapeutic drugs and radiation than mature spermatozoa (17). Spermatogenesis is arrested after treatment for hematological malignancies is initiated, while mature sperm may survive in semen early after the initiation of treatment (18). The duration of azoospermia is proportional to the number of dead spermatogonial stem cells. Spermatogenesis can recover provided that stem cells survive after treatment, whereas their absence can lead to permanent azoospermia.
Testicular toxicity depends on the amount and type of chemotherapeutic drugs and the radiation dose (Table 1) (6,11,19). Alkylating agents, including cyclophosphamide, chlorambucil, procarbazine and busulfan, platinums and radiation, are most gonadotoxic, so that transplantation conditioning regimens that include these interventions at very high doses render most men who undergo HSCT permanently infertile (Table 2) (20–23). Conditioning with cyclophosphamide alone, which has been used for allogeneic HSCT in aplastic anemia, frequently restores gonadal function, whereas cyclophosphamide combined with busulfan or total body irradiation (TBI) reduces the recovery rate of gonadal function (21,24). A total testicular dose of 2.5 Gy or more as fractionated radiation or 6 Gy or more as a single irradiation can cause permanent infertility at a high rate. Gonadal recovery occurs in less than 20% of patients who receive TBI (21,24). Men, who received TBI when they were younger than 25 years at HSCT, who survived for a long time afterward and who apparently have not developed chronic graft-versus-host disease (GVHD), have recovered spermatogenesis in semen examination even after standard-dose TBI (25). However, these patients have oligospermia and morphologic abnormalities, and few have fathered children. In reports with a small sample size, permanent infertility developed in patients with malignant lymphoma who received BEAM (carmustine, etoposide, cytarabine, melphalan) as the conditioning regimen in autologous transplantation (23).
Relationship between treatment for hematological malignancies and testicular toxicity (19)
| Intervention . | Indications . |
|---|---|
| High risk: prolonged azoospermia after treatment | |
| TBI | HSCT |
| Testicular radiation dose >2.5 Gy in men, >6 Gy in boys | Testicular lymphoma |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: prolonged azoospermia not common at standard dose | |
| Cumulative cisplatin dose >400 mg/m2 | Salvage regimen for lymphoma |
| Cumulative carboplatin dose >2 g/m2 | Salvage regimen for lymphoma |
| Low risk: temporary azoopenia after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
| Intervention . | Indications . |
|---|---|
| High risk: prolonged azoospermia after treatment | |
| TBI | HSCT |
| Testicular radiation dose >2.5 Gy in men, >6 Gy in boys | Testicular lymphoma |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: prolonged azoospermia not common at standard dose | |
| Cumulative cisplatin dose >400 mg/m2 | Salvage regimen for lymphoma |
| Cumulative carboplatin dose >2 g/m2 | Salvage regimen for lymphoma |
| Low risk: temporary azoopenia after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
TBI, total body irradiation; HSCT, hematopoietic stem cell transplantation; MOPP, mechlorethamine, vincristine, procarbazine and prednisone; COPP, cyclophosphamide, vincristine, procarbazine and prednisone; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; CNS, central nervous system; ABVD, doxorubicin, bleomycin, vinblastine and dacarbazine; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia.
Relationship between treatment for hematological malignancies and testicular toxicity (19)
| Intervention . | Indications . |
|---|---|
| High risk: prolonged azoospermia after treatment | |
| TBI | HSCT |
| Testicular radiation dose >2.5 Gy in men, >6 Gy in boys | Testicular lymphoma |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: prolonged azoospermia not common at standard dose | |
| Cumulative cisplatin dose >400 mg/m2 | Salvage regimen for lymphoma |
| Cumulative carboplatin dose >2 g/m2 | Salvage regimen for lymphoma |
| Low risk: temporary azoopenia after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
| Intervention . | Indications . |
|---|---|
| High risk: prolonged azoospermia after treatment | |
| TBI | HSCT |
| Testicular radiation dose >2.5 Gy in men, >6 Gy in boys | Testicular lymphoma |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: prolonged azoospermia not common at standard dose | |
| Cumulative cisplatin dose >400 mg/m2 | Salvage regimen for lymphoma |
| Cumulative carboplatin dose >2 g/m2 | Salvage regimen for lymphoma |
| Low risk: temporary azoopenia after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
TBI, total body irradiation; HSCT, hematopoietic stem cell transplantation; MOPP, mechlorethamine, vincristine, procarbazine and prednisone; COPP, cyclophosphamide, vincristine, procarbazine and prednisone; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; CNS, central nervous system; ABVD, doxorubicin, bleomycin, vinblastine and dacarbazine; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia.
Gonadal recovery following hematopoietic stem cell transplantation in male patients
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 109 | 24 (13–52) | 3 (1–19) | 61% | 51 live births, 4 spontaneous abortions and 7 elective abortions in 62 pregnancies of 28 patient’s partners | (21) |
| Allogeneic | CY | AA | 10 | Mean 23 | Mean 2.5 (1–15) | 90% | 4 live births in 4 patient’s partners | (22) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 23 (12–59) | 5 (1–19) | 20% | 8 live births in 8 pregnancies of 5 patient’s partners | (21) |
| CY/TBI (12 Gy) | HM | 226 | 31 (11–62) | 4 (1–14) | 16% | |||
| CY/TBI (>12 Gy) | HM | 166 | 26 (13–54) | 4 (1–13) | 18% | |||
| Allogeneic | CY/TBI (9.5–14.4 Gy) | HM | 11 | 33 (19–47) | 3 (1–9) | 9% | NA | (23) |
| Allogeneic | CY/TBI or CY/TAI | HM | 48 | mean 27 | mean5.6 (1–18) | 15% | 1 live birth in 1 patient’s partner | (22) |
| Allogeneic | BU/CY | HM | 46 | 34 (13–56) | 2 (1–5) | 17% | 4 live births in 4 pregnancies of 2 patient’s partners | (21) |
| Autologous | BEAM | HL, NHL | 10 | 30.5 (17–53) | 2.5 (0.1–5) | 0 | No recovery | (23) |
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 109 | 24 (13–52) | 3 (1–19) | 61% | 51 live births, 4 spontaneous abortions and 7 elective abortions in 62 pregnancies of 28 patient’s partners | (21) |
| Allogeneic | CY | AA | 10 | Mean 23 | Mean 2.5 (1–15) | 90% | 4 live births in 4 patient’s partners | (22) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 23 (12–59) | 5 (1–19) | 20% | 8 live births in 8 pregnancies of 5 patient’s partners | (21) |
| CY/TBI (12 Gy) | HM | 226 | 31 (11–62) | 4 (1–14) | 16% | |||
| CY/TBI (>12 Gy) | HM | 166 | 26 (13–54) | 4 (1–13) | 18% | |||
| Allogeneic | CY/TBI (9.5–14.4 Gy) | HM | 11 | 33 (19–47) | 3 (1–9) | 9% | NA | (23) |
| Allogeneic | CY/TBI or CY/TAI | HM | 48 | mean 27 | mean5.6 (1–18) | 15% | 1 live birth in 1 patient’s partner | (22) |
| Allogeneic | BU/CY | HM | 46 | 34 (13–56) | 2 (1–5) | 17% | 4 live births in 4 pregnancies of 2 patient’s partners | (21) |
| Autologous | BEAM | HL, NHL | 10 | 30.5 (17–53) | 2.5 (0.1–5) | 0 | No recovery | (23) |
CY, cyclophosphamide; AA, aplastic anemia; HM, hematological malignancy; NA, not assessed; TAI, thoracoabdominal irradiation; BU, busulfan; BEAM, carmustine, etoposide, cytarabine and melphalan.
Gonadal recovery following hematopoietic stem cell transplantation in male patients
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 109 | 24 (13–52) | 3 (1–19) | 61% | 51 live births, 4 spontaneous abortions and 7 elective abortions in 62 pregnancies of 28 patient’s partners | (21) |
| Allogeneic | CY | AA | 10 | Mean 23 | Mean 2.5 (1–15) | 90% | 4 live births in 4 patient’s partners | (22) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 23 (12–59) | 5 (1–19) | 20% | 8 live births in 8 pregnancies of 5 patient’s partners | (21) |
| CY/TBI (12 Gy) | HM | 226 | 31 (11–62) | 4 (1–14) | 16% | |||
| CY/TBI (>12 Gy) | HM | 166 | 26 (13–54) | 4 (1–13) | 18% | |||
| Allogeneic | CY/TBI (9.5–14.4 Gy) | HM | 11 | 33 (19–47) | 3 (1–9) | 9% | NA | (23) |
| Allogeneic | CY/TBI or CY/TAI | HM | 48 | mean 27 | mean5.6 (1–18) | 15% | 1 live birth in 1 patient’s partner | (22) |
| Allogeneic | BU/CY | HM | 46 | 34 (13–56) | 2 (1–5) | 17% | 4 live births in 4 pregnancies of 2 patient’s partners | (21) |
| Autologous | BEAM | HL, NHL | 10 | 30.5 (17–53) | 2.5 (0.1–5) | 0 | No recovery | (23) |
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 109 | 24 (13–52) | 3 (1–19) | 61% | 51 live births, 4 spontaneous abortions and 7 elective abortions in 62 pregnancies of 28 patient’s partners | (21) |
| Allogeneic | CY | AA | 10 | Mean 23 | Mean 2.5 (1–15) | 90% | 4 live births in 4 patient’s partners | (22) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 23 (12–59) | 5 (1–19) | 20% | 8 live births in 8 pregnancies of 5 patient’s partners | (21) |
| CY/TBI (12 Gy) | HM | 226 | 31 (11–62) | 4 (1–14) | 16% | |||
| CY/TBI (>12 Gy) | HM | 166 | 26 (13–54) | 4 (1–13) | 18% | |||
| Allogeneic | CY/TBI (9.5–14.4 Gy) | HM | 11 | 33 (19–47) | 3 (1–9) | 9% | NA | (23) |
| Allogeneic | CY/TBI or CY/TAI | HM | 48 | mean 27 | mean5.6 (1–18) | 15% | 1 live birth in 1 patient’s partner | (22) |
| Allogeneic | BU/CY | HM | 46 | 34 (13–56) | 2 (1–5) | 17% | 4 live births in 4 pregnancies of 2 patient’s partners | (21) |
| Autologous | BEAM | HL, NHL | 10 | 30.5 (17–53) | 2.5 (0.1–5) | 0 | No recovery | (23) |
CY, cyclophosphamide; AA, aplastic anemia; HM, hematological malignancy; NA, not assessed; TAI, thoracoabdominal irradiation; BU, busulfan; BEAM, carmustine, etoposide, cytarabine and melphalan.
Fertility is temporarily impaired by ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) or CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for malignant lymphoma, and multiagent therapies for acute leukemia, and then recovers several months or years after treatment. However, permanent azoospermia occurs at a high rate in non-HSCT patients after treatment including procarbazine such as MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) or COPP (cyclophosphamide, vincristine, procarbazine, and prednisone). There are still many unclear points regarding pregnancy in the partners of male patients taking tyrosine kinase inhibitor (TKI). C-Kit, which is inhibited by TKI, plays an important role in Leydig cell development, sperm migration and proliferation. Some reports have demonstrated that imatinib decreases the number of sperm and the intratesticular testosterone level in male mice (26,27), which impairs spermatogenesis. Meanwhile, more than 150 male human patients with imatinib had no increase in complications associated with conception, pregnancy or delivery in their partners and no increase in congenital abnormalities in their offspring (28). It remains controversial whether second-generation TKI affects spermatogenesis, due to the limited number of reports.
Other factors that cause infertility in men
Patients with hematological malignancies have an increased risk for a low sperm count and inadequate semen quality, such as abnormal morphology and DNA fragmentation, even before the initiation of treatment (29–34). This abnormal morphology and decreased number of sperm before treatment have been well confirmed, especially in patients with Hodgkin lymphoma (HL), and some previous reports demonstrated that the incidences of normal sperm number and azoospermia are 20–41% and 3–11%, respectively (29,33,34). In patients with HL treated with ABVD, the sperm concentration, total sperm number and abnormal forms before chemotherapy were comparable to those 2 years after chemotherapy, indicating that sperm quality had returned to pre-therapy values (30). Based on this result, it is unclear whether chemotherapy improves sperm abnormalities induced by hematological malignancies themselves. The control of GVHD is also an important factor in fertility preservation in patients who receive allogeneic SCT. In a sperm analysis to identify the risk factors for infertility in long-term male survivors after allogeneic HSCT, ongoing chronic GVHD is as strong an adverse factor for sperm recovery as a conditioning regimen with TBI and older age at HSCT (35). Irradiation of the hypothalamic–pituitary area at 35–40 Gy or more induced hypogonadotropic hypogonadism in male patients with central nervous system involvement (36). Moreover, high-dose radiation to the testis or brain causes testosterone deficiency, resulting in decreased libido and erectile dysfunction, which are not seen with the doses usually applied in TBI. Even if testicular function is maintained, mental stress, decreased strength and appearance changes due to GVHD may reduce libido. Therefore, not only physical treatment but also mental care is important for fertility preservation, so that support by psychologists and nurses is indispensable.
Methods for fertility preservation in men
Ideally, sperm should be collected and cryopreserved prior to the initiation of treatment that is associated with gonadal toxicity. As described above, good sperm may not be harvested due to abnormal spermatogenesis induced by hematological malignancies themselves even before treatment. In general, to obtain a sufficient number of good sperm, multiple collections should be performed after 3 days of abstinence (37). However, especially in acute leukemia, treatment must be started as soon as possible and there is often not enough time to retrieve good sperm. Furthermore, mental distress and poor general condition induced by infection or a hemorrhagic tendency can prevent collection before treatment. In such cases, because mature sperm may survive in semen early after the initiation of treatment (18), sperm may be harvested prior to the second course of chemotherapy. However, in this situation, healthcare providers should warn patients about the possibility that there may be an increased risk of DNA damage in sperm exposed to cytotoxic therapy (38). Intracytoplasmic sperm injection (ICSI), which bypasses several natural barriers, may contribute to fertilization by severely impaired sperm, resulting in abnormal embryo and implantation failure.
The standard procedure used to collect sperm samples in pubertal boys, adolescents, and young adults is masturbation, which is simple and non-invasive. However, mental distress and poor general condition interfere with erection and ejaculation, making sperm collection difficult. For these individuals, sperm can be retrieved by electroejaculation and microdissection testicular sperm extraction (micro-TESE). Electroejaculation is performed by inserting a transrectal probe in contact with the prostate and seminal vesicles (39). Pretreatment, adequate sperm were obtained by electroejaculation in patients whose sperm was not collected by masturbation; this procedure was performed under general anesthesia (39). Micro-TESE involves harvest of the microsurgical epididymal tubule, where spermatogenesis occurs, followed by the retrieval of sperm under a microscope (40). Micro-TESE was performed in long-surviving patients, including those with acute leukemia and malignant lymphoma, with persistent post-chemotherapy azoospermia, and sperm could be obtained in 40% or more of these, partially resulting in the fathering of a child with assisted fertilization by ICSI (41,42). However, it was difficult to retrieve sperm in patients who had undergone HSCT, even with the use of micro-TESE (42). Based on these findings, if good sperm cannot be collected before or during treatment, micro-TESE may be indicated before treatment to eradicate gonadal function. In prepubertal patients, spermatogenesis remains immature, and collection and cryopreservation of mature sperm cannot be performed by the procedures described above. In such patients, testicular tissue containing spermatogonial stem cells may be excised and cryopreserved in the hope that a method for producing sperm from this frozen tissue will be established with future developments in reproductive medicine, albeit this approach is still experimental (43). Most hematological malignancies carry a risk of leaving residual lesions in the testis (44). A previous report revealed that autotransplantation of testicular tissues or testicular cells caused the transmission of hematological malignant cells in an animal model (45). Since both micro-TESE and testicular tissue freezing are invasive procedures, it is desirable that patients should have no bleeding tendency and no increased susceptibility to infection. If treatment for hematological diseases can be greatly delayed, these procedures should not be performed because of the increased risk of recurrence. Testicular shielding may be an option for male patients receiving radiation therapy such as TBI, where the testes are covered with metallic blocks to reduce the radiation dose. However, testicular shielding may not be indicated for patients with hematological malignancies with a possibility of testicular infiltration. In practice, it is performed only in non-neoplastic diseases such as aplastic anemia and metabolic disorders (46–48).
Ovarian toxicities induced by chemotherapeutic drugs and radiation
The number of primordial follicles decreases with aging after birth, from 300 000 at birth, to 180 000 at menarche, 16 000 at 35 years and below 1000 at menopause (49). Primordial follicles grow into preantral follicles over 100 days independent of gonadotropin and then grow into antral follicles over another 70 days. Antral follicles become Graafian follicles in ~14–20 days, depending on changes in gonadotropins with the menstrual cycle (50). The follicle rapidly grows due to a surge in luteinizing hormone (LH), so that ovulation occurs and the ovulated ovum is caught by the fimbria of the fallopian tube. Although 1000 primordial follicles develop every month, only one egg eventually reaches ovulation over half a year.
Chemotherapeutic drugs damage granulosa cells in the growing follicles and eliminate mature follicles. When granulosa cells in growing follicles are reduced, anti-Müllerian hormones (AMH), which suppress the development of primordial follicles, are also reduced. As a result, primordial follicles are overdeveloped, which depletes their consumption and causes premature menopause (51). In addition, chemotherapeutic drugs caused ovarian cortical fibrosis and blood vessel damage, resulting in local ischemia, where primordial follicles are decreased. The radiosensitivity of follicles varies depending on their maturity; growing follicles are more sensitive than mature and primordial follicles, similar to their sensitivity to chemotherapeutic drugs. Residual fertility after treatment depends on the type and amounts of chemotherapeutic drugs, the radiation dose and the number of follicles patients possess before treatment. Therefore, permanent sterility occurs when gonadotoxic agents annihilate primordial follicles. Meanwhile, if a certain number of follicles remain after chemotherapy or radiotherapy, menstruation can recover, although many such patients develop premature menopause (52). Even if the number of follicles decreases, the ovary can maintain the menstrual cycle and ovulation to a certain extent (53). Therefore, recovery of a regular menstruation cycle alone does not mean that fertility is sufficiently maintained. The ideal tests to confirm residual ovarian function should be non-invasive, quickly interpretable, reproducible and less susceptible to menstruation. In practice, fertility is evaluated in terms of early follicular phase follicle-stimulating hormone (basal FSH) and antral follicular count (AFC) observed on ultrasound in the early follicular phase and AMH (54). Basal FSH and AFC are affected by the menstrual cycle, which complicates their use to measure ovarian reserve. However, AMH is less affected and has been used as a reliable marker that reflects the number of residual follicles. AMH is secreted from granulosa cells of the preantral and small antral follicles. AMH levels decrease steadily over time in healthy women (55). In cancer patients, AMH decreases rapidly with the initiation of chemotherapy but then recovers several months after the completion of chemotherapy if some primordial follicles remain (56–59). However, several reports have demonstrated that a low AMH level is not always predictive of reduced fecundity in patients who receive chemotherapy (60) or HSCT (61,62).
Menstrual recovery after chemotherapy was much lower in older women, especially those aged above 40 years (63). The gonadal toxicity of each agent toward the ovaries is basically the same as that toward the testis and is strong in regimens containing alkylating drugs, platinum and radiation (Table 3) (9,11,19). With the ABVD, CHOP, repetitive chemotherapy for acute leukemia and non-alkylating drug regimens, menstruation can recover following temporary amenorrhea, or patients may not develop amenorrhea at all. AMH was re-elevated even after chemotherapy in many patients who received these regimens, suggesting that follicles survived and fertility was maintained (57–59,64). Meanwhile, high doses of alkylating agents and procarbazine cause prolonged amenorrhea and delayed the recovery of AMH (57,59).
| Intervention . | Indications . |
|---|---|
| High risk: >80% of women develop amenorrhea after treatment | |
| TBI | HSCT |
| Abdominal or pelvic radiation dose >15 Gy in prepubertal girls | Malignant lymphoma |
| >10 Gy in postpubertal girls | |
| >6 Gy in adult women | |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 in female age <20 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: ~30–79% of women develop amenorrhea after treatment | |
| Protocols containing cisplatin | Salvage regimen for lymphoma |
| BEACOPP in female age <30 | HL |
| Low risk: <20% of women develop amenorrhea after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
| Intervention . | Indications . |
|---|---|
| High risk: >80% of women develop amenorrhea after treatment | |
| TBI | HSCT |
| Abdominal or pelvic radiation dose >15 Gy in prepubertal girls | Malignant lymphoma |
| >10 Gy in postpubertal girls | |
| >6 Gy in adult women | |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 in female age <20 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: ~30–79% of women develop amenorrhea after treatment | |
| Protocols containing cisplatin | Salvage regimen for lymphoma |
| BEACOPP in female age <30 | HL |
| Low risk: <20% of women develop amenorrhea after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
CNS, central nervous system; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone.
| Intervention . | Indications . |
|---|---|
| High risk: >80% of women develop amenorrhea after treatment | |
| TBI | HSCT |
| Abdominal or pelvic radiation dose >15 Gy in prepubertal girls | Malignant lymphoma |
| >10 Gy in postpubertal girls | |
| >6 Gy in adult women | |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 in female age <20 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: ~30–79% of women develop amenorrhea after treatment | |
| Protocols containing cisplatin | Salvage regimen for lymphoma |
| BEACOPP in female age <30 | HL |
| Low risk: <20% of women develop amenorrhea after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
| Intervention . | Indications . |
|---|---|
| High risk: >80% of women develop amenorrhea after treatment | |
| TBI | HSCT |
| Abdominal or pelvic radiation dose >15 Gy in prepubertal girls | Malignant lymphoma |
| >10 Gy in postpubertal girls | |
| >6 Gy in adult women | |
| Protocols containing procarbazine (MOPP, COPP) | HL |
| Alkylating chemotherapy for transplantation conditioning (cyclophosphamide, busulfan, melphalan) | HSCT |
| Cyclophosphamide >7.5 g/m2 in female age <20 | NHL |
| Cranial/brain radiation >40 Gy | CNS involvement |
| Intermediate risk: ~30–79% of women develop amenorrhea after treatment | |
| Protocols containing cisplatin | Salvage regimen for lymphoma |
| BEACOPP in female age <30 | HL |
| Low risk: <20% of women develop amenorrhea after treatment | |
| ABVD | HL |
| CHOP | NHL |
| Anthracycline + cytarabine | AML |
| Multiagent therapies for leukemia | ALL |
| Non-alkylating chemotherapy | |
| Unknown risk | |
| Tyrosine kinase inhibitors | CML, ALL with t(9;22)(q34.1;q11.2) |
CNS, central nervous system; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone.
Half of all oocytes are lost with a radiation dose of approximately 2 Gy (65). The dose of radiation required to produce prolonged amenorrhea was 15 Gy or more in prepubertal girls, 10 Gy or more in postpubertal girls (19) and 6 Gy or more in adult women (11). With regard to the conditioning regimen in HSCT (Table 4) (20,21,62,66–70), ovarian function recovers at a high rate with cyclophosphamide alone (21,24,66). However, when busulfan or TBI is used in combination with cyclophosphamide, the gonadal recovery rate is greatly reduced. It hardly recovers when busulfan is used in female patients. Unlike in the case of men, the use of BEAM for autologous SCT may restore gonadal function at a high rate in women (69).
Gonadal recovery following hematopoietic stem cell transplantation in female patients
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 103 | 24 (13–58) | 3 (1–17) | 54% | 44 live births, 4 spontaneous abortions, 5 elective abortions and 2 tubal pregnancies in 56 pregnancies of 28 patients | (21) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 21 (13–46) | 4 (1–14) | 27% | 8 live births, 6 spontaneous abortions and 2 elective abortions in | (21) |
| CY/TBI (12 Gy) | HM | 270 | 33 (11–58) | 3 (1–13) | 10% | 16 pregnancies of 13 patients | ||
| CY/TBI (>12 Gy) | HM | 203 | 33 (15–57) | 3 (1–10) | 5% | |||
| Allogeneic | TBI (8.5–13.2) based regimen | HM | 79 | NA | 4 (0.3–10) | 13.5% (>18 years), 100%(<18 years) | 1 live birth in 1 patient | (66) |
| Allogeneic | BU/CY | HM | 73 | 24 (14–57) | 2 (1–6) | 1% | No pregnancy in 1 patient | (21) |
| Allogeneic | FDR/MEL ± ETP ± CA ± TBI (3–4 Gy) | HM | 11 | 10 (1–18) | 8 (3–14) | 82% | NA | (67) |
| Allogeneic | FDR/MEL ± ATG | EBV-LPD, HM | 11 | 25 (15–38) at the examination | NA | 82% | NA | (68) |
| Autologous | BEAM | HL, NHL | 25 | 27 (17–40) | 8 (2–17) | 68% | 12 live births, 1 spontaneous abortion and 2 current pregnancies in 15 pregnancies of 10 patients | (69) |
| Autologous | MEL ± ETP | HL, NHL | 30 | 77% younger than 40 years | NA | NA | 16 live births (2 twins and 1 triplets) in 12 pregnancies of 10 patients. Abortions were not fully assessed. | (70) |
| Allogeneic Autologous Syngeneic | CY/TBI with OS or CA/TBI with OS | HM, AA | 19 | 23 (19–33) | 4 (0.2–10) | 78% | 2 live births, 2 spontaneous abortions and 1 current pregnancy in 5 pregnancies of 3 patients | (62) |
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 103 | 24 (13–58) | 3 (1–17) | 54% | 44 live births, 4 spontaneous abortions, 5 elective abortions and 2 tubal pregnancies in 56 pregnancies of 28 patients | (21) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 21 (13–46) | 4 (1–14) | 27% | 8 live births, 6 spontaneous abortions and 2 elective abortions in | (21) |
| CY/TBI (12 Gy) | HM | 270 | 33 (11–58) | 3 (1–13) | 10% | 16 pregnancies of 13 patients | ||
| CY/TBI (>12 Gy) | HM | 203 | 33 (15–57) | 3 (1–10) | 5% | |||
| Allogeneic | TBI (8.5–13.2) based regimen | HM | 79 | NA | 4 (0.3–10) | 13.5% (>18 years), 100%(<18 years) | 1 live birth in 1 patient | (66) |
| Allogeneic | BU/CY | HM | 73 | 24 (14–57) | 2 (1–6) | 1% | No pregnancy in 1 patient | (21) |
| Allogeneic | FDR/MEL ± ETP ± CA ± TBI (3–4 Gy) | HM | 11 | 10 (1–18) | 8 (3–14) | 82% | NA | (67) |
| Allogeneic | FDR/MEL ± ATG | EBV-LPD, HM | 11 | 25 (15–38) at the examination | NA | 82% | NA | (68) |
| Autologous | BEAM | HL, NHL | 25 | 27 (17–40) | 8 (2–17) | 68% | 12 live births, 1 spontaneous abortion and 2 current pregnancies in 15 pregnancies of 10 patients | (69) |
| Autologous | MEL ± ETP | HL, NHL | 30 | 77% younger than 40 years | NA | NA | 16 live births (2 twins and 1 triplets) in 12 pregnancies of 10 patients. Abortions were not fully assessed. | (70) |
| Allogeneic Autologous Syngeneic | CY/TBI with OS or CA/TBI with OS | HM, AA | 19 | 23 (19–33) | 4 (0.2–10) | 78% | 2 live births, 2 spontaneous abortions and 1 current pregnancy in 5 pregnancies of 3 patients | (62) |
FDR, fludarabine; MEL, melphalan; ETP, etoposide; CA, cytarabine; ATG, anti-thymocyte globulin; EBV-LPD, Epstein–Barr virus-associated lymphoproliferative disease; OS ovarian shielding.
Gonadal recovery following hematopoietic stem cell transplantation in female patients
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 103 | 24 (13–58) | 3 (1–17) | 54% | 44 live births, 4 spontaneous abortions, 5 elective abortions and 2 tubal pregnancies in 56 pregnancies of 28 patients | (21) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 21 (13–46) | 4 (1–14) | 27% | 8 live births, 6 spontaneous abortions and 2 elective abortions in | (21) |
| CY/TBI (12 Gy) | HM | 270 | 33 (11–58) | 3 (1–13) | 10% | 16 pregnancies of 13 patients | ||
| CY/TBI (>12 Gy) | HM | 203 | 33 (15–57) | 3 (1–10) | 5% | |||
| Allogeneic | TBI (8.5–13.2) based regimen | HM | 79 | NA | 4 (0.3–10) | 13.5% (>18 years), 100%(<18 years) | 1 live birth in 1 patient | (66) |
| Allogeneic | BU/CY | HM | 73 | 24 (14–57) | 2 (1–6) | 1% | No pregnancy in 1 patient | (21) |
| Allogeneic | FDR/MEL ± ETP ± CA ± TBI (3–4 Gy) | HM | 11 | 10 (1–18) | 8 (3–14) | 82% | NA | (67) |
| Allogeneic | FDR/MEL ± ATG | EBV-LPD, HM | 11 | 25 (15–38) at the examination | NA | 82% | NA | (68) |
| Autologous | BEAM | HL, NHL | 25 | 27 (17–40) | 8 (2–17) | 68% | 12 live births, 1 spontaneous abortion and 2 current pregnancies in 15 pregnancies of 10 patients | (69) |
| Autologous | MEL ± ETP | HL, NHL | 30 | 77% younger than 40 years | NA | NA | 16 live births (2 twins and 1 triplets) in 12 pregnancies of 10 patients. Abortions were not fully assessed. | (70) |
| Allogeneic Autologous Syngeneic | CY/TBI with OS or CA/TBI with OS | HM, AA | 19 | 23 (19–33) | 4 (0.2–10) | 78% | 2 live births, 2 spontaneous abortions and 1 current pregnancy in 5 pregnancies of 3 patients | (62) |
| Type of HSCT . | Regimen . | Disease . | Number . | Age at HSCT median (range), years . | Years post-transplant, median (range), years . | Gonadal recovery . | Outcome of pregnancies . | Reference . |
|---|---|---|---|---|---|---|---|---|
| Allogeneic | CY | AA | 103 | 24 (13–58) | 3 (1–17) | 54% | 44 live births, 4 spontaneous abortions, 5 elective abortions and 2 tubal pregnancies in 56 pregnancies of 28 patients | (21) |
| Allogeneic | CY/TBI (10 Gy) | HM | 59 | 21 (13–46) | 4 (1–14) | 27% | 8 live births, 6 spontaneous abortions and 2 elective abortions in | (21) |
| CY/TBI (12 Gy) | HM | 270 | 33 (11–58) | 3 (1–13) | 10% | 16 pregnancies of 13 patients | ||
| CY/TBI (>12 Gy) | HM | 203 | 33 (15–57) | 3 (1–10) | 5% | |||
| Allogeneic | TBI (8.5–13.2) based regimen | HM | 79 | NA | 4 (0.3–10) | 13.5% (>18 years), 100%(<18 years) | 1 live birth in 1 patient | (66) |
| Allogeneic | BU/CY | HM | 73 | 24 (14–57) | 2 (1–6) | 1% | No pregnancy in 1 patient | (21) |
| Allogeneic | FDR/MEL ± ETP ± CA ± TBI (3–4 Gy) | HM | 11 | 10 (1–18) | 8 (3–14) | 82% | NA | (67) |
| Allogeneic | FDR/MEL ± ATG | EBV-LPD, HM | 11 | 25 (15–38) at the examination | NA | 82% | NA | (68) |
| Autologous | BEAM | HL, NHL | 25 | 27 (17–40) | 8 (2–17) | 68% | 12 live births, 1 spontaneous abortion and 2 current pregnancies in 15 pregnancies of 10 patients | (69) |
| Autologous | MEL ± ETP | HL, NHL | 30 | 77% younger than 40 years | NA | NA | 16 live births (2 twins and 1 triplets) in 12 pregnancies of 10 patients. Abortions were not fully assessed. | (70) |
| Allogeneic Autologous Syngeneic | CY/TBI with OS or CA/TBI with OS | HM, AA | 19 | 23 (19–33) | 4 (0.2–10) | 78% | 2 live births, 2 spontaneous abortions and 1 current pregnancy in 5 pregnancies of 3 patients | (62) |
FDR, fludarabine; MEL, melphalan; ETP, etoposide; CA, cytarabine; ATG, anti-thymocyte globulin; EBV-LPD, Epstein–Barr virus-associated lymphoproliferative disease; OS ovarian shielding.
Interaction between the Kit ligand and c-Kit is required for the survival of follicles during early follicular development, so that patients with TKI may experience disturbed menstruation (71). In addition, some reports have shown an increased incidence of miscarriage, stillbirth and malformation in patients taking TKI (72,73), although it is difficult to judge whether these abnormalities are caused by TKI or some concomitant medication. Therefore, TKI should not be administered to pregnant patients.
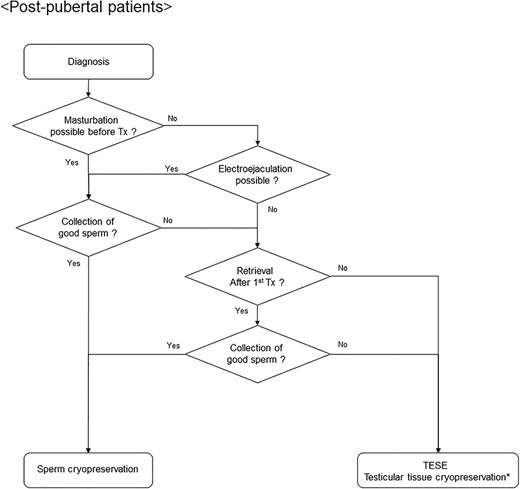
Algorithm for fertility preservation strategies in postpubertal male patients with hematological malignancies. Tx, therapy; TESE, testicular sperm extraction. Asterisk indicates that testicular tissue cryopreservation is considered to be in an experimental stage.
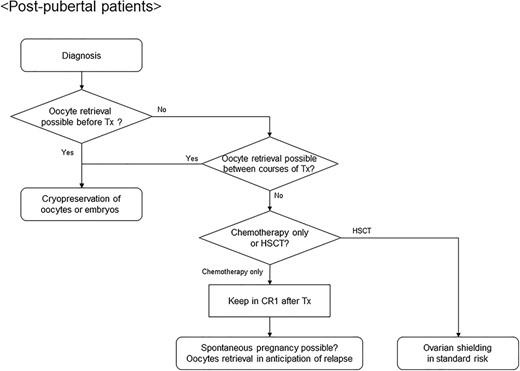
Algorithm for fertility preservation strategies in postpubertal female patients with acute leukemia or malignant lymphoma. CR1, first complete remission; HSCT, hematopoietic stem cell transplantation.
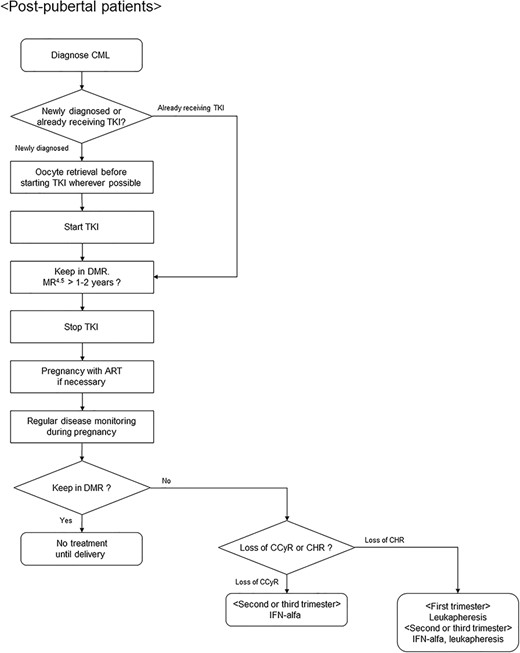
Algorithm for fertility preservation strategies in postpubertal female patients with chronic myeloid leukemia. CML, chronic myeloid leukemia; TKI, tyrosine kinase inhibitor; DMR, deep molecular response; MR, molecular response; ART, assisted reproductive technology; CCyR, complete cytogenetic response; CHR, complete hematological response; IFN, interferon. MR4.5 is defined as ≥4.5-log reduction in BCR-ABL1 transcript level.
Other factors that cause infertility in women
In the treatment of hematological malignancies with intracranial lesions, irradiation of the hypothalamus or pituitary gland at 40 Gy or higher increases the risk of hypogonadism due to gonadotropin secretion failure (36), causing infertility in postpubertal patients and a lack of secondary sexual characteristics in pediatric patients. If ovarian function itself has not been annihilated by chemotherapy or radiation, ovulation induction is required to restore fertility. In contrast, cranial radiation at a low dose of 24 Gy or more often results in early or precocious puberty by activating the hypothalamus.
Radiation of the uterus at 14–30 Gy can produce uterine dysfunction due to atrophy and fibrosis, resulting in an increased risk of miscarriage, preterm birth and the delivery of low-birthweight infants (74). The radiosensitivity of the uterus varies with age, and younger patients are more susceptible to radiation. Several reports have demonstrated that the average uterine volume in adolescent and adult patients who received TBI in childhood was 40–80% of that in a normal adult, even when these patients were given steroids (75,76).
Genital chronic GVHD can also affect fertility after allogeneic HSCT. The features of chronic GVHD in the external genitals and vagina have been defined in the guidelines (77). These include labial fusion, vaginal shortening, synechiae, dense sclerotic changes and complete vaginal stenosis, which can make sexual intercourse difficult. In addition, GVHD in the uterus and ovaries has been reported, which has not been discussed so far because conditioning regimens often eliminate fertility. Ovarian and uterine volumes were significantly lower in patients suffering from chronic GVHD (78). The ovaries following HSCT demonstrated donor T-cell infiltration in proximity to apoptotic granulosa cells in the ovarian follicles, resulting in impaired follicular hormone production and maturation of ovarian follicles in mice (79). Patients with extensive chronic GVHD experienced delayed recovery of menstruation and serum AMH (62). Therefore, the control of chronic GVHD is also an important factor for fertility preservation.
Methods for fertility preservation in women
The procedures for fertility preservation must be performed before the initiation of treatment for hematological malignancies that may lead to permanent infertility, depending on the patient’s condition and disease state. In a report comparing fertility in pediatric patients under 20 years of age who were diagnosed with cancer to that in their siblings, the rates of pregnancy and live birth in patients were similar to those in their siblings under 30 years old but gradually decreased relative to those in their siblings thereafter (52). Based on these findings, it is not sufficient merely for normal cyclic menstruation to recover; fertility preservation must be planned with a view to the possibility of premature menopause. Additionally, especially in women, the hemorrhagic tendency and increased susceptibility to infection caused by hematological malignancies themselves or myelosuppression greatly hinder the implementation of fertility preservation. Therefore, before, during and after treatment, hematologists need to carefully discuss the optimal method and timing for fertility preservation with various healthcare providers such as reproductive endocrinologists, nurses, clinical psychotherapist, embryologists and others.
Cryopreservation of embryos and oocytes
The cryopreservation of embryos is one of the most well-established methods in fertility preservation. In collecting oocytes for the cryopreservation of embryos or oocytes themselves, ovarian stimulation that promotes follicular development using an ovulation inducer is indispensable. Because conventional ovarian stimulation needs to be initiated early in the menstrual cycle, it may be necessary to wait 2–6 weeks to begin the administration of gonadotropin-releasing hormone (GnRH) agonist or GnRH antagonist. However, a random-start controlled ovarian stimulation, where patients can be stimulated regardless of the menstrual cycle, enabled the retrieval of oocytes within 2 weeks (80,81). Under transvaginal ultrasound guidance, the operator inserts a needle through the vaginal wall and into an ovarian follicle, which makes it possible to retrieve multiple oocytes. If the patient has a partner, oocytes are fertilized using co-incubation with sperm or ICSI, cultured to the embryo stage and cryopreserved thereafter. There are two methods for cryopreserving oocytes and embryos: slow freezing and vitrification. While slow freezing has been the conventional technique for years, oocytes are sensitive to cold injury when cooled slowly (82). However, vitrification enables the cryopreservation of oocytes that are difficult to cryopreserve by slow freezing (83). The outcomes in terms of the rate of fertilization, pregnancy and congenital abnormality with oocytes frozen and thawed by vitrification were equal to those with fresh oocytes (84), so that the cryopreservation of oocytes is also considered to be an established procedure (85). Since vitrification is superior to slow freezing with respect to these outcomes, it has recently become widespread (86). Oocyte cryopreservation is a feasible option for fertility preservation in patients without partners. However, it is difficult to retrieve oocytes in prepubertal girls because ovulation has not yet begun.
Additionally, patients with hematological malignancies have various obstacles that make it difficult to achieve the cryopreservation of oocytes and embryos. Invasive procedures, such as needle puncture for oocyte retrieval, are difficult to perform in patients who have hematological disorders due to the risks of bleeding and infection. Patients with hematological malignancies often need to start urgent chemotherapy, whereas oocyte retrieval can delay the initiation of chemotherapy by 2 weeks even with a random-start controlled ovarian stimulation. Moreover, if ovarian hyperstimulation syndrome develops, chemotherapy may be further delayed, resulting in exacerbation of the hematological malignancy. Therefore, in acute leukemia or highly aggressive lymphoma, oocyte retrieval is not permitted before or during chemotherapy. In practice, while oocyte retrieval can be achieved prior to chemotherapy in patients with HL, considerably less are retrieved than in those with acute leukemia (87). In anticipation of HSCT after relapse, it may be feasible to perform oocyte retrieval when patients achieve their first complete remission after the completion of chemotherapy and recover menstruation (88).
The clinical pregnancy rate per thawed embryo and oocyte ranges from 30 to 40% and 4.5 to 12%, respectively (89,90), which shows that the retrieval of multiple oocytes is needed to give birth to a child. Women diagnosed with cancer who have their oocytes cryopreserved before chemotherapy have good reproductive outcomes in terms of the oocyte survival rate, fertilization rate and pregnancy rate, without increasing the rate of malformations (91). Meanwhile, another report showed that the live birth rate was decreased even when oocytes were collected before the initiation of chemotherapy (92). Furthermore, there are few consequences in pregnancies with oocytes collected after exposure to chemotherapeutic drugs and radiation. Pregnancy using cryopreserved oocytes and embryos should be allowed on and 3–5 years after the completion of treatment, which reduces the risk of recurrence.
GnRH agonists
Continuous administration of GnRH agonists causes a temporary flare-up of gonadotropins and downregulates GnRH receptors in the pituitary gland 1–2 weeks after administration, resulting in decreased secretion of LH and FSH and suppression of granulosa cell proliferation and follicular growth (93). It may provide ovarian protection by leaving growing follicles at primordial follicles that are relatively less sensitive to chemotherapeutic drugs during chemotherapy. GnRH agonists can also reduce blood flow to the ovaries and reduce exposure to chemotherapeutic drugs. However, since the maturation of primordial follicles to secondary follicles is gonadotropin-independent, the true mechanism of ovarian protection by GnRH agonists remains unclear.
Various randomized controlled trials and a meta-analysis have examined the effects of GnRH administration during chemotherapy (8,94). In these trials, which were conducted mainly in breast cancer patients, the administration of GnRH agonist during chemotherapy reduced the incidence of premature menopause, but did not improve the pregnancy rate (95). However, there are still few data in patients with hematological malignancies. In randomized trials that allowed long-term follow-up for lymphoma, GnRH agonist was not effective at preventing chemotherapy-induced premature menopause and did not influence the pregnancy rate. Based on the above results, ASCO guidelines also indicated that GnRH agonist should not be considered a proven and established method for fertility preservation (8). Menstruation may not stop even after the initiation of chemotherapy. Therefore, the administration of GnRH agonist may be considered for the purpose of stopping menstruation and preventing massive bleeding induced by menstruation during thrombocytopenia.
Ovarian shielding
Most patients with hematological malignancies receive HSCT without any fertility-preserving procedures prior to HSCT. The conditioning regimen produces permanent infertility at a significant rate. To solve this problem, ovarian shielding with metal may be considered during TBI (62,96). Metallic ovarian shielding reduced the actual irradiation dose applied to the ovaries from 12 Gy to 2–3 Gy (97), which, by itself, is unlikely to result in permanent infertility (98). TBI with ovarian shielding is often combined with high-dose cyclophosphamide, which is unlikely to cause permanent infertility in young female patients. In a promising report, the 6-month and 1-year cumulative rates of menstrual recovery were 42 and 78%, respectively, in standard-risk female patients who received HSCT using TBI with ovarian shielding (62). In all patients with menstrual recovery, menstruation recovered within 1 year. The serum AMH level tended to gradually increase after menstrual recovery. The 5-year overall survival and relapse rates were 67 and 31%, respectively, which are comparable to transplantation outcomes in standard-risk patients without ovarian shielding. When the patients were grouped according to their background diseases, relapse was observed in only 2 of 14 patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), whereas 2 patients with acute unclassified leukemia and blastic plasmacytoid dendritic cell neoplasm both relapsed. In a report by the Seattle group, the relapse rate was not increased by a nonmyeloablative conditioning regimen that included only 2 Gy of TBI among patients with myelodysplastic syndrome (MDS) or AML in remission transformed from MDS (99). Based on this result, the relapse rate is not considered to be increased in standard-risk patients, since the ovaries are irradiated with at least 2 Gy. The effectiveness of fertility preservation has been demonstrated not only by the long source-to-axis distance method but also by the moving table method (100). In order to preserve the fertility, reduced intensity conditioning or the dose reduction of TBI has been investigated (67,101). However, conditioning regimen using TBI with ovarian shielding can preserve fertility without reducing treatment intensity on target organs such as bone marrow, indicating the hopeful strategy for female patients who wish to have a child in the future. In pediatric cases, however, the uterus is more susceptible to radiation than in adults and needs to be screened (75,76). However, it is difficult to shield the uterus without shielding the pelvic bone, and thus ovarian shielding with these methods may not be suitable in pediatric patients with hematological malignancies. Recently, intensity-modulated radiation therapy, which uses helical tomotherapy to protect organs to avoid radiation toxicity, has been demonstrated to more safely deliver TBI (102–104). In the future, this method may be used to more accurately shield the ovaries and uterus.
Cryopreservation of ovarian tissue
Since the first birth in a patient with HL was reported in 2004 (105), the cryopreservation of ovarian tissue has gradually increased (106,107). However, it is still considered an experimental procedure. A portion of unilateral ovarian tissue is excised laparoscopically prior to gonadotoxic treatment and cryopreserved by slow freezing or vitrification. After the treatment for cancer is completed, the thawed ovarian tissue is transplanted orthotopically or ectopically. Although orthotopic transplantation may allow natural pregnancy, pregnancy is often attempted using assisted reproduction. The successful live rate per transplantation is 20 to 30% (89). Ovarian tissue cryopreservation does not require ovarian stimulation for follicular development and delays treatment for cancer by 1–2 weeks. Even in prepubertal patients in whom ovulation has not yet started, it is theoretically possible to preserve a large number of oocytes.
However, as with oocyte retrieval, the removal of ovarian tissue is difficult prior to treatment in patients with hematological disorders who are at risk of infection or bleeding. In addition, subsequent reimplantation of ovarian tissue may carry a risk of introducing tumor cells into the patient. Immunodeficient mice were transplanted with some ovarian tissue collected from patients with ALL and thereafter relapsed with ALL (108), so that autotransplantation of ovarian tissue is not recommended for leukemia patients (109). Meanwhile, in patients with HL or non-HL, autotransplantation of ovarian tissue may be considered, provided that no residual disease exists by immunohistochemistry (IHC) or polymerase chain reaction (PCR) and lymphoma is not relapsed in xenotransplantation into immunodeficient mice (109). In a promising report, an AML survivor who underwent HSCT gave birth using cryopreserved ovarian tissue which was carefully evaluated by IHC, PCR, xenotransplantation and a next-generation sequencer before autotransplantation (110). However, it should be noted that these tests are performed on a portion of ovarian tissue and may not reflect the entire harvested tissue. In patients at high risk of tumor contamination, such as those with leukemia, ovarian tissue cryopreservation should not always be denied. Ovarian tissue cryopreservation may be performed in the hope that future advances in reproductive medicine will make it possible to obtain mature oocytes from cryopreserved ovarian tissue with in vitro maturation (111).
Optimized algorithm for fertility preservation
Male patients
Options for fertility preservation in male patients are described in Fig. 1. For postpubertal men, sperm retrieval should be attempted as much as possible prior to the initiation of gonadotoxic treatment, regardless of the type of hematological malignancy (7). If good sperm is not collected before the start of treatment, sperm retrieval is considered after the initial treatment, taking into account the effects of anticancer drugs on sperm (18). If collection is still difficult, TESE may be performed before treatment with strong gonadal toxicity, such as the conditioning regimen in HSCT (41,42). In patients who only receive chemotherapy, spermatogenesis will recover several months to years after the completion of chemotherapy, thereby providing the potential for spontaneous pregnancy. Meanwhile, if permanent azoospermia develops, ICSI using cryopreserved sperm may lead to pregnancy. However, for prepubertal men who have not completed spermatogenesis, there is no established, effective method of fertility preservation. In such cases, testicular tissue cryopreservation may be considered, albeit it is still in an experimental stage (43).
Female patients with acute leukemia and malignant lymphoma
Options for fertility preservation in female patients are described in Fig. 2. Ideally, for postpubertal women, oocytes should be collected prior to the initiation of gonadotoxic treatment (7). However, invasive procedures are difficult to perform in most patients who have hematological malignancies due to bleeding, infection or a need to start urgent chemotherapy. In such cases, oocyte retrieval between courses of chemotherapy can be considered if possible. Pregnancy should be allowed on and 3–5 years after the completion of treatment, which reduces the risk of recurrence. Against this background, it may be realistic to perform oocyte retrieval in anticipation of relapse during complete remission (112). In standard-risk patients, fertility may be preserved after HSCT by using TBI with ovarian shielding in the conditioning regimen, leading to the probability of spontaneous pregnancy (62). Prepubertal women cannot undergo oocyte retrieval due to immature oogenesis, and there are no effective, established procedures for fertility preservation, except for ovarian shielding. Ovarian tissue cryopreservation can be an option, in the expectation of future developments in reproductive medicine, although it is still in an experimental stage (8).
Female patients with chronic myeloid leukemia
Patients with chronic myeloid leukemia (CML) need to take TKIs for a long time, sometimes their whole life, to control their disease. However, TKI may have gonadal toxicity. Therefore, female patients who wish to have a child need special considerations regarding fertility preservation that are different from those in other hematological malignancies (Fig. 3). Patients with chronic phase CML often have no bleeding tendency and susceptibility to infection at the diagnosis, so that attempts can be made to retrieve oocytes before the start of TKIs whenever possible. A patient with accelerated or blastic phase CML should start chemotherapy immediately. Because TKIs may impair organogenesis in developing embryos and result in increased congenital abnormalities (72,73), pregnant patients should not take TKIs. Therefore, whether or not TKIs can be discontinued is an important issue in female patients. Recently, clinical trials on TKI discontinuation have been reported (113–118). In many of these trials, the cessation of TKIs was tried in patients who had maintained MR4.5 for 1–2 years or more, and TKI was resumed in cases with a loss of major molecular response (MMR) (113–116). Although 42–61% of patients lost MMR within 1 year after discontinuation, the remaining patients maintained deep remission for a long time. In addition, almost all patients who lost MMR regained MMR after resuming TKI. Fertility decreases naturally with age, so that second-generation TKIs that can achieve a deeper remission sooner may be appropriate for women who wish to become pregnant (119). Female patients who maintain MR4.5 over a fixed time period may discontinue TKI and try to conceive with or without assisted reproductive technology (ART). Real-time quantitative PCR should be performed to estimate the amount of CML cells every 4–8 weeks for prompt treatment in recurrence (28,120). Given the impact of treatment on the fetus, it remains debatable when to start treatment for CML during relapse. However, loss of complete cytogenetic response or complete hematological response may be one of the inception criteria (28,121). Interferon (IFN)-alfa can be an option in recurrence during pregnancy, since there have been no reports of congenital abnormalities in infants born to female CML patients treated with IFN-alfa (28,119). An alternative approach in patients who cannot tolerate IFN-alfa may be the reintroduction of imatinib only during the third trimester, when organogenesis has finished (28).
Pregnancy or fatherhood after HSCT
Several reports have demonstrated pregnancy and delivery in female patients and the partners of male patients who have received HSCT (21,122–125). Salooja et al. reported that 232 of 37 362 (0.6%) patients conceived after HSCT, consisting of 113 female patients and the partners of 119 male patients (122). In total, 323 conceptions resulted in 271 live births, whereas allograft recipients had significantly increased risks of delivery by Cesarean section, preterm birth and low-birthweight singleton offspring compared with those in the general population. Cesarean sections were frequently performed in female patients who had conceived by ART, which may be a preventive procedure to avoid complications during labor. Rates of congenital anomalies, developmental delay and malignant disease in the offspring of HSCT patients were not higher than those in the normal population. In a retrospective analysis by Carter et al. (123), 34 of 619 patients conceived after HSCT, consisting of 14 pregnancies in 8 female patients and 40 pregnancies in the partners of 26 male patients, resulting in 46 live births. The incidence of no conception was significantly increased in HCT survivors, whereas survivors were not significantly more likely to report miscarriages or stillbirths than their nearest-age siblings. Sanders et al. (21) reported that 146 of 1522 patients conceived after HSCT, consisting of 41 female patients and the partners of 35 male patients, resulting in 35 live births. Pregnancies in female patients who had received HSCT were more likely to be accompanied by preterm delivery and delivery of low-birthweight or very low-birthweight babies who did not seem to be at an increased risk of congenital anomalies. Pregnancies in female patients who received TBI were likely to be accompanied by spontaneous abortion, which may demonstrate that TBI prevents the development of the uterus and induces atrophy. However, the actual success rate of pregnancy depends mainly on how much patients try to conceive, which was not clarified in these reports. In one report, among patients of reproductive age at HSCT, 22% reported attempts to conceive and 10.3% reported success, although half of the participants received a reduced intensity conditioning regimen (126).
Information sharing and ethical issues
Fertility issues need to be fully informed not only to the patient but also to family members, such as partners and parents (127). In cryopreserving sperm, oocytes, embryos or ovarian tissues, the patient or married couple must address the handling for these products beforehand if not used for any reason, including the patient’s death. Reproductive products are often being stored for a long time in the absence of the patient’s consent to discard them, leading to social and financial problems in oncofertility. Therefore, before the preservation, it is necessary to sufficiently inform the patient and the family that the reproductive products will be discarded if the patient dies or if the intention to continue the preservation becomes unconfirmed.
Conclusion
Hematological malignancies complicate fertility preservation in childhood, AYA patients due to unique issues. Ideally, the procedures for fertility preservation should be performed before the initiation of gonadotoxic treatment. However, this is often impossible in practice. Even in such situations, there may be alternative approaches to preserve fertility, and healthcare providers should provide patients with enough information so that patients who wish to become pregnant can choose appropriate methods to preserve fertility. In addition, future advances in reproductive medicine may extend the application of fertility preservation to those in whom it is considered difficult to preserve fertility with current approaches. On the other hand, treatment of hematological malignancies should be prioritized over fertility preservation. Coordination and collaboration between hematologists and fertility specialists are important to optimize the strategy for fertility preservation in each patient through shared decision-making.
Conflict of interest statement
None.


