-
PDF
- Split View
-
Views
-
Cite
Cite
Kosuke Kojo, Koji Kawai, Takashi Kawahara, Tomokazu Kimura, Shuya Kandori, Yoshiyuki Nagumo, Satoshi Nitta, Takahiro Kojima, Ayako Okuyama, Takahiro Higashi, Hiroyuki Nishiyama, Recent malignant testicular tumor trend in Japan, a country with an aging population: a large-scale study of 2012–2015 hospital-based cancer registry data, Japanese Journal of Clinical Oncology, Volume 50, Issue 10, October 2020, Pages 1201–1208, https://doi.org/10.1093/jjco/hyaa110
Close - Share Icon Share
Abstract
Japan’s national database of hospital-based cancer registries is estimated to cover ~67% of all new cancer cases. Using this database, we analyzed the characteristics of the recently diagnosed testicular malignancy.
We obtained data for 6510 adult testicular malignancy patients diagnosed in 2012–2015. The distributions of patient ages, histological diagnoses and testicular germ cell tumor hospital care volumes were determined.
The most common histology was seminoma (60.3% of all testicular malignancies), followed by non-seminoma (24.1%) and diffuse large B-cell lymphoma (13.1%). The median and mean ages of the testicular germ cell tumor patients were high at 38 and 39.8 years, respectively. The age distribution peaked at 30–40 years, followed by 40–50 years. Approximately 18% of testicular germ cell tumor patients were ≥50 years. The ages of the diffuse large B-cell lymphoma patients peaked at 70–80 years (mean 67.7 years). When the analysis was limited to the testicular germ cell tumor patients who received first-course cancer treatment at the participating hospitals, the number of high-volume hospitals with ≥20 testicular germ cell tumor care volume was limited to 61 (10.0% of the 605 hospitals that treated ≥1 testicular germ cell tumor patient). However, when the patients who changed hospitals during treatment or relapsed after treatment completion were analyzed together, the number of high-volume hospitals increased to 104 (17.0% of 612 hospitals).
The testicular germ cell tumor patients’ mean age was nearly 40 years. The proportions of older testicular germ cell tumor patients and diffuse large B-cell lymphoma patients were higher than previously thought. The reasons for this trend are unknown, but it is important to address the trend identified herein in a country with a super-aging population.
Introduction
Although testicular germ cell tumor (TGCT) is the most common testicular malignancy (TM), it is rare. The reported incidence of TGCT in western countries is only 5–10 per 100 000 persons, and the incidence in Japan is even more rare at ~1.3 per 100 000 persons (1). Hematolymphoid tumors (HLTs), which are the most common non-germ cell TM in elderly men, are also rare (2). Population-based studies in western countries have estimated the annual incidence of HLT at 0.09–0.26 per 100 000 persons (2), but the HLT incidence in Japan is unknown. Other testicular malignancies are extremely rare.
Several investigations have described the increasing age of TGCT patients. Ruf et al. (3) reported that the mean age of TGCT patients in Germany increased from 28 years (before 1990) to 36 years (2005–2010). In Japan, Miki et al. (4) reported that the median age of 1121 patients with TGTC was 37 years, based on the Japanese Urological Association (JUA) registration data in 2005 and 2008. Although some investigators suggested that the increasing incidence of seminoma relative to non-seminoma might be associated with this trend (3,5), the exact reason(s) for the increasing age of these patients are unknown. When considering TGCT chemotherapy, it should be noticed that older men are at a higher risk for complications (6,7).
The super-aging of the population is occurring in many countries; among them, Japan has the highest rate of aging in the world, followed by Germany. In 2016, Japan’s aging rate (here, the ratio of elderly [aged ≥65 years] to the total population) was high at 27.3%. The corresponding rates in 2000 and 2010 were 17.4 and 23%, respectively (8). It is thus possible that the continued progression of aging has affected the age distribution of TGCT patients and the proportions of non-gem cell testicular malignancies such as HLTs and other cancers.
To clarify these clinical characteristics of recently diagnosed TM patients, we analyzed the cases of the 6510 patients in Japan diagnosed with TM between January 2012 and December 2015 by obtaining their records from Japan’s hospital-based cancer registry (HBCR), which archives newly diagnosed cancer cases in designated cancer care hospitals (DCCHs) and other prefectural recommended hospitals (9–11). Using this database, we also analyzed the TM care volume in 623 participating hospitals during the same 4-year period.
Patients and methods
Data source and selection of data
Since 2007, Japan’s national database has been collecting HBCR data from the DCCHs, which were assigned by the Ministry of Health, Labor and Welfare. An increasing number of prefectural governments have also been designating a wide range of community cancer hospitals. The number of DCCHs and other hospitals submitting their data increased from 422 in 2012 to 751 in 2015. As a result, the HBCR database covers ~67% of all new cancer cases in Japan (9–11). We collected all of the HBCR data of the patients with a malignant testicular tumor diagnosed between January 2012 and December 2015 in order to determine the clinical characteristics of individuals who were recently diagnosed with a TM.
In the HBCR, trained cancer registrars at each hospital register each cancer case based on standardized rules and criteria. The HBCRs collect both demographic and cancer characteristic data, including topology and morphology codes of the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3), the UICC TNM classification (7th edition) and the initial treatments. The malignant testicular tumor cases other than HLT were classified as stages I–III according to the TNM classification for TGCT. Since most of the HLTs were malignant lymphomas, the HLTs were classified as stages I–IV according to the Ann Arbor classification for malignant lymphoma (12).
Identification of malignant testicular tumors
We included the cases of the patients who met the following criteria (Fig. 1): (i) the tumor(s) were diagnosed between 2012 and 2015, (ii) the registered site was the testis with the ICD-O-3 topology code C62, (iii) the patient underwent cancer treatment at the participating hospital, (iv) the tumor(s) were classified with the ICD-O-3 morphology code as carcinoma in situ or malignant, primary site and (v) the case was picked up with the ICD-O-3 morphology code and classified according to the General Rule for Clinical and Pathological Studies on Testicular Tumors (4th edition) (13) as a germ cell tumor, sex cord-stromal tumor, miscellaneous tumor of the testis, HLT or tumor of collecting ducts and rete testis. We set aside the code registered as described below: paratesticular tumors, mesenchymal tumors of the spermatic cord and testicular adnexa and other tumors that cannot be classified according to the general rule.
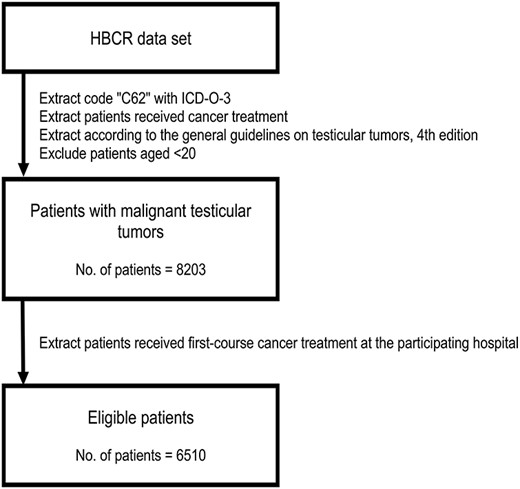
Data selection
The single exclusion criterion was age <20 years (Fig. 1); this was because some pediatric DCCHs were not part of the database.
Classification by courses of treatment
The registration classified the cases into three groups based on the courses of treatment: ‘A’ patients were diagnosed and started first-course cancer therapy at the participating hospital; ‘B’ patients were diagnosed at another hospital and referred to begin the first-course treatment at the participating hospital and ‘C’ patients started the first-course cancer therapy at another hospital and were referred to continue or change the treatment at the participating hospital or relapsed as detected at the participating hospital after they completed their cancer therapy.
We extracted the cases of the group A and group B patients as eligible for the present analyses because they received first-course cancer treatment at the participating hospitals, which were primarily responsible for these groups (Fig. 1). In addition, the appropriate data such as cancer stage were more accurate for groups A and B than for group C. To prevent the double registration of any patients, we did not use the data of the group C patients for our analysis of the patient characteristics, but we reviewed the group C patients in our analysis of the distribution of hospital care.
Cancer staging
In the HBCR, both the clinical and pathological UICC TNM classifications are registered. We typically used the clinical stage to analyze the distribution of stages. In cases with missing clinical stage data, we used the pathological stage in substitution for the clinical stage in accordance with a previous study (11).
The number of patients requiring hospital care
We calculated the distribution of hospitals according to the number of group A and group B patients who received first-course cancer treatment. We used the data during the 4 years beginning with 2012, but some of the participating hospitals were not involved in the HBCR throughout the entire 4-year (2012–2015) period. We thus modified the number of patients per 4 years according to how long the respective hospitals had participated in the HBCR.
Statistical analyses
We used the χ2 test to evaluate differences in categorical variables. P values <0.05 were considered significant. All χ2 statistics were calculated in the Categorical Response Analysis module of JMP 14 software (SAS, Cary, NC).
Ethical considerations
The study protocol and data processing were approved by the Tsukuba University Hospital Ethical Board (H29–267). If the histology was rare and the group of patients was extremely small, i.e. n < 10, we present only the approximate number in order to avoid identifying personal information according to recommendation from the Ministry of Health, Labour and Welfare.
Results
We analyzed the cases of a total of 6510 patients with malignant testicular tumors from 623 participating hospitals (Fig. 1); among the 623 hospitals, 605 treated at least one TGCT patient. The most common histology was seminoma (60.3%), followed by non-seminoma (24.1%) and diffuse large B-cell lymphoma (DLBCL, 13.1%). Table 1 shows the age distribution according to tumor histology. The median and mean ages of the 5577 TGCT patients were 38 and 39.8 years, respectively. The mean age of the seminoma patients was 41.4 years, higher than that of the non-seminoma patients, 35.4 years. The mean age of the HLT patients was 67.8 years. DLBCL was the predominant histology, accounting for 94.8% of all of the HLTs. Other types of lymphomas were extremely rare. Only 14 patients had malignant sex cord-stromal tumors; their mean age was 57.8 years.
| . | . | n (%) . | Median age, years . | Mean age, years . | Age range, years . | Age IQR, years . |
|---|---|---|---|---|---|---|
| Malignant testicular tumors | 6510 (100%) | >40 | >43.8 | 20–95 | 33–52 | |
| Germ cell tumors | 5577 (85.7%) | >38 | >39.8 | 20–92 | 32–46 | |
| Seminomaa | 3925 (60.3%) | >40 | >41.4 | 20–88 | 34–48 | |
| Non-seminomab | 1570 (24.1%) | >34 | >35.4 | 20–83 | 27–41 | |
| Spermatocytic tumorc | 28 (0.4%) | >65 | >61.7 | 31–92 | 53–72 | |
| Well-differentiated neuroendocrine tumord | 13 (0.2%) | >66 | >57.8 | 23–76 | 47–71 | |
| Other germ cell tumorse | Each <10 (<0.2%) | |||||
| Sex cord-stromal tumorsf | 14 (0.2%) | >64 | >58.4 | 37–76 | 43–72 | |
| HLTs | 911 (14.0%) | >69 | >67.8 | 21–95 | 61–77 | |
| DLBCLsg | 856 (13.1%) | >69 | >67.7 | 21–94 | 61–76 | |
| Other HLTsh | Each <10 (<0.2%) | |||||
| Othersi | Each <10 (<0.2%) | |||||
| . | . | n (%) . | Median age, years . | Mean age, years . | Age range, years . | Age IQR, years . |
|---|---|---|---|---|---|---|
| Malignant testicular tumors | 6510 (100%) | >40 | >43.8 | 20–95 | 33–52 | |
| Germ cell tumors | 5577 (85.7%) | >38 | >39.8 | 20–92 | 32–46 | |
| Seminomaa | 3925 (60.3%) | >40 | >41.4 | 20–88 | 34–48 | |
| Non-seminomab | 1570 (24.1%) | >34 | >35.4 | 20–83 | 27–41 | |
| Spermatocytic tumorc | 28 (0.4%) | >65 | >61.7 | 31–92 | 53–72 | |
| Well-differentiated neuroendocrine tumord | 13 (0.2%) | >66 | >57.8 | 23–76 | 47–71 | |
| Other germ cell tumorse | Each <10 (<0.2%) | |||||
| Sex cord-stromal tumorsf | 14 (0.2%) | >64 | >58.4 | 37–76 | 43–72 | |
| HLTs | 911 (14.0%) | >69 | >67.8 | 21–95 | 61–77 | |
| DLBCLsg | 856 (13.1%) | >69 | >67.7 | 21–94 | 61–76 | |
| Other HLTsh | Each <10 (<0.2%) | |||||
| Othersi | Each <10 (<0.2%) | |||||
IQR, interquartile range; HLT, hematolymphoid tumor; DLBCL, diffuse large B-cell lymphoma. ICD-O-3 morphology codes are as follows.
a9061/3, 9062/3.
b9065/3, 9070/3, 9071/3, 9080/3, 9084/3, 9085/3, 9100/3, 9101/3.
c9063/3.
d8240/3.
e9064/2 9064/3 9081/3.
f8631/3, 8640/3, 8650/3.
g9680/3.
h9590/3, 9591/3, 9599/3, 9670/3, 9673/3, 9679/3, 9687/3, 9687/3, 9688/3, 9690/3, 9702/3, 9719/3, 9728/3, 9731/3, 9734/3, 9755/3, 9761/3, 9827/3, 9930/3.
i8140/3, 8470/3.
| . | . | n (%) . | Median age, years . | Mean age, years . | Age range, years . | Age IQR, years . |
|---|---|---|---|---|---|---|
| Malignant testicular tumors | 6510 (100%) | >40 | >43.8 | 20–95 | 33–52 | |
| Germ cell tumors | 5577 (85.7%) | >38 | >39.8 | 20–92 | 32–46 | |
| Seminomaa | 3925 (60.3%) | >40 | >41.4 | 20–88 | 34–48 | |
| Non-seminomab | 1570 (24.1%) | >34 | >35.4 | 20–83 | 27–41 | |
| Spermatocytic tumorc | 28 (0.4%) | >65 | >61.7 | 31–92 | 53–72 | |
| Well-differentiated neuroendocrine tumord | 13 (0.2%) | >66 | >57.8 | 23–76 | 47–71 | |
| Other germ cell tumorse | Each <10 (<0.2%) | |||||
| Sex cord-stromal tumorsf | 14 (0.2%) | >64 | >58.4 | 37–76 | 43–72 | |
| HLTs | 911 (14.0%) | >69 | >67.8 | 21–95 | 61–77 | |
| DLBCLsg | 856 (13.1%) | >69 | >67.7 | 21–94 | 61–76 | |
| Other HLTsh | Each <10 (<0.2%) | |||||
| Othersi | Each <10 (<0.2%) | |||||
| . | . | n (%) . | Median age, years . | Mean age, years . | Age range, years . | Age IQR, years . |
|---|---|---|---|---|---|---|
| Malignant testicular tumors | 6510 (100%) | >40 | >43.8 | 20–95 | 33–52 | |
| Germ cell tumors | 5577 (85.7%) | >38 | >39.8 | 20–92 | 32–46 | |
| Seminomaa | 3925 (60.3%) | >40 | >41.4 | 20–88 | 34–48 | |
| Non-seminomab | 1570 (24.1%) | >34 | >35.4 | 20–83 | 27–41 | |
| Spermatocytic tumorc | 28 (0.4%) | >65 | >61.7 | 31–92 | 53–72 | |
| Well-differentiated neuroendocrine tumord | 13 (0.2%) | >66 | >57.8 | 23–76 | 47–71 | |
| Other germ cell tumorse | Each <10 (<0.2%) | |||||
| Sex cord-stromal tumorsf | 14 (0.2%) | >64 | >58.4 | 37–76 | 43–72 | |
| HLTs | 911 (14.0%) | >69 | >67.8 | 21–95 | 61–77 | |
| DLBCLsg | 856 (13.1%) | >69 | >67.7 | 21–94 | 61–76 | |
| Other HLTsh | Each <10 (<0.2%) | |||||
| Othersi | Each <10 (<0.2%) | |||||
IQR, interquartile range; HLT, hematolymphoid tumor; DLBCL, diffuse large B-cell lymphoma. ICD-O-3 morphology codes are as follows.
a9061/3, 9062/3.
b9065/3, 9070/3, 9071/3, 9080/3, 9084/3, 9085/3, 9100/3, 9101/3.
c9063/3.
d8240/3.
e9064/2 9064/3 9081/3.
f8631/3, 8640/3, 8650/3.
g9680/3.
h9590/3, 9591/3, 9599/3, 9670/3, 9673/3, 9679/3, 9687/3, 9687/3, 9688/3, 9690/3, 9702/3, 9719/3, 9728/3, 9731/3, 9734/3, 9755/3, 9761/3, 9827/3, 9930/3.
i8140/3, 8470/3.
As shown in Fig. 2a, the age distribution of all TGCT patients peaked at 30–40 years, followed by a peak at 40–50 years. The age distribution of the seminoma patients showed the same pattern, but the age distribution of the non-seminoma patients showed a major peak at 30–40 years, followed by 20–30 years. Thus, the proportion of seminomas among TGCT cases was increased in the elderly patients; it was 69.7% in the patients aged 30–40 years and increased to 80.4% in the patients aged ≥50 years.
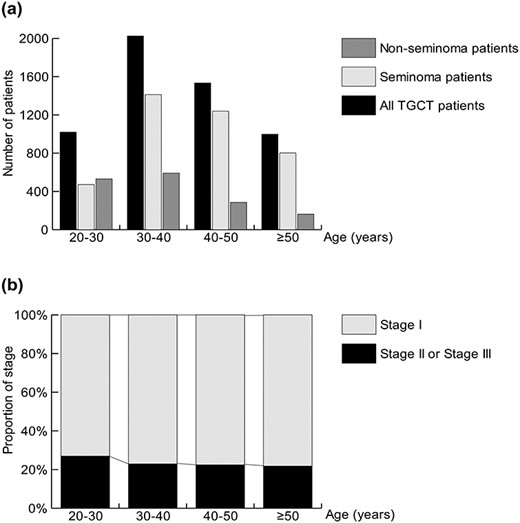
Patient number (a) and clinical stage (b) of TGCT stratified by age groups.
The TGCT stage data were available for 5373 patients. The proportion of stage II or III disease in the TGCT group was significantly higher in the patients aged 20–30 years compared to the expected value (Fig. 2b, 27.0%, 263 of 975 patients, P = 0.019). The proportion of stage II or III disease was quite similar in the patients aged ≥30 years at ~22%.
A total of 1860 malignant testicular tumor patients aged ≥50 years were identified, with seminoma (802 of 1860 patients, 43.1%), non-seminoma (162 of 1860 patients, 8.7%) and DLBCL (794 of 1860 patients, 42.7%) accounting for 94.5% of all testicular malignancies in patients aged ≥50 years. Figure 3 shows the distribution of tumor histology stratified by age group. Seminoma was predominant in the patients aged 50–60 years (566 of 820 patients, 69.0%). In contrast, DLBCL was the most common histology among the patents aged ≥60 years, which peaked at 70–80 years.

Patients’ number and histology of testicular tumors among patients aged ≥50 years. DLBCL, diffuse large B-cell lymphoma.
The DLBCL stage data were available for 722 patients. Figure 4 shows the proportion of stage III or IV disease in the DLBCL patients according to age. Stage III or IV disease accounted for ~40% of the DLBCL patients aged ≥70 years.
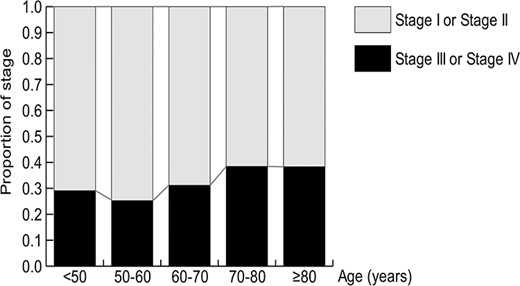
Table 2 summarizes the data of patients and stages stratified by hospital TGCT case volume during the 4-year study period. When we dealt with the hospitals that had participated in the HBCR for <4 years, we converted the number of hospitals treating patients to the number per 4 years. The numbers of hospitals treating 20–29 patients and ≥30 patients were limited to 42 (6.9%) and 19 (3.1%), respectively. The proportion of stage II or III disease was not significantly different among hospitals (Fig. 5). However, when we analyzed the patients who changed hospitals during treatment or relapsed after completing their treatment together, as shown in Table 3, the numbers of hospitals treating 20–29 patients and ≥30 patients increased to 66 (10.8%) and 38 (6.2%), respectively. Moreover, 625 (47.5%) of the TGCT patients in the group were managed in hospitals treating ≥30 cases.
Discussion
The results of our analyses of the large-scale HBCR data of 5577 patients revealed that the median and mean ages of the TGCT patients diagnosed during the 4 years from 2012 to 2015 were 38 and 39.8 years, respectively. Our analyses uncovered several relevant findings regarding the trends of testicular malignancies in Japan, which has the highest rate of aging worldwide.
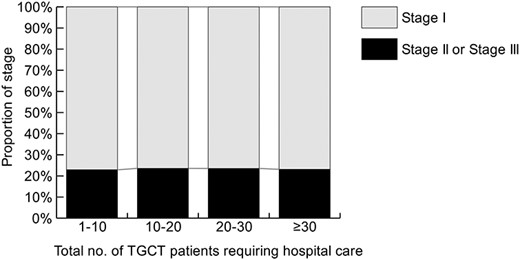
Clinical stage of testicular germ cell tumor (TGCT) stratified by the hospital group.
| No. of cases . | No. of hospitals . | Total no. of patients . |
|---|---|---|
| 1–9 | 349 (57.7%) | 1550 (27.8%) |
| 10–19 | 195 (32.2%) | 2465 (44.2%) |
| 20–29 | 42 (6.9%) | 854 (15.3%) |
| ≥30 | 19 (3.1%) | 708 (12.7%) |
| All | 605 | 5577 |
| No. of cases . | No. of hospitals . | Total no. of patients . |
|---|---|---|
| 1–9 | 349 (57.7%) | 1550 (27.8%) |
| 10–19 | 195 (32.2%) | 2465 (44.2%) |
| 20–29 | 42 (6.9%) | 854 (15.3%) |
| ≥30 | 19 (3.1%) | 708 (12.7%) |
| All | 605 | 5577 |
| No. of cases . | No. of hospitals . | Total no. of patients . |
|---|---|---|
| 1–9 | 349 (57.7%) | 1550 (27.8%) |
| 10–19 | 195 (32.2%) | 2465 (44.2%) |
| 20–29 | 42 (6.9%) | 854 (15.3%) |
| ≥30 | 19 (3.1%) | 708 (12.7%) |
| All | 605 | 5577 |
| No. of cases . | No. of hospitals . | Total no. of patients . |
|---|---|---|
| 1–9 | 349 (57.7%) | 1550 (27.8%) |
| 10–19 | 195 (32.2%) | 2465 (44.2%) |
| 20–29 | 42 (6.9%) | 854 (15.3%) |
| ≥30 | 19 (3.1%) | 708 (12.7%) |
| All | 605 | 5577 |
Patients restratified by the hospital group when the cases of changing hospitals and relapse are included
| No. of cases . | No. of hospitals . | No. of eligible patients . | No. of patients changing hospitals or relapsed . |
|---|---|---|---|
| 1–9 | 316 (51.6%) | 1249 (22.4%) | 122 (9.3%) |
| 10–19 | 192 (31.4%) | 2215 (37.9%) | 271 (20.6%) |
| 20–29 | 66 (10.8%) | 1117 (20.0%) | 299 (22.7%) |
| ≥30 | 38 (6.2%) | 1096 (19.7%) | 625 (47.5%) |
| All | 612 | 5577 | 1317 |
| No. of cases . | No. of hospitals . | No. of eligible patients . | No. of patients changing hospitals or relapsed . |
|---|---|---|---|
| 1–9 | 316 (51.6%) | 1249 (22.4%) | 122 (9.3%) |
| 10–19 | 192 (31.4%) | 2215 (37.9%) | 271 (20.6%) |
| 20–29 | 66 (10.8%) | 1117 (20.0%) | 299 (22.7%) |
| ≥30 | 38 (6.2%) | 1096 (19.7%) | 625 (47.5%) |
| All | 612 | 5577 | 1317 |
Patients restratified by the hospital group when the cases of changing hospitals and relapse are included
| No. of cases . | No. of hospitals . | No. of eligible patients . | No. of patients changing hospitals or relapsed . |
|---|---|---|---|
| 1–9 | 316 (51.6%) | 1249 (22.4%) | 122 (9.3%) |
| 10–19 | 192 (31.4%) | 2215 (37.9%) | 271 (20.6%) |
| 20–29 | 66 (10.8%) | 1117 (20.0%) | 299 (22.7%) |
| ≥30 | 38 (6.2%) | 1096 (19.7%) | 625 (47.5%) |
| All | 612 | 5577 | 1317 |
| No. of cases . | No. of hospitals . | No. of eligible patients . | No. of patients changing hospitals or relapsed . |
|---|---|---|---|
| 1–9 | 316 (51.6%) | 1249 (22.4%) | 122 (9.3%) |
| 10–19 | 192 (31.4%) | 2215 (37.9%) | 271 (20.6%) |
| 20–29 | 66 (10.8%) | 1117 (20.0%) | 299 (22.7%) |
| ≥30 | 38 (6.2%) | 1096 (19.7%) | 625 (47.5%) |
| All | 612 | 5577 | 1317 |
The median and mean ages of the patients in this study were both 2 years higher than those of the JUA registration study using data collected in 2005 and 2008 (36 and 37.0 years, respectively) (4). This may be due to differences in study design such as the sample size (5572 vs. 1121 patients) and/or the participating institutions. However, the increase in patients’ ages between the study periods might be partly responsible for the older age in this study. In addition, large-scale TGCT registry data in the United States revealed that the mean ages of Hispanic and Caucasian TGCT patients were 29.7 and 35.7 years, respectively (14). In European countries, the reported mean age of TGCT patients was 30.8–31.8 years (15–17), while the New Zealand Cancer Registry reported the median age of 36 years in a European cohort (18). Our present findings thus document the highest mean and median ages for TGCT patients.
As this was a cross-sectional study, we cannot draw conclusions about whether there is a tendency of increasing age among TGCT patients. However, several German studies showed a significant shift toward older age at TGCT diagnosis; for example, Ruf et al. (3) reported that the mean age increased from 28 years before 1990 to 36 years in 2005–2010. They pointed out that the proportion of seminoma patients also increased from 30.9 to 56.0%. Stang et al. (19) read the Ruf et al.’s article and reported in their Letter to Editor that the mean age increased from 36.3 years in 1996 to 38.4 years in 2008. Stang et al. also expressed their hypothesis that the age of TGCT patients might be influenced by the age distribution of the underlying population at risk, as the mean age of the male population at risk in the country changed from 37.9 years in 1996 to 43.2 years in 2008. In this study, the proportion of seminoma patients was 70.7%, and this proportion was higher than the proportion in the previous study in Japan, which was 61.9% (4). According to the national census in Japan, the mean age of Japanese men increased to 44.8 years in 2015 from 41.9 years in 2005 (20). Therefore, both factors (i.e. the proportion of seminoma patients in TGCT and the increase of the mean age of male population) may be responsible for the older age of the patients in this study. As another explanation, there is a possibility that the increase of seminoma patient age may be larger than that of non-seminoma. Using JUA registration data in 2005 and 2008, Kawai et al. (21) reported that the proportion of patients aged 40 years or older in adult seminoma patients was 42.6%, whereas that of non-seminoma was 26.2%. In this study, the proportions of patients aged 40 years or older in seminoma and non-seminoma were 52.0 and 28.5%, respectively. However, other reasons—such as unknown environmental or occupational risk exposure—might also contribute to the older age of TGCT patients.
HLT is known as a major TM in men aged ≥70 years. In this study, HLT accounted for 14.0% of all testicular malignancies, which is higher than the reported values, i.e. <5–7% of testicular malignancies (2,22). The median and mean ages of the present HLT patients were 69 and 67.8 years, respectively. Most of the HLT cases were DLBCLs, accounting for 94.0% of the HLT cases; this is similar to previous results (2). The age distribution of the DLBCL patients peaked at 70–80 years, followed by a peak at 60–70 years (Fig. 3). Therefore, the increasing elderly population at risk for developing DLBCL may be responsible for the high proportion of HLTs.
As shown in Fig. 3, DLBCL was the most common TM in men aged ≥60 years, in sharp contrast to the men aged 50–60 years, in whom seminoma was the major histology type. There was an increasing tendency of patients to have advanced disease at ages 70–80 and ≥80 years. Therefore, when testicular malignancies are suspected in men aged ≥60 years, a consultation with a hematologist is mandatory.
The results of this study demonstrate that many more elderly TGCT patients are being treated in Japan than was previously recognized. Our analyses revealed that 27.5 and 17.9% of the TGCT patients were aged 40–50 years and ≥50 years, respectively. The proportion of patients aged ≥50 years was higher than that reported at 5.6–11.6% (5,6,21). As shown in Fig. 2b, the proportion of patients with advanced disease was not significantly different among the age groups ≥30 years. In fact, during the 4-year study period, 206 patients aged ≥50 years were diagnosed with advanced TGCT. Older age is a risk factor for chemotherapy-related complications and poorer outcomes. Age ≥40 years was associated with a higher risk of relapse and death from metastatic TGCT (22).
Feldman et al. (6) reported that ~60% of patients aged ≥50 years who received first-line chemotherapy experienced complications, leading to treatment discontinuation, treatment delay or regimen change. Moreover, aging is a definite risk factor for bleomycin pulmonary toxicity. Simpson et al. (7) found that 3 of 11 patients aged ≥50 years developed fatal bleomycin pulmonary toxicity (7). For patients aged ≥50 or ≥40 years with other risk factors such as impaired renal function, alternative regimens such as etoposide, ifosfamide and cisplatin should therefore be considered (23).
Finally, we evaluated the TGTC case volume of hospital where patients received the first-course treatment and the case volume of hospital accepted patients after receiving first-course treatment. As shown in Table 2, only 60 hospitals treated ≥20 patients with TGCT who required hospital care (10.0% of the participating hospitals). However, when we analyzed the patients who changed hospitals during treatment or relapsed after treatment completion together, the hospitals treating 20–29 patients and ≥30 patients increased to 66 (10.8%) and 38 (6.2%), respectively (Table 3). Most of the patients (70.2%) were registered in hospitals treating ≥20 patients. In the HBCR, the detailed information of the patients who changed hospitals or relapsed was unavailable, and we could not analyze the actual situation of centralization of TGTC management, which should be defined by the number of advance cases. In fact, the centralization of management has resulted in improved survival outcomes of advanced cases (24,25). To further understanding the centralization of management in Japan, future investigations with more detailed information regarding the referral pattern is needed.
This study has several limitations due to the unavailability of data in the HBCR. Detailed information is not available regarding the patients’ clinical condition or treatment. The calculated age distribution and mean/median age were obtained by the dataset excluding age <20, since HBCR did not fully register pediatric patients. The data contained details of only the registered information provided at the registering facility. Moreover, although the HBCR covers 67% of all cancer patients in Japan, additional data from other hospitals may be required to gain a more accurate national profile of testicular malignancies.
Conclusions
This study obtained important findings regarding the trends of testicular malignancies in Japan, the country with the highest rate of aging. The findings are also informative for many other countries where the elderly population is increasing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
None.


