-
PDF
- Split View
-
Views
-
Cite
Cite
Tom A. Royer, Bonnie B. Pendleton, Norman C. Elliott, Kristopher L. Giles, Greenbug (Hemiptera: Aphididae) Biology, Ecology, and Management in Wheat and Sorghum, Journal of Integrated Pest Management, Volume 6, Issue 1, March 2015, 19, https://doi.org/10.1093/jipm/pmv018
Close - Share Icon Share
Abstract
Greenbug, Schizaphis graminum (Rondani), is a major insect pest of wheat and sorghum on the southern Great Plains. In this review, we outline greenbug life history and biology, describe direct and indirect crop injury to wheat and sorghum, and discuss current management strategies such as biological control, cultural control focusing on host plant resistance, monitoring, and chemical control that can be incorporated into an integrated pest management program.
Greenbug, Schizaphis graminum (Rondani) (Hemiptera: Aphididae), is one of several aphids that infest cereal grains (Teetes and Pendleton 2000, Royer 2007). It is among the most important insect pests of winter wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor (L.) Moench) in the southern Great Plains (Starks and Burton 1977, Webster 1995). Greenbug nymphs and adults use piercing-sucking mouthparts to feed on aboveground stems and leaves. Their feeding inhibits plant growth or kills plants, resulting in less yield and lower economic return (Burton et al. 1985, Royer et al. 2004). Adults have a high reproductive capacity and greenbug abundance increases rapidly when abiotic factors (temperature and scotophase) are favorable and biotic factors (natural enemies and resistant plants) are not present (Starks and Burton 1977).
Infestation by greenbugs occurs annually in winter wheat and sorghum on the southern Great Plains, but large-scale outbreaks are rare, occurring every 5–10 yr (Starks and Burton 1977; Giles et al. 2000, 2003). Seasonal infestation patterns have been described in wheat (Rogers et al. 1972, Brooks 1989) and sorghum (Daniels 1977a, b), but greenbug is also a pest of other small grains and some turfgrasses (Blackman and Eastop 2000).
Greenbug management in winter wheat and sorghum has been optimized during the past 20 yr through the conservation of natural enemies, development of resistant varieties, adoption of reduced tillage, deployment of insecticide-treated seed, and improved sampling protocols that allow for judicious suppression of greenbugs. Here, we offer an overview of greenbug ecology and discuss integrated pest management (IPM) tactics for this insect pest in wheat and sorghum.
Origin, Geographical Distribution, and Hosts
While the specific origin of greenbug remains in doubt, it is likely of Palearctic origin and now widely distributed throughout southern Europe, the Middle East, central western and central Asia, Africa, and North and South America (Blackman and Eastop 2000). Greenbug was first identified as a pest of wheat in the United States in 1882 (Webster and Phillips 1912). Although sorghum was known as a host for greenbug as early as 1863 (Webster and Phillips 1912) and an outbreak was reported in sorghum in 1916 (Hayes 1922), greenbug did not become a prominent pest of sorghum in the United States until 1968 (Harvey and Hackerott 1969). Numerous (more than 70) grasses and cereals serve as hosts for greenbug, including cultivated barley, Hordeum vulgare L.; maize, Zea mays L.; oats, Avena sativa L.; rice, Oryza sativa L.; rye, Secale cereale L.; sorghum, Sorghum bicolor (L.) Moench; wheat, Triticum aestivum L.; and the turfgrasses Kentucky bluegrass, Poa pratensis L.; (Street et al. 1978, Bowed 1983, Kindler et al. 1983), as well as, more recently, seaside paspalum, Paspalum vaginatum L. (Nuessly et al. 2008).
Weedy hosts include species of wheatgrass (Agropyron spp.), bentgrass (Agrostis spp.), foxtail (Alopecurus spp.), bluestem and broomsedge (Andropogon spp.), old-world bluestem (Bothrichloa spp.), grama grass (Bouteloua spp.), bromegrass (Bromus spp.), Chloris spp., bermudagrass (Cynodon spp.), orchardgrass (Dactylis spp.), crabgrass (Digitaria spp.), barnyardgrass (Echinochloa spp.), goosegrass (Elusine spp.), wildrye (Elymus spp.), lovegrass (Eragrostis spp.), fescue (Festuca spp.), ryegrass (Lolium spp.), panicgrass (Panicum spp.), fountaingrass (Pennisetum spp.), timothy (Phleum spp.), Setaria spp., Indiangrass (Sorghastrum spp.), johnsongrass (Sorghum spp.), needlegrass (Stipa spp.), Tripsacum spp., and teosinte (Zea spp.), (Dahms et al. 1954, Michels 1986).
Description and Life History
Greenbug was first described by Rondani (1852) in Italy. Both wingless (Fig. 1) and winged (Fig. 2) forms of greenbug can be found on their host plants. Adults and nymphs are oval-shaped with light-green bodies and a darker green stripe down the back. They possess two cornicles (commonly called “tailpipes”) with dark tips on the posterior end of the abdomen, and they have green legs with dark tarsi (commonly called “feet”) and dark antennae. All wingless greenbugs are female and give birth to live young via parthenogenesis. Winged forms may be parthenogenic females or winged male and female sexual morphs that can mate and produce eggs. Winged and wingless greenbugs measure 1.3–2.1 mm (Blackmon and Eastop 2000), but Washburn (1908) in describing the winged sexual forms, indicated that males were slightly smaller than females. Winged forms have a brownish-yellow head and prothorax with black lobes on the thorax and a yellowish-green to dark-green abdomen and transparent wings that extend past the abdomen.

Wingless greenbugs are green with a darker green stripe along the top of their back. They have dark-tipped cornicles and tarsi and dark antennae. Photo by Richard Grantham, Oklahoma State University.

Winged greenbugs will colonize new sorghum or wheat fields. Photo by Richard Grantham, Oklahoma State University.
Greenbugs are monoecious (do not alternate to a non-grass host to reproduce sexually) on true grasses (Family Poaceae). They are anholocyclic (produce only females that reproduce asexually) and overwinter as nymphs or adults in southern regions of the United States, but are holocyclic (produce male and female aphids that reproduce sexually and lay eggs) and overwinter as eggs in northern areas of the United States. Webster and Phillips (1912) and Daniels (1956) indicated that the dividing line between anholocyclic and holocyclic reproduction was “the 35th parallel,” suggesting that day length and temperature might regulate these cycles. A combination of temperature and scotophase has been shown to regulate the production of sexuales, which corresponds to Webster and Phillips’ observation (Wadley 1931, Mayo and Starks 1972, 1974; Puterka and Slosser 1983, 1986; Mittler and Gorder 1991).
The biology of greenbug has been studied extensively. Greenbug survival, growth, and reproduction are influenced significantly by temperature (Kirkland et al. 1981, Walgenbach et al. 1988). Jones et al. (2008) found that greenbugs supercool at approximately −26°C and winter survival would therefore not be possible in geographical areas with this temperature. Based on historical data, −26°C has been observed in the Texas Panhandle and north of Oklahoma, but not in North-Central Oklahoma and south through Central Texas where greenbug infestations are common throughout winter months (Giles et al. 2003, Oklahoma Mesonet 2015). Although greenbugs overwinter as nymphs and adults in Oklahoma and Texas, Arnold (1981) found that greenbug survival during the winter can be significantly reduced when nighttime temperatures cool to −12.3°C for 3 d coupled with day temperatures that remained colder than 0°C for 2 d.
The lower development threshold (temperature where greenbugs begin to develop) is approximately 3.5–6.0°C and upper development temperature (temperature where reproduction ceases) is approximately 37°C (Kirkland et al. 1981, Walgenbach et al. 1988). The optimal development threshold for greenbug was lower for a wheat-adapted biotype (Walgenbach et al. 1988) compared with a sorghum-adapted biotype (Kirkland et al. 1981). Under optimal temperatures of 24–30°C, a newborn nymph will attain adulthood in 5 d, begin giving birth to live young in less than 0.5 d, and live for 25 d. One female can produce 60–80 offspring (Walgenbach et al. 1988). Under ideal conditions, numbers could double every 2 d; however, greenbug population abundance fluctuates because of mortality and emigration influenced by weather, natural enemies, and host plant conditions (Rogers et al. 1972, Wallin and Loonan 1977, Hamilton et al. 1982, Summy and Gilstrap 1983, Sumner et al. 1986, Kieckhefer and Gellner 1988). Development and reproduction are also affected by resistant crop varieties (Wood 1961, Kirkland et al. 1981, Kindler and Spomer 1986, Sumner et al. 1986, Kerns et al. 1989) and alternate grass hosts (Stoner and Kieckhefer 1979, Sambaraju and Pendleton 2005).
Greenbug Feeding and Injury to Small Grains and Sorghum
Greenbugs use needle-like, piercing-sucking mouthparts to extract plant sap from the phloem of their host. As they feed, greenbugs inject saliva into the plants to enhance food uptake. Greenbug feeding imparts visible injury to wheat and sorghum. Early feeding damage in wheat appears as yellow or orange spots that have a dark necrotic lesion in the center (Fig. 3a and b). Sorghum reacts similarly, but the damaged spots first appear more reddish and then yellow (Fig. 4a and b). Feeding can result in death of sorghum leaves (Teetes and Johnson 1974) or death of seedling sorghum or wheat (Dahms and Wood 1957).

Typical damage caused by greenbugs feeding on wheat. (a) greenbugs and mummies on wheat leaf. (b) Stunted wheat from greenbug feeding (left) and uninfested wheat because greenbugs were controlled with an insecticide seed treatment (right). Photos by Bob Hunger, Oklahoma State University.

Typical damage caused by greenbugs feeding on sorghum. (a) visible damage on the topside of the leaf. (b) greenbug colony on the underside of the leaf. Photos by Phil Sloderbeck, Kansas State University.
The health of the host plant is generally affected by this feeding, and it is believed that greenbug possesses substances in its saliva that are toxic to the host plant, reducing shoot and root mass development and tillering in wheat (Burton 1986, Burton and Burd 1993, Riedell and Kieckhefer 1995). Indeed, Burd (2002) showed that some salivary material is translocated in the root and shoot of wheat seedlings. Wheat plants apparently respond to feeding by increased production of ethylene (Anderson and Peters 1994). Al-Mousawi et al. (1983) showed that feeding by biotype C greenbugs on sorghum caused structural damage to plant cell organelles and damage to chloroplast membranes that resulted in collapsed mesophyll. Morgham et al. (1994) reported similar types of injury from greenbug feeding on wheat. Greenbug feeding can result in less chlorophyll (Ryan et al. 1987, Girma et al. 1998), carbon assimilation, and transpiration (Ryan et al. 1987).
Economic loss caused by greenbug feeding on cereal crops across the U.S. Great Plains likely exceeds $100 million annually (Webster et al. 2000, Giles et al. 2008). In spring and winter wheat, greenbug feeding results in plants with fewer fertile tillers and seeds (Burton et al. 1985, Kieckhefer et al. 1994, Kindler et al. 2002). Winter wheat grown during severe drought (Dorschner et al. 1986, Kindler et al. 2002) or cold temperatures (Kantack and Dahms 1957) suffers greater yield loss and plant death from greenbug feeding. Aside from direct losses of plant stand, indirect losses are a result of reductions in carbohydrate reserves, particularly fructans (Holmes et al. 1991). Yield reductions from greenbug feeding occur from fall and spring infestations (Burton et al. 1985), but economic damage to wheat is greater from fall than from spring infestations (Burton et al. 1985).
Greenbug is also a vector of the viruses that cause barley yellow dwarf disease in wheat (Johnson and Rachow 1972, Gray et al. 1998) and was shown to predispose sorghum to charcoal rot (Teetes et al. 1973). The viruses that cause barley yellow dwarf disease are transmitted by greenbug and other aphid vectors in a nonpropagative way; the viruses do not replicate in the vector but do circulate through the aphid’s body. Despite its ability to transmit the viruses, greenbug is a less important vector than other cereal grain aphids, such as bird cherry- oataphid, Rhopalosiphum padi (L.), or English grain aphid, Sitobion avenae (F.) (Hemiptera: Aphididae) (Chapin et al. 2001).
Gibson and Painter (1957) demonstrated that greenbugs could transport the vector of wheat steak mosaic virus, the wheat curl mite Aceria tosichella Keifer (Acari: Eriophyidae), to new plants via phoresis. Interestingly, greenbugs that feed on wheat that is infected with wheat streak mosaic disease do not reproduce as well as they do on noninfected plants (Michels et al. 1994).
Biological Control
Greenbugs are prey or hosts to numerous natural enemies that can potentially suppress greenbug colonization and abundance in wheat and sorghum (Brewer and Elliott 2004). Natural enemies include predators such as lady beetles (Coleoptera: Coccinellidae), lacewings (Neuroptera: Chrysopidae), damsel bugs (Hemiptera: Nabidae), spiders (Araneae), ground beetles (Coleoptera: Carabidae), syrphid flies (Diptera: Syrphidae) (Elliott et al. 2006), wasp parasitoids (Hymenoptera: Aphelinidae, Aphidiidae, Braconidae) (Walker et al. 1973, Archer et al. 1974, Gilstrap et al. 1984), and entomopathogenic fungi (Entomophthorales and Hypocreales) (Feng et al. 1990).
Generalist predators, particularly some species of lady beetles, have a major impact on greenbug numbers in sorghum and winter wheat in Kansas (Rice and Wilde 1988) and the High Plains of Texas (Kring and Gilstrap 1984, Kring et al. 1986, Michels et al. 2001). Indigenous coccinellids, primarily Hippodamia spp. (Coleoptera: Coccinelidae) (Fig. 5a and b), consistently reduced greenbug numbers by 63% in winter wheat in the Texas High Plains (Michels et al. 2001). An important mechanism for their effectiveness in sorghum depends on the early appearance of corn leaf aphids, Rhopalosiphum maidis (Fitch) (Hemiptera: Aphididae), which provide an alternative food source that maintains high numbers of Hippodamia spp. that then feed on greenbugs as sorghum panicles emerge (Kring and Gilstrap 1986). The abundance of arthropod predators also seems to be influenced by tillage (Rice and Wilde 1991); surprisingly, predators (except spiders) were fewer in reduced tillage systems.

Lady beetles play a key role in suppressing greenbugs in sorghum and wheat. (a) Hippodamia convergens adult. (b) Hippodamia convergens larva. Photos by Phil Sloderbeck, Kansas State University.
Lysiphlebus testaceipes (Cresson) (Hymenoptera: Aphidiidae) is the most abundant parasitoid of greenbug in wheat and sorghum in the southern Great Plains (Fig. 6) (Walker et al. 1973, Archer et al. 1974, Gilstrap et al. 1984). L. testaceipes is vulnerable to typical winter temperatures north of Oklahoma, and most wasps likely disperse as larvae each spring within parasitized winged aphids as they migrate northward (Jackson et al. 1970, Jones et al. 2008). Fernandes et al. (1998) suggested that augmentative releases of L. testaceipes could be effective in preventing greenbugs from reaching economic thresholds in sorghum grown on the northern plains. However, L. testaceipes overwinters in Oklahoma and Texas, where it plays a major role in the suppression of greenbug outbreaks in winter wheat (Jones 2001, Giles et al. 2003) and sorghum (Jackson et al. 1970). In fact, it has been incorporated into control recommendations for greenbugs in winter wheat (Giles et al. 2003, Royer et al. 2004). Jones et al. (2003, 2007) demonstrated that L. testaceipes can parasitize greenbugs in Oklahoma wheat throughout the winter, even when temperatures are below greenbug’s developmental threshold. This ovipositional behavior/ability allows L. testaceipes to effectively maintain greenbugs below economic injury levels in Oklahoma and Texas most years. In addition, it appears that greenbug-resistant winter wheat varieties are compatible with conservation of L. testaceipes (Starks et al. 1972, Fuentes-Granados et al. 2001) and there is little possibility for interference between coccinellids and L. testaceipes for greenbug prey owing to intraguild predation (Royer et al. 2008, Mullins et al. 2013).

Greenbug mummies parasitized by Lysiphlebus testaceipes. They are accounted for in determining the need to control greenbugs in winter wheat. Photo by Richard Grantham, Oklahoma State University.
Feng et al. (1990) reported seven different species of entomopathogenic fungi, including Pandora neosphidi (Remaudiere & Hennebert) Humber (Entomophthorales: Entomophthoraceae), Conidiobolus coronatus (Costantin) Batko, Conidiobolus obscurus (Hall & Dunn) Remaudiere & Keller, Conidiobolus thromboides Drechesler (Entomophthorales: Ancylistaceae), Neozygites fresentii (Nowakowski) Remaudiere & Keller (Entomophtorales: Neozygitaceae), Beauveria bassiana (Bals.-Criv.) Vuill (Hypocreales: Clavicpitaceae), and Verticillium licanii (now Lecanicillium lecanii) (Zimmerman) Zare & W. Gams (Hypocreales: Clavicpitaceae), that attacked greenbug in irrigated wheat. While entomopathogenic fungi were found in greenbug, fungal epizootics were generally associated with other cereal aphid species. Epizootics of these entomopathogens were sporadic and most often associated with the number and timing of rainy days, cumulative precipitation, and other factors (Feng et al. 1992).
Cultural Control
Winter wheat is grown in parts of the southern Great Plains to be used as forage (wheat pasture) for stocker cattle and for grain production. Wheat for grazing is typically planted earlier to accumulate forage biomass (Hossain et al. 2003). Continued winter grazing by cattle can reduce greenbug abundance as much as 98% (Arnold 1981, Ismail et al. 2003) and lower risk of barley yellow dwarf disease by 70% (Ismail et al. 2003). There is an association between planting date and greenbug abundance in winter wheat. Earlier-planted wheat tends to support early colonization and greater numbers of greenbugs compared with later plantings (Pike and Schaffner 1985, Hesler et al. 2005, Royer et al. 2005) and is more at risk to becoming infected with viruses that cause barley yellow dwarf disease (Hesler et al. 2005, Royer et al. 2005).
Tillage also impacts greenbug colonization of wheat and sorghum. Greenbugs were less abundant when wheat was grown under cultivation that preserved surface crop residues when compared with conventional clean tilling, and it was hypothesized that such surface residue might serve as reflective mulch that either repels colonization or masks the attractiveness of the crop (Burton and Krenzer 1985). These results contrast with those observed by Hesler and Berg (2003), who showed that conservation tillage did not impact abundance of greenbug.
Sorghum is grown as a full-season summer crop or as a double-crop (Crabtree et al. 1990), and tillage practices and planting date affect colonization by greenbugs. Burton et al. (1990) found that greenbugs were less abundant in sorghum grown in no-tillage compared with conventional tillage plots. They also showed that greenbugs were less abundant in late-planted versus early-planted sorghum and indicated that planting date and tillage approaches to reduce greenbug abundance were compatible with use of resistant hybrids.
Plant Resistance and Biotypes
The search for plants resistant to greenbug has been an important component of variety development in winter wheat (Tyler et al. 1987) and hybrid development in sorghum (Teetes and Pendleton 2000). The first efforts at evaluating for resistance were reported by Dahms et al. (1955). Painter and Peters (1956) described methods for evaluating germplasm for resistance. The first resistant wheat varieties were developed and deployed in the 1950s (Porter et al. 1997), and numerous sources of winter wheat resistant to greenbugs have been documented (Martin 1981, Joppa and Williams 1982, Martin et al. 1982, Tyler et al. 1985, 1987; Porter et al. 1994, Porter and Mornhinweg 2004).
Sorghum germplasm was identified with resistance to biotype “C” greenbug in the early 1970’s (Schuster and Starks 1973) and subsequently included in commercial sorghum hybrids (Young and Teetes 1977). Expression of resistance in sorghum has been shown to vary with temperature (Schweissing and Wilde 1978, Thindwa and Teetes 1994, Pendleton et al. 2009) and development stage (Starks and Wood 1974). Later efforts focused on characterization of resistance at the molecular level (Katsar et al. 2002). Additional research examined the categorization of resistance (Zhu et al. 2005), impact of resistance on greenbug biology (Kirkland et al. 1981), the compatibility of resistance with biological control (Starks et al. 1972), and identification of markers for marker-assisted selection of resistance (Weng et al. 2005, 2007; Wu and Huang 2008).
Concurrent with development of resistant germplasm was the need to classify phenotypes of greenbugs that were virulent to a specific resistance genotype. The term biotype was defined as “a phenotypic expression of an indefinite number of genotypes” (Puterka and Peters 1990). Wood (1961) described a biotype, referred to as “B,” that was able to damage “Dickinson Selection 28A,” a germplasm that was the source of greenbug resistance in most of the wheat varieties grown at that time. The original susceptible greenbug biotype was called biotype A. The next biotype was “C,” a warm-temperature-tolerant greenbug that could attack sorghum as well as all deployed greenbug-resistant wheat varieties. Additional biotypes have been described based on their ability to overcome a specific source of resistance in wheat or sorghum (Table 1).
Chronology of greenbug biotype designations (from Porter et al. 1997, Nuessly et al. 2008)
| Biotype . | Host collected from . | Year collected or reported . |
|---|---|---|
| A | Wheat | 1961 |
| B | Wheat | 1961 |
| C | Sorghum | 1968 |
| Da | Sorghum | 1975 |
| E | Wheat | 1979 |
| F | Canada bluegrass | 1986 |
| G | Wheat | 1987 |
| H | Wheat | 1987 |
| I | Sorghum | 1990 |
| Jb | Wheat | 1995 |
| K | Sorghum | 1992 |
| Florida isolatec | Seashore paspalum | 2003 |
| Biotype . | Host collected from . | Year collected or reported . |
|---|---|---|
| A | Wheat | 1961 |
| B | Wheat | 1961 |
| C | Sorghum | 1968 |
| Da | Sorghum | 1975 |
| E | Wheat | 1979 |
| F | Canada bluegrass | 1986 |
| G | Wheat | 1987 |
| H | Wheat | 1987 |
| I | Sorghum | 1990 |
| Jb | Wheat | 1995 |
| K | Sorghum | 1992 |
| Florida isolatec | Seashore paspalum | 2003 |
a Biotype D was originally classified as a biotype that was resistant to organophosphate insecticides (Teetes et al. 1975); however, it is no longer considered a biotype because the designation was not based on insect–plant relationships (Porter et al 1997).
b Biotype J was described as a distinct biotype because it was avirulent to all known resistant wheat and barley varieties, including the susceptible wheat control “Triumph 64’. (Beregovoy and Peters 1995).
c The Florida isolate was not given a letter designation, but is considered to be a unique biotype because it attacks seashore paspalum, the greenbug resistant wheat variety ‘GRS1201’, and all identified resistant sorghum varieties (Nuessly et al 2008).
Chronology of greenbug biotype designations (from Porter et al. 1997, Nuessly et al. 2008)
| Biotype . | Host collected from . | Year collected or reported . |
|---|---|---|
| A | Wheat | 1961 |
| B | Wheat | 1961 |
| C | Sorghum | 1968 |
| Da | Sorghum | 1975 |
| E | Wheat | 1979 |
| F | Canada bluegrass | 1986 |
| G | Wheat | 1987 |
| H | Wheat | 1987 |
| I | Sorghum | 1990 |
| Jb | Wheat | 1995 |
| K | Sorghum | 1992 |
| Florida isolatec | Seashore paspalum | 2003 |
| Biotype . | Host collected from . | Year collected or reported . |
|---|---|---|
| A | Wheat | 1961 |
| B | Wheat | 1961 |
| C | Sorghum | 1968 |
| Da | Sorghum | 1975 |
| E | Wheat | 1979 |
| F | Canada bluegrass | 1986 |
| G | Wheat | 1987 |
| H | Wheat | 1987 |
| I | Sorghum | 1990 |
| Jb | Wheat | 1995 |
| K | Sorghum | 1992 |
| Florida isolatec | Seashore paspalum | 2003 |
a Biotype D was originally classified as a biotype that was resistant to organophosphate insecticides (Teetes et al. 1975); however, it is no longer considered a biotype because the designation was not based on insect–plant relationships (Porter et al 1997).
b Biotype J was described as a distinct biotype because it was avirulent to all known resistant wheat and barley varieties, including the susceptible wheat control “Triumph 64’. (Beregovoy and Peters 1995).
c The Florida isolate was not given a letter designation, but is considered to be a unique biotype because it attacks seashore paspalum, the greenbug resistant wheat variety ‘GRS1201’, and all identified resistant sorghum varieties (Nuessly et al 2008).
It was originally believed that greenbug biotypes were selected by widespread deployment of resistance (Eisenbach and Mittler 1987, Lazar et al. 1995). However, Porter et al. (1997) proposed that greenbug populations in nature diverged through genetic recombination of host-adapted races on native grasses. Genetic recombination originates via continual sexual reproduction primarily in colder climates. Thus, biotypes are “pre-adapted opportunists” and are present independently of any single deployment of a resistant host genotype. This hypothesis has been supported by Shufran et al. (1997a, 2000), Anstead et al. (2003), Burd and Porter (2006) and Shufran (2011).
Sampling and Economic Thresholds
The development of accurate and usable sampling plans based on defined economic thresholds is important for making economically justified control decisions for greenbugs (Elliott et al. 1994). Economic thresholds have been defined in winter wheat (Kindler et al. 2003) and sampling plans have been developed in several states based on greenbug distribution (Kring and Gilstrap 1983, Elliott and Kieckhefer 1986, Elliott et al. 1990, Feng and Nowierski 1992, Boeve and Weiss 1997, Giles et al. 2000). Giles et al. (2000, 2003) developed a sampling system that included L. testaceipes and seasonal differences in aphid distribution (fall vs. spring). His work was developed into a user-friendly binomial sequential sampling plan called Glance n’ Go (Royer et al. 2004) that is adjustable for the price of wheat, controls costs, and considers the amount of L. testaceipes activity (Fig. 7). It has also been converted into an Internet-based decision support system (Elliott et al. 2004) and is now delivered through myFields at http://myfields.info/dashboard, which was developed by Dr. Brian McCornack and Dr. Wendy Johnson at Kansas State University.
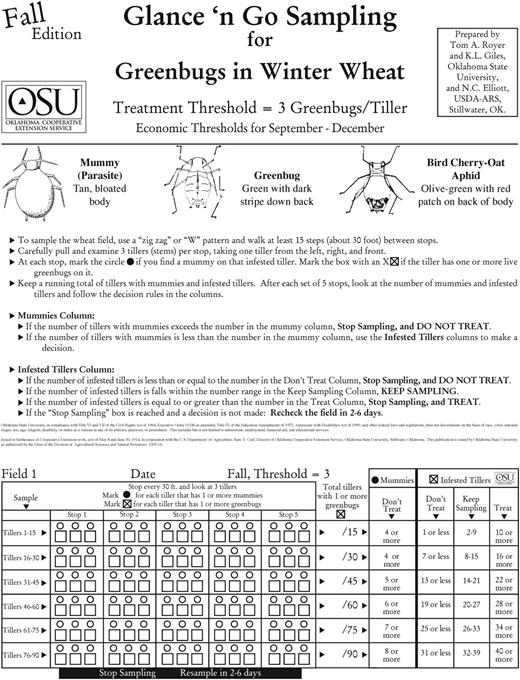
A Glance n’ Go form used to sample winter wheat for greenbugs, fall threshold.
Economic thresholds for sorghum were developed by Teetes and Johnson (1973, 1974). Greenbug distribution has been characterized in sorghum (Archer and Bynum 1986, Michels and Undersander 1986), and current sampling recommendations are adapted from that work but modified among the sorghum-producing states of the southern Great Plains (Table 2; Wright et al. 2006, Cronholm et al. 2007, Michaud et al. 2014, Royer, 2015). While some encourage documentation of natural enemies, and sampling strategies have been evaluated for coccinellids (Michels and Behle 1992), there is no formal incorporation of natural enemy abundance into insecticide treatment decisions other than to suggest that when 20% of greenbugs are parasitized mummies, an insecticide application might not be warranted.
Treatment threshold recommendations for greenbug in sorghum within the southern Great Plains (from Wright et al. 2006, Cronholm et al. 2007, Michaud et al. 2014, Royer 2015)
| Plant stage . | When to treat . | ||
|---|---|---|---|
| 0–1-leaf stage | Kansas thresholds | Nebraska thresholds | Arkansas, Oklahoma, Texas thresholds |
| 25–50 greenbugs/plant. Colonies or numerous winged adults present on majority of plants. Risky to wait until visible damage is obvious. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. | |
| 3-leaf stage | 50–100 greenbugs/plant. As above, before general signs of stress are visible. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. |
| 5-leaf stage | 150–300 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies of greenbugs and initial signs of reddening appear. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Mid-whorl stage | 300–600 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies, but before leaves begin to die. | Treat if greenbug colonies are beginning to cause red or yellow leafspotting on lower leaves of most plants AND parasite numbers are less than 20% of greenbugs parasitized. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Boot | 700–1,000 greenbugs/plant Some lower leaves wet and sticky with honeydew. Some leaves yellowing/reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of one functional leaf. |
| Heading through soft dough | 700–1000 greenbugs/plant. Some lower leaves wet and sticky with honeydew. Some leaves yellowing and reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of two functional leaves. |
| Plant stage . | When to treat . | ||
|---|---|---|---|
| 0–1-leaf stage | Kansas thresholds | Nebraska thresholds | Arkansas, Oklahoma, Texas thresholds |
| 25–50 greenbugs/plant. Colonies or numerous winged adults present on majority of plants. Risky to wait until visible damage is obvious. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. | |
| 3-leaf stage | 50–100 greenbugs/plant. As above, before general signs of stress are visible. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. |
| 5-leaf stage | 150–300 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies of greenbugs and initial signs of reddening appear. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Mid-whorl stage | 300–600 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies, but before leaves begin to die. | Treat if greenbug colonies are beginning to cause red or yellow leafspotting on lower leaves of most plants AND parasite numbers are less than 20% of greenbugs parasitized. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Boot | 700–1,000 greenbugs/plant Some lower leaves wet and sticky with honeydew. Some leaves yellowing/reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of one functional leaf. |
| Heading through soft dough | 700–1000 greenbugs/plant. Some lower leaves wet and sticky with honeydew. Some leaves yellowing and reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of two functional leaves. |
Treatment threshold recommendations for greenbug in sorghum within the southern Great Plains (from Wright et al. 2006, Cronholm et al. 2007, Michaud et al. 2014, Royer 2015)
| Plant stage . | When to treat . | ||
|---|---|---|---|
| 0–1-leaf stage | Kansas thresholds | Nebraska thresholds | Arkansas, Oklahoma, Texas thresholds |
| 25–50 greenbugs/plant. Colonies or numerous winged adults present on majority of plants. Risky to wait until visible damage is obvious. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. | |
| 3-leaf stage | 50–100 greenbugs/plant. As above, before general signs of stress are visible. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. |
| 5-leaf stage | 150–300 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies of greenbugs and initial signs of reddening appear. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Mid-whorl stage | 300–600 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies, but before leaves begin to die. | Treat if greenbug colonies are beginning to cause red or yellow leafspotting on lower leaves of most plants AND parasite numbers are less than 20% of greenbugs parasitized. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Boot | 700–1,000 greenbugs/plant Some lower leaves wet and sticky with honeydew. Some leaves yellowing/reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of one functional leaf. |
| Heading through soft dough | 700–1000 greenbugs/plant. Some lower leaves wet and sticky with honeydew. Some leaves yellowing and reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of two functional leaves. |
| Plant stage . | When to treat . | ||
|---|---|---|---|
| 0–1-leaf stage | Kansas thresholds | Nebraska thresholds | Arkansas, Oklahoma, Texas thresholds |
| 25–50 greenbugs/plant. Colonies or numerous winged adults present on majority of plants. Risky to wait until visible damage is obvious. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. | |
| 3-leaf stage | 50–100 greenbugs/plant. As above, before general signs of stress are visible. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | 20% of leaves visibly damaged. |
| 5-leaf stage | 150–300 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies of greenbugs and initial signs of reddening appear. | Treat if greenbug colonies are present on 10–20% of plants and visibly yellowing or sporting on leaves is present. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Mid-whorl stage | 300–600 greenbugs/plant. Majority of plants are infested with rapidly increasing colonies, but before leaves begin to die. | Treat if greenbug colonies are beginning to cause red or yellow leafspotting on lower leaves of most plants AND parasite numbers are less than 20% of greenbugs parasitized. | Visible damage on leaves (red spots, yellow leaves) but before an entire leaf has been killed on 20% of plants. |
| Boot | 700–1,000 greenbugs/plant Some lower leaves wet and sticky with honeydew. Some leaves yellowing/reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of one functional leaf. |
| Heading through soft dough | 700–1000 greenbugs/plant. Some lower leaves wet and sticky with honeydew. Some leaves yellowing and reddening with occasional leaves dying. Small to large increasing colonies present on the majority of plants. | Treat if greenbug colonies are on most plants, before one lower leaf has been killed AND parasite numbers are less than 20% of greenbugs parasitized. | Death of two functional leaves. |
There is interest in using remote sensing to detect greenbug injury to wheat plants. Laboratory and greenhouse studies indicated reflected light or temperature in green, red, and several near-infrared wavelength bands were sensitive to the injury greenbugs cause to wheat (Michels et al. 1999; Riedell and Blackmer 1999; Yang et al. 2005, 2009; Mirik et al. 2006a, b). These studies documented an increase in light reflectance in the visible wavelengths and a decrease in near-infrared reflectance from wheat injured by greenbugs compared with non-injured wheat. Field studies documented a similar pattern of light reflectance (Mirik et al. 2006a, 2006b; Elliott et al. 2009).
The observed reflectance pattern is very similar to that seen in typical plant stress response, making it difficult to identify greenbug-stressed wheat based only on reflectance. Backoulou et al. (2015) explored the possibility of combining spatial pattern identification techniques with multispectral imagery to discern greenbug induced plant stress from other stress-causing factors, such as drought and inadequate soil fertility. They were able to successfully differentiate plant stress caused by greenbug from other kinds of plant stress in commercial wheat fields.
The above research demonstrated the potential of remote sensing to detect greenbug infestations in the field. Unmanned aerial vehicles equipped with multi-spectral imaging systems at specific wavelengths could be developed to provide cost-effective systems for detecting greenbug infested fields. This could prove useful for monitoring large, multiple field areas, or for developing precision approaches to application of insecticides for greenbug control within a field.
Chemical Control
Insecticides are important tools for managing greenbug in wheat and sorghum. During the past 50 yr, many insecticides have been used but have generally been restricted to four insecticide classes: broad-spectrum carbamates, organophosphates, and pyrethroids, and the more selective neonicotinoids. Neonicotinoids are typically applied as seed treatments and the other classes as seed treatment, layby, in-furrow, or foliar applications via ground or aerial equipment. Many growers are interested in “saving trips across the field” by including an insecticide with a top-dress fertilization during the fall and winter. Routine applications with top-dress can be avoided by using the Glance n’ Go system to determine the need for an application. When an application is justified, greenbug control can be accomplished at most temperatures that allow adequate application (Bauernfeind 1983, Royer et al. 2011).
Resistance of greenbugs to organophosphorus and carbamate insecticides has been identified (Teetes et al. 1975, Shufran et al. 1997b, Wilde et al. 2001), and the genetic mechanisms described (Siegfried and Ono 1993, Ono et al. 1994). Greenbugs resistant to organophosphate insecticides were widely distributed within the southern Great Plains (Teetes et al. 1975; Shufran et al. 1997b, c; Wilde et al. 2001). While it appears that insecticide-resistant greenbug numbers have subsided, current management recommendations encourage growers to use insecticide resistance management strategies such as scouting coupled with use of defined economic thresholds, planting resistant varieties, use of insecticides with selective modes of action, and rotation of insecticide modes of action that minimize/delay the selection of insecticide-resistant greenbugs (Cronholm et al. 2007, Bynum et al. 2012, Michaud 2015, Royer 2015, Royer and Giles 2015).
Acknowledgments
We thank Gerrit Cuperus for encouraging us to jump in and work with greenbugs in Oklahoma. We thank Eric Rebek and Jackie Lee for providing a review of earlier versions of this manuscript. This manuscript was approved for publication by the Vice President, Dean, and Director, Division of Agriculture and Natural Resources, Oklahoma State University, Stillwater.



