-
PDF
- Split View
-
Views
-
Cite
Cite
Alicja Sadowska-Klasa, Wendy M Leisenring, Ajit P Limaye, Michael Boeckh, Cytomegalovirus Viral Load Threshold to Guide Preemptive Therapy in Hematopoietic Cell Transplant Recipients: Correlation With Cytomegalovirus Disease, The Journal of Infectious Diseases, Volume 229, Issue 5, 15 May 2024, Pages 1435–1439, https://doi.org/10.1093/infdis/jiad386
Close - Share Icon Share
Abstract
A systematic review of randomized and observational studies from 2013 to 2023 demonstrated that antiviral preemptive therapy started at cytomegalovirus viral load thresholds between 2 and 3 log10 IU/mL was associated with similar cytomegalovirus disease rates. Thus, viral thresholds in this range appear to effectively protect patients not receiving prophylaxis.
Polymerase chain reaction (PCR)–guided preemptive therapy (PET) is highly effective in preventing cytomegalovirus (CMV) disease and has been used in randomized controlled trials (RCTs) to evaluate novel vaccines and therapeutics as part of the clinically significant CMV infection end point [1]. However, there is no consensus on the specific CMV viral load (VL) threshold for starting antiviral PET. The differences between reported thresholds are relatively small on a log10 scale and were within the assay variability when calibrated to the World Health Organization international standard [2]. Nevertheless, consensus on thresholds for multicenter clinical trials has proved difficult, and international guidelines are vague about the issue [3].
Although the introduction of the World Health Organization international standard has improved interassay comparability, the median variance of results from CMV-positive samples was 1.5 log10 IU/mL, with a range of 1.22–2.82 log10 IU/mL [2]. Thus, the VL thresholds used in most trials and studies (Table 1) are within the interassay variability of the quantitative PCR tests. We hypothesized that different VL thresholds used in RCTs and observational studies (OSs) for PET administration are similarly effective for prevention of CMV disease, as they were within the range of PCR assay variability in most cases. The aim of this study was to systematically review the incidence of early CMV disease across the range of CMV VL thresholds used for initiation of antiviral PET in RCTs and OSs among patients not receiving prophylactic agents.
Randomized Clinical Trials and Observational Studies Included in the Final Analysis
| Authors . | Year and Journal . | Pts Receiving Placebo, No. . | CMV IgG Status . | Donor Type . | CMV IgG(-) Donors . | Use of ATG; Ex Vivo TCD; Alemtuzumab . | Criteria for Defining HR Pts . | PCR Test . | PET VL Threshold(s), Log10 IU/mL . | Pts With CMV Disease at d 100, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Marty et al [4] | 2013; N Engl J Med | 59 | R+ | All types, CB, 6.8%; haplo, no data | 44.1% | ATG, 15.2%; TCD, 6.8% | Not defined | Viracor-IBT | 2.57 | 3 |
| Marty et al [1] | 2017; N Engl J Med | 192 | R+ | All types; CB, 5.7%; haplo, 10.9% | 41.6% | ATG, 30.2%; TCD, 2.6%; alemtuzumab, 5.7% | mMRD/mMUD, haplo, CB, TCD, GvHD ≥2 with Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (28.1%) HR; 2.42 (71.9%) LR | 1.2 |
| Marty et al [5] | 2019; Biol Blood Marrow Transplant | 149 | R+ | All types; CB, 7.4%; haplo, 5.4% | 43.6% | ATG, 31.5%; TCD, 13.4%; alemtuzumab, 8.1% | CB, TCD, MUD, mismatched, haplo, ATG, alemtuzumab, Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (73.2%) HR; 2.95 (26.8%) LR | 2 |
| Green et al [6] | 2016; Lancet Haematol. | 936 | R+, R−/D+ | All types; CB, 11%; haplo, 5% | 50% | No data | Pred ≥1 mg/kg, CB | Laboratory-developed assay (UW)a | 1.3 HR; 2.09 LR (most) | 5 |
| Johnsrud et al [7] | 2020; Biol Blood Marrow Transplant | 637 | R+, R−/D+ | All types; CB, 7.5%; haplo, 4.6% | 26.7% | ATG, 36.9% | Haplo, CB, ATG, TCD | Roche Amplicor CMV PCR Detection Systems | 2.6b | 5.3 |
| Anderson et al [8] | 2020; Clin Transplant | 106 | R+ | All types; CB, 3%; haplo, 7%, | 48% | ATG, 86% | Haplo, CB, mMUD, acute GvHD with Pred ≥ 1 mg/kg, MUD/ATG | Until 2015, test with LLOD of 96 IU/mL (Viracor-IBT); later, Roche COBAS AmpliPrep/COBAS | 2.3c | 3 |
| Zavras et al [9] | 2020; Biol Blood Marrow Transplant | 368 | R+ | No CB; haplo, no data | 39.4% | ATG, 50.3%; TCD, 42.4% | Haplo, mismatched, TCD | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (52.5%) HR; 2.47 (47.8%) LR d | 3.2 |
| this values should be in row above (52.5%); 2.47 (47.8%)d | ||||||||||
| Derigs et al [10] | 2021; Ann Hematol | 80 | R+ | No CB; haplo, 5% | 23% | ATG, 69% | Not defined | Laboratory-developed assay | 3.5 | 2.5 |
| Ueda Oshima et al [11] | 2023; Blood Adv | 780 | R+ | No CB; no haplo | 52% | Excluded | Pred ≥1 mg/kg | Laboratory-developed assay (UW)a | 1.69 HR; 2.17 LR (most) | 4 |
| Authors . | Year and Journal . | Pts Receiving Placebo, No. . | CMV IgG Status . | Donor Type . | CMV IgG(-) Donors . | Use of ATG; Ex Vivo TCD; Alemtuzumab . | Criteria for Defining HR Pts . | PCR Test . | PET VL Threshold(s), Log10 IU/mL . | Pts With CMV Disease at d 100, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Marty et al [4] | 2013; N Engl J Med | 59 | R+ | All types, CB, 6.8%; haplo, no data | 44.1% | ATG, 15.2%; TCD, 6.8% | Not defined | Viracor-IBT | 2.57 | 3 |
| Marty et al [1] | 2017; N Engl J Med | 192 | R+ | All types; CB, 5.7%; haplo, 10.9% | 41.6% | ATG, 30.2%; TCD, 2.6%; alemtuzumab, 5.7% | mMRD/mMUD, haplo, CB, TCD, GvHD ≥2 with Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (28.1%) HR; 2.42 (71.9%) LR | 1.2 |
| Marty et al [5] | 2019; Biol Blood Marrow Transplant | 149 | R+ | All types; CB, 7.4%; haplo, 5.4% | 43.6% | ATG, 31.5%; TCD, 13.4%; alemtuzumab, 8.1% | CB, TCD, MUD, mismatched, haplo, ATG, alemtuzumab, Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (73.2%) HR; 2.95 (26.8%) LR | 2 |
| Green et al [6] | 2016; Lancet Haematol. | 936 | R+, R−/D+ | All types; CB, 11%; haplo, 5% | 50% | No data | Pred ≥1 mg/kg, CB | Laboratory-developed assay (UW)a | 1.3 HR; 2.09 LR (most) | 5 |
| Johnsrud et al [7] | 2020; Biol Blood Marrow Transplant | 637 | R+, R−/D+ | All types; CB, 7.5%; haplo, 4.6% | 26.7% | ATG, 36.9% | Haplo, CB, ATG, TCD | Roche Amplicor CMV PCR Detection Systems | 2.6b | 5.3 |
| Anderson et al [8] | 2020; Clin Transplant | 106 | R+ | All types; CB, 3%; haplo, 7%, | 48% | ATG, 86% | Haplo, CB, mMUD, acute GvHD with Pred ≥ 1 mg/kg, MUD/ATG | Until 2015, test with LLOD of 96 IU/mL (Viracor-IBT); later, Roche COBAS AmpliPrep/COBAS | 2.3c | 3 |
| Zavras et al [9] | 2020; Biol Blood Marrow Transplant | 368 | R+ | No CB; haplo, no data | 39.4% | ATG, 50.3%; TCD, 42.4% | Haplo, mismatched, TCD | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (52.5%) HR; 2.47 (47.8%) LR d | 3.2 |
| this values should be in row above (52.5%); 2.47 (47.8%)d | ||||||||||
| Derigs et al [10] | 2021; Ann Hematol | 80 | R+ | No CB; haplo, 5% | 23% | ATG, 69% | Not defined | Laboratory-developed assay | 3.5 | 2.5 |
| Ueda Oshima et al [11] | 2023; Blood Adv | 780 | R+ | No CB; no haplo | 52% | Excluded | Pred ≥1 mg/kg | Laboratory-developed assay (UW)a | 1.69 HR; 2.17 LR (most) | 4 |
Abbreviations: ATG, antithymocyte globulin; CB, cord blood; CMV, cytomegalovirus; D−, donor CMV seronegative; D+, donor CMV seropositive; GvHD, graft-vs-host disease; haplo, haploidentical; HR, high-risk; IgG, immunoglobulin G; LLOD, lower limit of detection; LR, low-risk; mMRD, mismatched related donor; mMUD, mismatched unrelated donor; MUD, matched unrelated donor; PCR, polymerase chain reaction; PET, preemptive therapy; Pred, prednisone or equivalent; Pts, patients; R−, recipient CMV seronegative, R+, recipient CMV seropositive; TCD, ex vivo T-cell depletion; UW, University of Washington, Seattle.
aPCR assay was recalibrated into international standards. The LLOD and quantitation differed slightly between these studies and analysis periods. Studies included mostly separate patient populations.
bBefore 2018, CB recipients received valacyclovir (2000 mg thrice daily); this population consisted of 48 patients (7.5%) over the entire study period (2013–2019).
cMonitoring twice weekly; the PET viral load cutoff was >200 IU/mL on 2 consecutive tests.
dMonitoring at least weekly; the PET cutoff was 1 result ≥137 IU/mL or any 2 positive test results for HR patients or 2 consecutive results >300 IU/mL for LR patients.
Randomized Clinical Trials and Observational Studies Included in the Final Analysis
| Authors . | Year and Journal . | Pts Receiving Placebo, No. . | CMV IgG Status . | Donor Type . | CMV IgG(-) Donors . | Use of ATG; Ex Vivo TCD; Alemtuzumab . | Criteria for Defining HR Pts . | PCR Test . | PET VL Threshold(s), Log10 IU/mL . | Pts With CMV Disease at d 100, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Marty et al [4] | 2013; N Engl J Med | 59 | R+ | All types, CB, 6.8%; haplo, no data | 44.1% | ATG, 15.2%; TCD, 6.8% | Not defined | Viracor-IBT | 2.57 | 3 |
| Marty et al [1] | 2017; N Engl J Med | 192 | R+ | All types; CB, 5.7%; haplo, 10.9% | 41.6% | ATG, 30.2%; TCD, 2.6%; alemtuzumab, 5.7% | mMRD/mMUD, haplo, CB, TCD, GvHD ≥2 with Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (28.1%) HR; 2.42 (71.9%) LR | 1.2 |
| Marty et al [5] | 2019; Biol Blood Marrow Transplant | 149 | R+ | All types; CB, 7.4%; haplo, 5.4% | 43.6% | ATG, 31.5%; TCD, 13.4%; alemtuzumab, 8.1% | CB, TCD, MUD, mismatched, haplo, ATG, alemtuzumab, Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (73.2%) HR; 2.95 (26.8%) LR | 2 |
| Green et al [6] | 2016; Lancet Haematol. | 936 | R+, R−/D+ | All types; CB, 11%; haplo, 5% | 50% | No data | Pred ≥1 mg/kg, CB | Laboratory-developed assay (UW)a | 1.3 HR; 2.09 LR (most) | 5 |
| Johnsrud et al [7] | 2020; Biol Blood Marrow Transplant | 637 | R+, R−/D+ | All types; CB, 7.5%; haplo, 4.6% | 26.7% | ATG, 36.9% | Haplo, CB, ATG, TCD | Roche Amplicor CMV PCR Detection Systems | 2.6b | 5.3 |
| Anderson et al [8] | 2020; Clin Transplant | 106 | R+ | All types; CB, 3%; haplo, 7%, | 48% | ATG, 86% | Haplo, CB, mMUD, acute GvHD with Pred ≥ 1 mg/kg, MUD/ATG | Until 2015, test with LLOD of 96 IU/mL (Viracor-IBT); later, Roche COBAS AmpliPrep/COBAS | 2.3c | 3 |
| Zavras et al [9] | 2020; Biol Blood Marrow Transplant | 368 | R+ | No CB; haplo, no data | 39.4% | ATG, 50.3%; TCD, 42.4% | Haplo, mismatched, TCD | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (52.5%) HR; 2.47 (47.8%) LR d | 3.2 |
| this values should be in row above (52.5%); 2.47 (47.8%)d | ||||||||||
| Derigs et al [10] | 2021; Ann Hematol | 80 | R+ | No CB; haplo, 5% | 23% | ATG, 69% | Not defined | Laboratory-developed assay | 3.5 | 2.5 |
| Ueda Oshima et al [11] | 2023; Blood Adv | 780 | R+ | No CB; no haplo | 52% | Excluded | Pred ≥1 mg/kg | Laboratory-developed assay (UW)a | 1.69 HR; 2.17 LR (most) | 4 |
| Authors . | Year and Journal . | Pts Receiving Placebo, No. . | CMV IgG Status . | Donor Type . | CMV IgG(-) Donors . | Use of ATG; Ex Vivo TCD; Alemtuzumab . | Criteria for Defining HR Pts . | PCR Test . | PET VL Threshold(s), Log10 IU/mL . | Pts With CMV Disease at d 100, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Marty et al [4] | 2013; N Engl J Med | 59 | R+ | All types, CB, 6.8%; haplo, no data | 44.1% | ATG, 15.2%; TCD, 6.8% | Not defined | Viracor-IBT | 2.57 | 3 |
| Marty et al [1] | 2017; N Engl J Med | 192 | R+ | All types; CB, 5.7%; haplo, 10.9% | 41.6% | ATG, 30.2%; TCD, 2.6%; alemtuzumab, 5.7% | mMRD/mMUD, haplo, CB, TCD, GvHD ≥2 with Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (28.1%) HR; 2.42 (71.9%) LR | 1.2 |
| Marty et al [5] | 2019; Biol Blood Marrow Transplant | 149 | R+ | All types; CB, 7.4%; haplo, 5.4% | 43.6% | ATG, 31.5%; TCD, 13.4%; alemtuzumab, 8.1% | CB, TCD, MUD, mismatched, haplo, ATG, alemtuzumab, Pred ≥1 mg/kg | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (73.2%) HR; 2.95 (26.8%) LR | 2 |
| Green et al [6] | 2016; Lancet Haematol. | 936 | R+, R−/D+ | All types; CB, 11%; haplo, 5% | 50% | No data | Pred ≥1 mg/kg, CB | Laboratory-developed assay (UW)a | 1.3 HR; 2.09 LR (most) | 5 |
| Johnsrud et al [7] | 2020; Biol Blood Marrow Transplant | 637 | R+, R−/D+ | All types; CB, 7.5%; haplo, 4.6% | 26.7% | ATG, 36.9% | Haplo, CB, ATG, TCD | Roche Amplicor CMV PCR Detection Systems | 2.6b | 5.3 |
| Anderson et al [8] | 2020; Clin Transplant | 106 | R+ | All types; CB, 3%; haplo, 7%, | 48% | ATG, 86% | Haplo, CB, mMUD, acute GvHD with Pred ≥ 1 mg/kg, MUD/ATG | Until 2015, test with LLOD of 96 IU/mL (Viracor-IBT); later, Roche COBAS AmpliPrep/COBAS | 2.3c | 3 |
| Zavras et al [9] | 2020; Biol Blood Marrow Transplant | 368 | R+ | No CB; haplo, no data | 39.4% | ATG, 50.3%; TCD, 42.4% | Haplo, mismatched, TCD | Roche COBAS AmpliPrep/COBAS TaqMan | 2.13 (52.5%) HR; 2.47 (47.8%) LR d | 3.2 |
| this values should be in row above (52.5%); 2.47 (47.8%)d | ||||||||||
| Derigs et al [10] | 2021; Ann Hematol | 80 | R+ | No CB; haplo, 5% | 23% | ATG, 69% | Not defined | Laboratory-developed assay | 3.5 | 2.5 |
| Ueda Oshima et al [11] | 2023; Blood Adv | 780 | R+ | No CB; no haplo | 52% | Excluded | Pred ≥1 mg/kg | Laboratory-developed assay (UW)a | 1.69 HR; 2.17 LR (most) | 4 |
Abbreviations: ATG, antithymocyte globulin; CB, cord blood; CMV, cytomegalovirus; D−, donor CMV seronegative; D+, donor CMV seropositive; GvHD, graft-vs-host disease; haplo, haploidentical; HR, high-risk; IgG, immunoglobulin G; LLOD, lower limit of detection; LR, low-risk; mMRD, mismatched related donor; mMUD, mismatched unrelated donor; MUD, matched unrelated donor; PCR, polymerase chain reaction; PET, preemptive therapy; Pred, prednisone or equivalent; Pts, patients; R−, recipient CMV seronegative, R+, recipient CMV seropositive; TCD, ex vivo T-cell depletion; UW, University of Washington, Seattle.
aPCR assay was recalibrated into international standards. The LLOD and quantitation differed slightly between these studies and analysis periods. Studies included mostly separate patient populations.
bBefore 2018, CB recipients received valacyclovir (2000 mg thrice daily); this population consisted of 48 patients (7.5%) over the entire study period (2013–2019).
cMonitoring twice weekly; the PET viral load cutoff was >200 IU/mL on 2 consecutive tests.
dMonitoring at least weekly; the PET cutoff was 1 result ≥137 IU/mL or any 2 positive test results for HR patients or 2 consecutive results >300 IU/mL for LR patients.
METHODS
Studies eligible for this systematic review included both prospective placebo-controlled clinical trials and OSs of PCR-guided PET with ≥50 participants, were published in English between 2013 and 2023, and provided sufficient information on the PCR thresholds and the CMV disease end point. End-organ CMV disease was diagnosed based on previously published criteria [3, 12]. Potentially eligible studies were identified via PubMed search (see Supplementary Figure 1 for terms used). Specifically, criteria for inclusion included CMV monitoring in plasma or serum with international standard–validated quantitative PCR assay, conversion factor available for results presented in copies per milliliter (data presented in an article or collected through public sources for commercially available tests), threshold for PET provided, CMV serostatus of donor and recipient reported in the study, and information on CMV disease incidence by day 100 after transplantation. RCTs comprised phase 2 or 3 placebo control studies, and only data from the placebo group were used for this analysis.
For studies meeting all inclusion criteria, detailed information was collected from published material, supplementary data, or study protocols. PET strategies that were difficult to categorize—for example, those based on CMV DNA doubling time—were excluded from analysis. Study authors reviewed 3 RCTs [1, 4, 5] and 6 OSs feasible for final analysis [6–11]. A listing of excluded articles is provided in Supplementary Table 1, and reasons for exclusion are summarized in Supplementary Figure 1.
All VL thresholds were converted into logarithmic scale (log10). In 5 of 9 studies, 2 suggested thresholds for initiating PET were provided for patients at low risk (LR) or high risk (HR) for CMV disease. Definitions of the HR population varied (Table 1), and the denominator for LR/HR patients was available for 3 of the 5 studies. We performed 2 separate analyses using the LR or HR threshold values provided in the study as a sensitivity analysis.
The primary outcome of analysis was CMV disease incidence by day 100 after hematopoietic cell transplantation for each study. Spearman correlation coefficients (r values) and associated P values for testing the null hypothesis that r = 0 were used to assess the correlation between VL thresholds as indicated in the clinical trial protocols and/or methods section of the publications (and converted to log10) and the incidence of CMV disease at day 100 after hematopoietic cell transplantation. A linear regression line is imposed on figures as a visual representation of the relationship.
RESULTS
Characteristics of trials selected for this analysis and those excluded (with reasons) are summarized in Table 1 and Supplementary Figure 1. Three clinical trials and 6 OSs, involving 400 and 2897 patients, respectively, were available for the final analysis. The patient sample size ranged from 59 to 192 in RCTs and from 80 to 926 in cohort studies. All studies comprised CMV-seropositive patients, except 2 that also included recipient-negative/donor-positive patients. The median proportion of CMV-seronegative donors was 43.6% (range, 23%–52%). Six studies had no restrictions based on donor type, while 2 studies excluded cord blood donors and 1 excluded both cord blood and haploidentical donors.
Overall, the median suggested viral load threshold for initiation of PET was 2.13 log10 IU/mL (range, 1.3–3.5 log10 IU/mL) for all predefined HR groups (when applicable; RCTs, 2.13 [, 2.13–2.57] log10 IU/mL; OSs, 2.22 [1.3–3.5] log10 IU/mL) and 2.47 (2.09–3.5) log10 IU/mL for predefined LR patients (when applicable; RCTs, 2.57 [2.43–2.95] log10 IU/mL; OSs 2.39 [2.09–3.5] log10 IU/mL).
Of the 5 studies with separate thresholds, 3 included predominantly LR and 2 included predominantly HR patients. The median incidence of CMV disease at day 100 was 3% across all studies, 2% (range, 1.2%–3%) in RCTs and 4% (2.5%–5.3%) in OSs. In both groups, there was no evidence of a positive correlation (Spearman correlation: r = −0.16; P = 0.68 and r = −0.37; P = 0.31, respectively) between CMV thresholds for PET initiation and the incidence of CMV disease, using either the lower or higher VL threshold for studies where the population was mixed (Figure 1A and 1B).
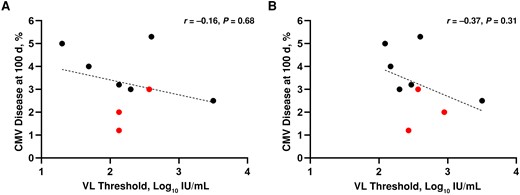
Correlation between the incidence of cytomegalovirus (CMV) disease at 100 days and viral load (VL) thresholds. For the 5 studies that provided different thresholds for low-risk and high-risk populations (Table 1), we assessed the use of either threshold. The lower VL thresholds used for high-risk patients (A) and the higher thresholds used for low-risk patients (B) were uniformly applied. Red dots represent randomized controlled trials; black dots, observational studies; dashed line, the linear regression line. Abbreviation: NS, not significant.
DISCUSSION
In the present study we summarized different PET VL thresholds with associated incidence of early CMV disease across contemporary clinical trials and OSs and found no apparent correlation with CMV disease incidence rates across thresholds ranging between 2 and 3 log10 IU/mL. In all but 1 study PET was introduced at ≤3 log10 IU/mL, and the highest incidence of CMV disease, although not significantly correlated, was observed in studies that used the lowest threshold values. We conducted 2 separate analyses for each group, and even with the approach of applying the higher VL thresholds to start PET regardless of the proportion of HR populations in a particular study, the correlation was not statistically significant (Figure 1). The somewhat lower incidence of CMV disease observed in RCTs may result from more rigorous monitoring in these population than in retrospective studies. Among the included trials we observed varying numbers of patients receiving both ex vivo and in vivo T-cell depletion; some of the investigated groups were more homogeneous, including only LR patients, which may account for the heterogeneity of reported results.
The optimal VL threshold to start PET for CMV disease prevention has not been defined and is often debated when multicenter clinical trial protocols are developed. Indeed, no trials were performed to prospectively compare PCR cutoff values with CMV disease in a multicenter fashion and to assess the impact of different assays in this setting. The present analysis suggests that antiviral PET started at CMV VL thresholds between 2 and 3 log10 IU/mL is associated with similar CMV disease rates. Thus, viral thresholds in this range appear to similarly prevent CMV disease by day 100 in patients not receiving prophylaxis in clinical trials.
For human immunodeficiency virus PCR monitoring, differences >0.5 log10 IU/mL between 2 assays or 2 separate VL samples are considered to be clinically important. However, even after the introduction of the international laboratory standards, CMV DNA plasma and serum PCR assays still have a substantially higher variability. Assay characteristics, specimen type, extraction method, genetic target, amplicon size, and the standard used contribute to the variability of results across different tests [2, 13]. Indeed, intralaboratory and interlaboratory analyses suggest that for clinical CMV DNA-positive samples only approximately 60% were within ±0.5 log10 IU/mL of the mean of all results for the samples tested [2, 13]. Moreover, variance for individual clinical samples—defined as the difference between the highest and the lowest result—was large (median, 1.5 log10 IU/mL; range, 1.22–2.82 log10 IU/mL) between laboratories [2]. Translated into clinical practice, this means, for example, that a PCR result of 300 IU/mL (2.47 in log10 scale) may vary from 1.97 (95 IU/mL) to 2.97 (950 IU/mL) among different assays, assuming a variability of ±0.5 log10, and for >40% of samples results the expected variability may be even greater.
This analysis has several limitations, including the relatively small number of studies fulfilling inclusion criteria (Supplementary Figure 1) and the unknown proportion and varying definitions of HR versus LR patients in some studies, which affected the precision of our analysis related to the actual VL threshold used in some studies. Only 1 study reported the distribution of CMV disease among LR and HR subgroups [7]. We addressed this issue by conducting 2 separate analyses including LR/HR thresholds. Another limitation is the frequency of PCR monitoring between studies, as some centers performed testing twice weekly [8, 10], and for 2 positive consecutive tests were required initiation of PET [8, 9]. In the studies included in the current analysis, gastrointestinal involvement was the most common manifestation of CMV disease; however, there was no information on concomitant gastrointestinal graft-vs-host disease that could affect the effectiveness of PET.
Two cohort studies included recipient-negative/donor-positive patients. This may be one of the limitations, but it is now a predominant situation where PET is used, and the association of VL with CMV disease is generally particularly strong, because CMV is acquired through the stem cell product. It is also unknown whether our findings can be extrapolated to PCR testing in whole blood. Finally, we did not include solid organ transplant studies because the number of qualifying studies would have been small owing to widespread use of prophylaxis. However, the general principle of interassay variability likely applies to these settings as well.
In conclusion, plasma or serum CMV VL thresholds between 2 and 3 log10 IU/mL for starting antiviral PET, as used at most centers and in multicenter RCTs, are associated with similar CMV disease rates at day 100 after hematopoietic cell transplantation. Thus, any VL-guided PET in this range appears to provide adequate protection from CMV disease and can be used safely in clinical trial protocols that include a placebo or a control group not receiving prophylaxis and could be extrapolated to late CMV. Further research should focus on the incidence and risk factors for late CMV disease and the proper viral threshold to initiate PET. Once such data are available a similar analysis should be performed for the late setting and in patients receiving letermovir prophylaxis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Polish National Agency for Academic Exchange (A. S. K.), the Joel D. Meyers Scholarship Endowment financed by Fred Hutchinson Cancer Center (A. S. K.), and the National Institutes of Health (grants HHSN272201600015C to W. M. L., A. P. L, and M. B. and grants HHSN272201600015C, AI163090, and HL147011 to A. P. L.).
References
Author notes
Potential conflicts of interest. A. P. L. has received research support from Merck and Takeda; served as a consultant for GSK, Vera, AiCuris, Merck, and Moderna; and served on a data safety and monitoring board for Novartis, Syneos, and Nobelpharma. M. B. received research support from Merck and served as a consultant for Allovir, Symbio Pharmaceuticals, Moderna, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




