-
PDF
- Split View
-
Views
-
Cite
Cite
Ethel D Weld, Ian McGowan, Peter Anton, Edward J Fuchs, Ken Ho, Alex Carballo-Dieguez, Lisa C Rohan, Rebecca Giguere, Rhonda Brand, Stacey Edick, Rahul P Bakshi, Teresa Parsons, Madhuri Manohar, Aaron Seigel, Jared Engstrom, Julie Elliott, Cindy Jacobson, Christina Bagia, Lin Wang, Amer Al-khouja, Douglas J Hartman, Namandje N Bumpus, Hans M L Spiegel, Mark A Marzinke, Craig W Hendrix, Tenofovir Douche as HIV Preexposure Prophylaxis for Receptive Anal Intercourse: Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics (DREAM 01), The Journal of Infectious Diseases, Volume 229, Issue 4, 15 April 2024, Pages 1131–1140, https://doi.org/10.1093/infdis/jiad535
Close - Share Icon Share
Abstract
Despite highly effective HIV preexposure prophylaxis (PrEP) options, no options provide on-demand, nonsystemic, behaviorally congruent PrEP that many desire. A tenofovir-medicated rectal douche before receptive anal intercourse may provide this option.
Three tenofovir rectal douches—220 mg iso-osmolar product A, 660 mg iso-osmolar product B, and 660 mg hypo-osmolar product C—were studied in 21 HIV-negative men who have sex with men. We sampled blood and colorectal tissue to assess safety, acceptability, pharmacokinetics, and pharmacodynamics.
The douches had high acceptability without toxicity. Median plasma tenofovir peak concentrations for all products were several-fold below trough concentrations associated with oral tenofovir disoproxil fumarate (TDF). Median colon tissue mucosal mononuclear cell (MMC) tenofovir-diphosphate concentrations exceeded target concentrations from 1 hour through 3 to 7 days after dosing. For 6–7 days after a single product C dose, MMC tenofovir-diphosphate exceeded concentrations expected with steady-state oral TDF 300 mg on-demand 2-1-1 dosing. Compared to predrug baseline, HIV replication after ex vivo colon tissue HIV challenge demonstrated a concentration-response relationship with 1.9 log10 maximal effect.
All 3 tenofovir douches achieved tissue tenofovir-diphosphate concentrations and colorectal antiviral effect exceeding oral TDF and with lower systemic tenofovir. Tenofovir douches may provide a single-dose, on-demand, behaviorally congruent PrEP option, and warrant continued development.
Clinical Trials Registration. NCT02750540.
Effective preexposure prophylaxis (PrEP) options for sexually acquired human immunodeficiency virus (HIV) include daily oral tenofovir (TFV)/emtricitabine (FTC), monthly dapivirine vaginal ring, and bimonthly injectable cabotegravir [1–7]. Advocates for more choice in PrEP options hope to increase overall PrEP uptake in light of the contraceptive experience, in which each additional contraceptive option contributes to greater population-level contraception [8–10]. Reducing duration of drug exposure, on-demand, short-acting PrEP (3-day, 4-dose 2-1-1 IPERGAY study regimen) demonstrated equivalence in efficacy and participant choice when compared to daily oral dosing of TFV disoproxil fumarate (TDF)/FTC in men who have sex with men (MSM) and transgender women [11, 12]. Reducing systemic drug exposure, CAPRISA 004, an on-demand vaginal microbicide, 1% TFV gel, proved effective in participants with high adherence [13, 14].
Behaviorally congruent PrEP formulations, in which commonly used sex-related products (eg, sexual lubricants or douches) are medicated to prevent HIV, remain untested. Surveys of PrEP-naive populations indicate substantial interest in rectal PrEP and, in an experiential study comparing rectal formulations, a douche topped the list as the most desired [15, 16]. Several reports indicate 80% or more MSM use a cleansing rectal douche regularly before receptive anal intercourse (RAI), suggesting a medicated douche may provide a behaviorally congruent PrEP option for MSM [17–20]. Despite scant rectal douching data in women, the population attributable fraction of RAI-acquired HIV is approximately 40% in women, suggesting a possible role for rectal microbicides in women as well [21–24].
Previously, we demonstrated the safety of hypo- and iso-osmolar rectal douches [25]. The TFV douches achieved high colorectal tissue concentrations in mice and macaques and protection from ex vivo Simian-Human Immunodeficiency Virus (SHIV) challenge of macaque colorectal biopsies [26, 27]. Hypo-osmolar TFV formulations achieved increased tissue concentrations of TFV and its active metabolite, TFV diphosphate (TFV-DP) compared to iso-osmolar formulations, attributed to hypo-osmolar advection [26–29]. Using a weekly macaque rectal SHIV challenge model, we demonstrated the superior SHIV protection of a single hypo-osmolar rectal douche applied 1 hour prior to each weekly SHIV challenge, when compared to steady-state daily oral human-equivalent TDF/FTC dosing [30].
Encouraged by our preclinical program results, the unmet medical need of on-demand, nonsystemic, and behaviorally congruent PrEP choice, we undertook the current phase 1 study to describe the safety, pharmacokinetics (PK), and ex vivo HIV protective effect of TFV douche formulations, comparing dose and osmolality impact on outcomes.
METHODS
Study Design and Participants
We conducted a phase 1, single ascending dose study (NCT02750540) to identify the dose and formulation of a TFV douche that was acceptable and achieved HIV-protective colon mucosal mononuclear cell (MMC) TFV-DP concentration (estimated 100 fmol/million cell). We recruited HIV-negative MSM assigned male at birth, at least 18 years of age, at 3 research sites. Inclusion criteria were a history of RAI at least 5 times in a lifetime, RAI in the prior 3 months, and rectal douching experience. Exclusion criteria included chronic hepatitis B infection, creatinine clearance <50 mL/min, ≥ grade 2 chemistry and hematology panel abnormalities, colorectal symptoms, and rectal or reproductive tract infection requiring treatment within 2 months. Ethical standards accorded with the 1975 Helsinki Declaration (2000 revision) and the study was approved by the 3 institutional review boards at Johns Hopkins University, University of Pittsburgh, and the University of California at Los Angeles.
Study Products
Each dose of the 3 study products was prepared individually by a research pharmacist or compounding pharmacy following step-wise compounding procedures. Specifications included: appearance (transparent and clear solution), pH (7.0), osmolality (290 or 145 mOsm/kg for iso-osmolar or hypo-osmolar), and TFV concentration: product A, iso-osmolar 220 mg TFV with 0.862% w/v NaCl; product B, iso-osmolar 660 mg TFV with 0.797% w/v NaCl; product C, hypo-osmolar 660 mg TFV with 0.330% w/v NaCl. Final volume for all products was 125 mL in a 4-oz douche bottle. Confirmatory quality testing was performed for all doses. Nonmedicated douches, consisting of osmolalities mimicking the study products (0.9% and 0.45% NaCl), were taken home with instructions to use them prior to anticipated RAI.
Study Procedures
Each of a planned 18 evaluable research participants were to receive a single dose of each of 3 TFV douches followed by 1 week of evaluations (Figure 1) with at least 1 month washout between doses. Participants were asked to retain the douche for 5 minutes before expulsion. Adverse events were assessed at each visit. Plasma, peripheral blood mononuclear cells (PBMC), rectal fluid, and colorectal tissue biopsies were collected predose and over 168 hours after study product dosing. For each product, each participant had 2 separate postdose biopsies for histology and explant challenge comparison to baseline biopsies. Sampling included plasma and PBMC via phlebotomy, rectal fluid via Dacron swab, and flexible sigmoidoscopy with pinch biopsy 12–15 cm proximal to the anal verge. Biopsies were processed for histology, explant HIV challenge, and PK (tissue homogenate and MMC). PBMC (from cell separation tubes) and colorectal MMC were washed, counted, and lysed in 70% ice cold methanol. Samples, except histology and radioisotopes, were stored at ≤ −70°C.
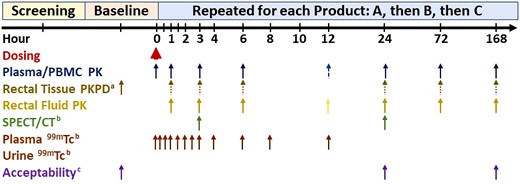
Study schema. Blood was collected for safety assessment predose and at 24 hours. Dashed arrow indicates blood (plasma/PBMC) collection at 12 hours at Johns Hopkins University School of Medicine only. Dotted arrows indicate sparse sampling (only two sampling times for biopsies per participant). aOnly baseline, 72-hour, and 168-hour biopsy was preceded by nonmedical cleansing douche. bJohns Hopkins University School of Medicine only. cAcceptability at 168 hours was a global comparative assessment only after all products had been received. Abbreviations: PBMC, peripheral blood mononuclear cell; PD, pharmacodynamics; PK, pharmacokinetics; SPECT/CT, single-photon emission computed tomography/computed tomography.
Colorectal Lumen Distribution
To assess colorectal lumen distribution, study products were 99mTc-DTPA (99m-technetium-diethylenetriamine pentaacetate)-labeled (1.8 millicuries), serving as a soluble small molecule drug (TFV) surrogate, and single-photon emission computed tomography (SPECT)/computed tomography (CT) imaging was performed at 2, 6, and 24 hours after each dose (Johns Hopkins University School of Medicine only, n = 6). Imaging used a dual-head VG series system equipped with a Hawkeye CT unit (GE Healthcare). CT images provided an anatomical reference and attenuation correction of SPECT images. SPECT images were reconstructed and fused with CT images into a 128 × 128 × 128 matrix with (3.453 mm)3 voxels using the GE Xeleris Functional Imaging Workstation, software version 3.1 (GE Healthcare).
Principal curves with a length penalty were used for 3-dimensional colonic curve fitting of SPECT images to generate concentration-distance curves from fitted centerlines as previously described [31]. Concentration-distance parameters were estimated using noncompartmental analysis (WinNonLin) by replacing time with distance (relative to the anorectal junction): Dmin, most distal signal; Dmax, most proximal signal; DCmax, maximum radiolabel concentration distance; and Dave, mean residence distance (Supplementary Figure 1).
Differences within participants over time for each product and across all 3 products at each imaging time were assessed for each concentration-distance parameter using the Skillings-Mack test, a variant of the Friedman ANOVA accounting for missing data [32] (Stata; StataCorp).
Pharmacokinetic Analysis
TFV, TFV-DP, FTC, and FTC triphosphate (FTC-TP) were measured using validated liquid chromatographic-tandem mass spectrometric (LC-MS/MS) methods described previously [33–35]. Assay lower limits of quantification (LLOQ) were plasma TFV and FTC, 0.31 ng/mL; rectal fluid TFV, 0.625 ng/swab; colorectal tissue homogenate TFV, 0.05 ng/sample; and TFV-DP in PBMC and MMC lysate and colorectal tissue homogenate, 50 fmol/sample. Adjusting for swab weight, cell number, or biopsy mass, median LLOQs were rectal fluid, TFV 0.037 ng/mg; PBMC TFV-DP, 5 fmol/million cells; tissue TFV-DP, 5 fmol/mg; and tissue TFV 0.006 ng/mg. We measured 99mTc-DTPA in plasma and urine using a gamma counter (2480 Wizard2; PerkinElmer). MMC TFV-DP was compared to estimated values for on-demand oral TDF 2-1-1 dosing based on PBMC TFV-DP modeling and simulation and PBMC to colon MMC TFV-DP ratio [35–41].
We assessed FTC analytes only in predose samples to identify oral PrEP use. TFV analyte and 99mTc data were used to estimate area under the concentration-time curve (AUC), peak concentration (Cmax), time to Cmax (Tmax), and terminal half-life (t1/2) (Pharsight WinNonLin version 8.3; Certara). We used WinNonlin sparse sampling calculation method to determine tissue point estimates.
Douche bottle pre/postdose and douche effluent weights were used to estimate dose delivered. For the SPECT/CT participants, 99mTc-DTPA dosimetry of pre/postdose douche bottle, douche effluent, gloves, and absorptive materials used during dosing were used to estimate the dose delivered and retained.
Explant Infectability Assay
Ex vivo colorectal tissue susceptibility to HIV infection was assessed by placing 3 biopsies, separately, in tissue culture media, exposing to 105 50% tissue culture infectious dose (TCID50) HIVBaL for 2 hours, followed by supernatant replacement. At 3, 7, 10, and 14 days post-HIV inoculation, supernatant was sampled for HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) assay (PerkinElmer) with 30 pg/mL LLOQ. Cumulative p24 antigen was calculated as the sum of 4 p24 antigen supernatant concentrations for each biopsy divided by the biopsy weight with values below the LLOQ imputed as LLOQ/2. The unit of analysis was median weight-adjusted cumulative p24 antigen for 3 biopsies. PK/PD data were fit to a sigmoid Imax model exploring 2 to 4 parameters with imputation of TFV-DP values below the LLOQ as LLOQ/2 and predose values as LLOQ/10 (WinNonlin).
Histology
One predose and 2 postdose biopsies were processed for histologic examination using paraffin embedding, microtome sectioning, and hematoxylin/eosin staining. A pathologist, DJT, scored slides using a modified inflammatory bowel disease grading scale, grade of epithelial denudation, and degree of lamina propria hemorrhage [42].
Acceptability
Each participant completed a Baseline Behavioral Questionnaire of prestudy sexual practices and likelihood of use of a rectal microbicide douche. After each of 3 study product doses, participants completed a research unit dose acceptability questionnaire to assess each product's acceptability. After each take-home douche use, participants completed the take-home dose acceptability questionnaire. Finally, at study completion participants underwent an in-depth interview to explore their experiences having used all 3 products.
Statistical Methods
We used median and interquartile range for descriptive statistics of most readouts. Paired comparisons among study products used the Friedman test, which, if statistically significant, was followed by the Wilcoxon rank sum test for differences between 2 study products with exact 2-tailed P values. Correlations between variables used the Spearman correlation test. A P value ≤.05 was considered statistically significant.
RESULTS
Enrollment
Twenty-eight MSM provided written informed consent of whom 21 entered the study and received at least 1 dose of study product defining the safety cohort (Figure 2). Eligible participants identified as white 16, black 4, and Asian 1; 4 identified as Hispanic. Median age was 38 years (range, 25–64 years), weight 78 kg (range, 60–125 kg), body mass index 26 (range, 21–38), and creatinine clearance 107 mL/min (range, 75–216 mL/min). Based upon predose PrEP drug concentrations (not available real-time), we excluded 5 participants (9 doses) leaving 20 participants (51 doses) for the PK cohort.
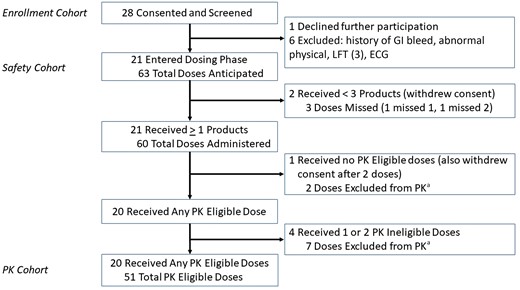
Research participants enrollment, dosing, and PK eligibility. aAll PK ineligible doses were excluded on the basis of detectable tenofovir or emtricitabine analytes prior to receiving the study product, as test results were available only after completion of the clinical phase. Abbreviations: ECG, electrocardiogram; GI, gastrointestinal; LFT, liver function test; PK, pharmacokinetics.
Product Delivery and Retention
The median TFV dose delivered for product A, B, and C was 188 mg (interquartile range [IQR], 176–198 mg), 543 mg (IQR, 525–589 mg), and 516 mg (IQR, 484–570 mg), respectively, with overall percent of prescribed dose delivered 80% (75%–86%). Based on radiolabel data available in 6 participants, there was an additional 12% (8%–18%) loss in the douche effluent overall.
Colorectal Lumen Distribution
Forty-one (76%) of 54 planned scans (6 participants, 3 times, 3 products) were evaluable; one 2-hour scan was lost due to scanner malfunction while one 6-hour and eleven 24-hour scans were not evaluable due to insufficient remaining radiolabel. SPECT/CT imaging showed all 3 products distributed throughout the rectosigmoid in all 6 participants with further distribution proximally into the descending colon in 3 of these (Figure 3). We identified no statistically significant differences for any concentration-distance parameter among products or within participants over time (P > .05; Supplementary Table 1). All concentration-distance parameters assessed at 24 hours were positively and moderately correlated (R range 0.60–0.67, P < .007) with duration of time until first bowel movement since initial douche evacuation, and modestly and negatively correlated with total number and time-adjusted accumulation of bowel movements (R range −0.62 to −0.68, P < .005).
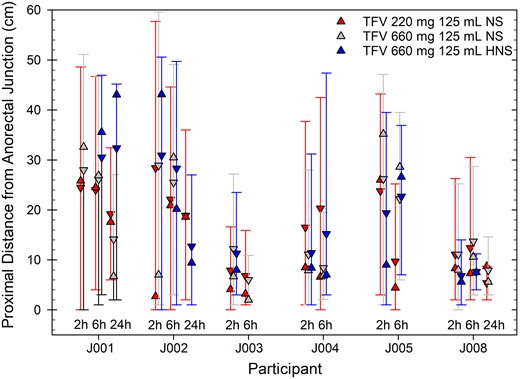
Intraluminal colonic distribution of radiolabel in each study product indicated by jittered location in cluster and color (product A, left, red; product B, middle, gray; product C, right, blue) based on SPECT/CT imaging and tube fitting (described in Supplementary Figure 1). Each participant imaged is shown over time (2, 6, and 24 hours); one 2-hour scan was lost due to scanner malfunction, while one 6-hour and eleven 24-hour scans were not evaluable due to insufficient remaining radiolabel. Distances are synchronized to the anorectal junction, setting y-axis = 0 cm, which serves as the reference location. Distributions are summarized here using the following radiolabel concentration-distance parameters: Dmax (maximal proximal radiolabel distance) upper error bar, Dmin (most distal radiolabel distance) lower error bar, Dave (mean residence radiolabel distance) down triangle, DCmax (location of peak radiolabel concentration) up triangle. Abbreviations: HNS, half normal saline; NS, normal saline; SPECT/CT, single-photon emission computed tomography/computed tomography; TFV, tenofovir.
Pharmacokinetics
Figure 4 displays concentration-time plots for all PK matrices with PK parameters in Supplementary Table 2. All pairwise combinations of concentrations in each biological matrix were positively and strongly correlated with each other (R range 0.75–0.89, P < .05)
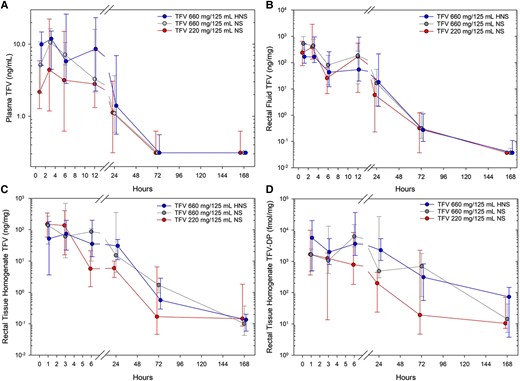
Tenofovir analyte concentration versus time for (A) plasma TFV, (B) rectal fluid, (C) colorectal tissue homogenate TFV, and (D) colorectal tissue homogenate TFV-DP. Each study product dose and osmolality are indicated in the inset key by color and jittered position at each sample time cluster: product A (left, red), product B (middle, gray), product C (right, blue). Values are median concentration (closed circle) with upper and lower quartiles (asymmetric capped error bars) for all participants sampled at a given nominal time for each study product. Abbreviations: HNS, half normal saline; NS, normal saline; TFV, tenofovir; TFV-DP, TFV diphosphate.
Blood
Comparing study products, product C median plasma concentration was greater than product A at 1, 3, and 6 hours, and Cmax and AUC0-6 were also greater; product C median plasma concentration was greater than product B at 1 hour (all P < .05). Product A and B were not different at any time for any PK parameter. PBMC TFV-DP was quantifiable in 21 of 364 (6%) samples with the highest overall value 11.6 fmol/million cells.
Rectal Fluid
Rectal fluid TFV concentrations fell mostly in a monotonic fashion from 1 to 168 hours with no differences among study products.
Rectal Tissue
Rectal tissue homogenate TFV concentration did not differ among products (Figure 4). Over the first 6 hours, both homogenate and MMC TFV-DP for product C were greater than product A (all P < .05); product B held an intermediate position without statistically significant differences compared to product A or C. Product C rectal tissue MMC TFV-DP concentration exceeded those targeted and presumed protective (131 fmol/million cells)—based upon modeling and simulation of a steady-state oral TDF 300 mg 2-1-1 regimen—for a median (range) 156 hours (range, 72 to > 168; Figure 5).
![Rectal tissue MMC TFV-DP concentration versus time by study product indicated by jittered location within each sample time cluster and color: product A (left, red), product B (middle, gray), product C (right, blue) with inset key noting dose and osmolality of each formulation. Values are median concentration (closed circle) with upper and lower quartiles indicated by asymmetric error bars for all participants sampled at a given nominal time for each study product. For comparison with oral dosing, the solid lines without error bars indicate the point estimate of simulated MMC TFV-DP with oral TDF 300 mg dosing using the on-demand 2-1-1 IPERGAY regimen, initiating dosing at time equal to zero (dark cyan) and at steady-state (dark yellow). Oral TDF 2-1-1 dosing simulations are based upon the Tanaudommongkon et al [36] model of PBMC TFV-DP and the Zhang et al [37] estimate of log-weighted geometric mean local to PBMC ratio (Table S3.1 in [37]) using data (Figure S3.2A in [37]) from 5 clinical studies with oral TDF dosing and colon tissue pharmacokinetics data [35, 38–41]. Abbreviations: HNS, half normal saline; MMC, mucosal mononuclear cell; NS, normal saline; PBMC, peripheral blood mononuclear cell; TDF, tenofovir disoproxil fumarate; TFV-DP, tenofovir diphosphate.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/229/4/10.1093_infdis_jiad535/1/m_jiad535f5.jpeg?Expires=1750045113&Signature=hNH6EJ5FbQ6pN4TJ02AuHquZdBqBSuLIpi0lwRfPqT~32PpYxP1ecqcNbv2Gsqkb0n5H0sBfU~T7CN1HKTFtLwPiPq6-EqysCPX1~bsudyashnH543EaroQb7xhoxe7OJAUbdd8wVAy9TMki27kurrJT~3k9rueMTsjS8VwToRbAw2dXLubefwgOjhSoastZqduxsGkdyFi0zuN-9g9d6CU6CuGfeC63OYD-RmkctM4vr4WDhkb5u9leJNftYJXrA9syGJVX~hWKLGJkjePNt9bdjNXsqAtQzxVXL~KyyUwAehOY9wDmX-7S~cIzD4MFaDV~VyTrjeG~uECEoB6pUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Rectal tissue MMC TFV-DP concentration versus time by study product indicated by jittered location within each sample time cluster and color: product A (left, red), product B (middle, gray), product C (right, blue) with inset key noting dose and osmolality of each formulation. Values are median concentration (closed circle) with upper and lower quartiles indicated by asymmetric error bars for all participants sampled at a given nominal time for each study product. For comparison with oral dosing, the solid lines without error bars indicate the point estimate of simulated MMC TFV-DP with oral TDF 300 mg dosing using the on-demand 2-1-1 IPERGAY regimen, initiating dosing at time equal to zero (dark cyan) and at steady-state (dark yellow). Oral TDF 2-1-1 dosing simulations are based upon the Tanaudommongkon et al [36] model of PBMC TFV-DP and the Zhang et al [37] estimate of log-weighted geometric mean local to PBMC ratio (Table S3.1 in [37]) using data (Figure S3.2A in [37]) from 5 clinical studies with oral TDF dosing and colon tissue pharmacokinetics data [35, 38–41]. Abbreviations: HNS, half normal saline; MMC, mucosal mononuclear cell; NS, normal saline; PBMC, peripheral blood mononuclear cell; TDF, tenofovir disoproxil fumarate; TFV-DP, tenofovir diphosphate.
99mTc-DTPA Pharmacokinetics
The pattern of plasma Tc-DTPA versus time for iso-osmolar product A and B indicated a 4-hour peak concentration followed by gradual decay. By contrast, product C demonstrated a rapid rise and fall within the first hour after which it reached a second peak with similar shape as the other products (Supplementary Figure 2). Higher Cmax and AUC0-12 of product C compared to product A (P = .09 and P = .06, respectively) and product B (P = .03 and P = .06, respectively) achieved or approached statistical significance (Supplementary Table 3). The dose accumulation in urine was not different among products.
HIV Explant Challenge
There were no statistically significant differences in p24 values among products. Pooling study products, biopsies collected at each sample time from 1 to 24 hours reduced HIV viral replication compared to predose baseline (all P < .002); peak viral suppression came 3 hours after dosing, median 1.3 log10 pg/mg (IQR, 0.6–1.5), and remained statistically significant through 24 hours after dosing, 0.6 log10 pg/mg (IQR, 0.0–1.5). The relationship between MMC TFV-DP concentration and p24 antiviral response (Figure 6) best fit a 3 parameter Imax viral suppression model: predose effect, E0, mean 2.2 log10 pg/mg (95% confidence interval [CI], 1.9–2.5), concentration at half-maximal effect, IC50, 2192 fmol/106 cells (95% CI, 595–3789), and maximal inhibitory response, Imax, 1.9 log10 pg/mg (95% CI, 1.6–2.3).
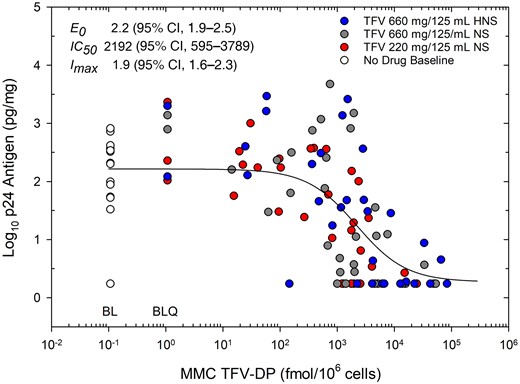
Concentration of MMC TFV-DP versus antiviral response of colon tissue explants after ex vivo HIV challenge. Response readout is log10 median (of 3 biopsies) cumulative (14 day) weight-adjusted p24 antigen. All biopsies from the pharmacokinetics eligible cohort are pooled indicating predose baseline values (open circles) and study products (closed circles): product A red, product B gray, product C blue. Fully suppressed p24 values (all 4 supernatants in 2 of 3 biopsies at a given sample time) are imputed as p24 LLOQ/2. MMC TFV-DP values below the assay LLOQ are imputed (for display purposes) as LLOQ/2 and indicated as BLQ; predose baseline values are imputed (for display purposes) as 1/10th the TFV-DP assay LLOQ value indicated as BL. Pharmacodynamic antiviral-concentration response model parameters (upper inset) indicate mean (95% CI) for fitted values. Parameters include: E0, baseline effect without drug; IC50, inhibitory concentration 50%, or concentration where antiviral effect is half-maximal; Imax, maximum antiviral effect relative to baseline. Abbreviations: CI, confidence interval; HNS, half normal saline; LLOQ, lower limit of quantification; MMC, mucosal mononuclear cell; NS, normal saline; TFV-DP, tenofovir diphosphate.
Acceptability
At baseline, 88% of participants indicated they would be likely or very likely to use a medicated rectal douche before RAI for HIV prevention. All participants had used a rectal douche in the past, and most had douched before sex within 3 months prior to study enrollment. In the context of actual use during the study, 95% of participants found all 3 products highly or somewhat acceptable, including those participants who rated themselves unlikely to use a rectal microbicide douche at baseline, indicating study product experience improved acceptability and likelihood of use (Supplementary Table 4). Overall, 94% indicated they would be likely to use the products before RAI if proven efficacious for HIV prevention and at least 61% indicated likely use both before and after RAI. Confidence in cleanliness after a single dose of douche varied, with 66%, 84%, and 83% of participants somewhat or completely confident in cleanliness after products A, B, and C, respectively. Take-home douches had no negative effects on sexual pleasure.
Safety
All 3 products were well tolerated without adverse event differences among study products (all P > .64; Table 1). One unrelated serious adverse event, grade 3 appendicitis, was reported. There were no grade 4 or 5 adverse events and of the 3 grade 3 events—1 during each study product phase—none were deemed product related. Two adverse events were deemed related to the study, both after product A: grade 1 blood-tinged rectal mucus in douche effluent in a participant with internal hemorrhoids and grade 1 rectal dryness.
| Adverse Event Category . | Product A . | Follow-up 1 . | Product B . | Product C . | Follow-up 2 . | Total . |
|---|---|---|---|---|---|---|
| Cardiovascular | 1 | … | 1 | … | … | 2 |
| Gastrointestinal | 3b | … | … | 1a | … | 4 |
| Genitourinary | … | 1 | … | … | … | 1 |
| HEENT | … | 1 | 1 | … | … | 2 |
| Infectious | 2 | 1 | … | 2 | 3 | 8 |
| Musculoskeletal | 1 | 1 | 2a | … | … | 4 |
| Laboratory | 8a | 8 | 6 | 3 | 4 | 29 |
| Total | 15 | 12 | 10 | 6 | 7 | 50 |
| Adverse Event Category . | Product A . | Follow-up 1 . | Product B . | Product C . | Follow-up 2 . | Total . |
|---|---|---|---|---|---|---|
| Cardiovascular | 1 | … | 1 | … | … | 2 |
| Gastrointestinal | 3b | … | … | 1a | … | 4 |
| Genitourinary | … | 1 | … | … | … | 1 |
| HEENT | … | 1 | 1 | … | … | 2 |
| Infectious | 2 | 1 | … | 2 | 3 | 8 |
| Musculoskeletal | 1 | 1 | 2a | … | … | 4 |
| Laboratory | 8a | 8 | 6 | 3 | 4 | 29 |
| Total | 15 | 12 | 10 | 6 | 7 | 50 |
All events were grade 1 or 2 and judged unrelated to study drug unless noted. χ2 tests for differences between products not statistically significant (all P > .64).
Abbreviation: HEENT, head, eyes, ears, nose, and throat.
aGrade 3 (n = 3): hypoglycemia (laboratory) predose product A dosing visit; fracture with reconstruction (musculoskeletal) after trauma, 3 months after product B and 2 months before product C dosing; appendicitis (gastrointestinal) 7 days after dosing.
bRelated to study product (n = 2): grade 1 blood-tinged mucus in douche effluent in participant with internal hemorrhoids and grade 1 rectal dryness in different participant, both after product A; neither adverse event recurred after product B or product C dosing.
| Adverse Event Category . | Product A . | Follow-up 1 . | Product B . | Product C . | Follow-up 2 . | Total . |
|---|---|---|---|---|---|---|
| Cardiovascular | 1 | … | 1 | … | … | 2 |
| Gastrointestinal | 3b | … | … | 1a | … | 4 |
| Genitourinary | … | 1 | … | … | … | 1 |
| HEENT | … | 1 | 1 | … | … | 2 |
| Infectious | 2 | 1 | … | 2 | 3 | 8 |
| Musculoskeletal | 1 | 1 | 2a | … | … | 4 |
| Laboratory | 8a | 8 | 6 | 3 | 4 | 29 |
| Total | 15 | 12 | 10 | 6 | 7 | 50 |
| Adverse Event Category . | Product A . | Follow-up 1 . | Product B . | Product C . | Follow-up 2 . | Total . |
|---|---|---|---|---|---|---|
| Cardiovascular | 1 | … | 1 | … | … | 2 |
| Gastrointestinal | 3b | … | … | 1a | … | 4 |
| Genitourinary | … | 1 | … | … | … | 1 |
| HEENT | … | 1 | 1 | … | … | 2 |
| Infectious | 2 | 1 | … | 2 | 3 | 8 |
| Musculoskeletal | 1 | 1 | 2a | … | … | 4 |
| Laboratory | 8a | 8 | 6 | 3 | 4 | 29 |
| Total | 15 | 12 | 10 | 6 | 7 | 50 |
All events were grade 1 or 2 and judged unrelated to study drug unless noted. χ2 tests for differences between products not statistically significant (all P > .64).
Abbreviation: HEENT, head, eyes, ears, nose, and throat.
aGrade 3 (n = 3): hypoglycemia (laboratory) predose product A dosing visit; fracture with reconstruction (musculoskeletal) after trauma, 3 months after product B and 2 months before product C dosing; appendicitis (gastrointestinal) 7 days after dosing.
bRelated to study product (n = 2): grade 1 blood-tinged mucus in douche effluent in participant with internal hemorrhoids and grade 1 rectal dryness in different participant, both after product A; neither adverse event recurred after product B or product C dosing.
Histology
Histology score, epithelial denudation, or lamina propria hemorrhage in colon biopsies collected after each study product were not statistically different from baseline biopsies (all P > .2; Supplementary Table 5).
DISCUSSION
We developed single-dose, on-demand rectal microbicide candidates in a behaviorally congruent douche formulation to provide an alternative to systemic PrEP options, and to overcome prior rectal product limitations. All 3 TFV douche products were well tolerated, highly acceptable, with minor attributable adverse events and no histologic changes. Participants also indicated a high likelihood of using such a product in the future should it be proven effective as PrEP. Proof of these single-dose findings await extended safety and acceptability trials.
Critically, the douches achieved our goals for low systemic TFV, rapid high colon tissue cell concentrations of TFV-DP, and colon product distribution covering likely HIV distribution based on prior HIV surrogate studies [43, 44]. Peak plasma TFV medians for all products were several-fold below the median plasma TFV trough associated with both daily TDF (52 ng/mL) and similar to TFV trough associated with TFV alafenamide (10.2 ng/mL); all PBMC medians were below the LLOQ [35]. One hour after douching, product C achieved tissue cell TFV-DP concentrations more than 1 log10 higher than steady-state concentrations achieved by (weekly) on-demand oral TDF 2-1-1 dosing—an advantage that diminished gradually until concentration parity nearly 7 days later. We chose the oral TDF/FTC 2-1-1 regimen as the most relevant comparator as a proven, highly effective, on-demand PrEP regimen. Importantly, this tissue TFV-DP comparison ignores additional protective effects of FTC in the oral regimen and the potential protective effects of higher systemic concentrations of active drug with oral dosing. In this regard, the superior macaque protection from SHIV rectal challenge with the product C TFV douche, when compared to steady-state oral daily TDF/FTC, provides key reassurance in advancing a rectal douching approach [30].
Previously, we reported results of colorectal tissue drug distribution using matrix-assisted laser desorption/ionization (MALDI), which indicated heterogeneity of TFV and TFV-DP in colon biopsies, including regions in which TFV and TFV-DP were not detectable. As a control for tissue composition and effects of tissue processing, phosphatidyl choline (16:0/OH) exhibited relatively homogeneous distribution across the tissue sections examined [45].
We found no clear differences among products in terms of acceptability, adverse events, histology, or luminal distribution to recommend advancing one over the others. There were no statistically significant product differences in explant p24 suppression, although far more of the concentrations above the IC50 and associated with the 2.2 log Imax response were associated with products B and C than with product A.
Examination of PK differences, however, favored product C, which achieved the highest plasma concentrations across the first 6 hours—statistically significantly greater than product A with product B in the intermediate position—indirectly indicating higher tissue penetration. Product C also rose the fastest in plasma, evidenced by the highest plasma concentrations at the very first plasma sampling time (1 hour). More importantly, for both colorectal tissue homogenate and MMC concentration of the active TFV-DP analyte, product C achieved higher concentrations than product A, with product B again in an intermediate position over the first 6 hours, indicating the product C advantage in the critical first 6 hours after dosing and anticipated sex.
While we did not have any plasma or tissue assessment of TFV or TFV-DP earlier than 1 hour, there is a suggestion that the differences between formulations may be greatest within the first hour, as the biophysical properties would predict. For the 6 Johns Hopkins University School of Medicine participants who underwent SPEC/CT imaging, the plasma 99mTc-DTPA concentration for product C was markedly higher than the other products in the first hour, including a statistically significant 4 times higher Cmax when compared to the iso-osmolar products; following this time point, all product's patterns were quite similar. The pattern is consistent with a bolus of 99mTc-DTPA absorbed across the tissue (seen only in hypo-osmolar product C and likely due to advection, which moves the drug rapidly to the mucosal surface as water is rapidly absorbed), followed by a prolonged period of much slower absorption across tissue from the persistent colonic reservoir of 99mTc-DTPA seen in all products. We did not assess how different rates of tissue absorption and systemic elimination differ between DTPA and TFV. However, the early, rapid plasma TFV and tissue TFV-DP concentration advantages of the hypo-osmolar product C, when compared to the iso-osmolar product B (both 600 mg doses), suggest osmolality as the primary cause. The more rapid uptake of product C provides an advantage over the iso-osmolar products.
Favorably for the TFV douche, the SPECT/CT-defined luminal colorectal distribution of our radiolabeled TFV surrogate, in all participants, overlapped the typical location of peak HIV surrogate distribution (6–7 cm above the anorectal junction) following simulated RAI using similar methods [43, 44]. However, those prior studies did not take account of the variety of parasexual behaviors like douching [44]. We previously reported that, among MSM who have RAI, 80% report douching before RAI, with a varied number of repeated douches, and 27% report douching following RAI [18]. The most rigorous test of any rectal microbicide candidate distribution—douche, gel, or insert—should include imaging of drug and viral surrogates simultaneously following a variety of sequences of simulated sex, pre- and post-RAI douching, and rectal microbicide candidate administration to inform product optimization and patient counseling matched to the heterogeneity of sexual practices.
CONCLUSIONS
A single dose of these TFV douches achieved colon tissue TFV-DP concentrations and HIV antiviral effect exceeding similar measures after oral TDF dosing, although with far lower systemic TFV exposure. All formulations were safe and highly acceptable. Hypo-osmolar product C demonstrated early PK advantages. In combination with the superiority of product C compared to oral daily steady-state TDF/FTC in macaques after SHIV rectal challenge, these data support advancing product C for additional clinical development as an on-demand, nonsystemic, behaviorally congruent PrEP candidate.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health, Department of Allergy and Infectious Disease, Division of AIDS Integrated Preclinical/Clinical Program for HIV Topical Microbicides (grant number U19 AI113127) and Clinical Pharmacology Training Program (grant number T32 GM066691); and Center for AIDS Research, Johns Hopkins University (grant number P30AI094189).
References
Author notes
Presented in part: HIV Research for Prevention (HIVR4P), 22–25 October 2018, Madrid, Spain. Tenofovir douche for PrEP: on demand, behaviorally congruent douche rapidly achieves colon tissue concentration targets (DREAM 01 study). Weld ED, Fuchs EJ, Marzinke MA, et al, abstract OA20.03.
Potential conflicts of interest. C. W. H. has received clinical research funding from Gilead Sciences and Merck; he is a coinventor of 2 issued US patents related to microbicides; and is founder of Prionde BioPharma, LLC, a microbicide company. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




