-
PDF
- Split View
-
Views
-
Cite
Cite
Paul N Zivich, Stephen R Cole, Jessie K Edwards, David V Glidden, Moupali Das, Bonnie E Shook-Sa, Yongwu Shao, Megha L Mehrotra, Adaora A Adimora, Joseph J Eron, HIV Prevention Among Men Who Have Sex With Men: Tenofovir Alafenamide Combination Preexposure Prophylaxis Versus Placebo, The Journal of Infectious Diseases, Volume 229, Issue 4, 15 April 2024, Pages 1123–1130, https://doi.org/10.1093/infdis/jiad507
Close - Share Icon Share
Abstract
While noninferiority of tenofovir alafenamide and emtricitabine (TAF/FTC) as preexposure prophylaxis (PrEP) for the prevention of human immunodeficiency virus (HIV) has been shown, interest remains in its efficacy relative to placebo. We estimate the efficacy of TAF/FTC PrEP versus placebo for the prevention of HIV infection.
We used data from the DISCOVER and iPrEx trials to compare TAF/FTC to placebo. DISCOVER was a noninferiority trial conducted from 2016 to 2017. iPrEx was a placebo-controlled trial conducted from 2007 to 2009. Inverse probability weights were used to standardize the iPrEx participants to the distribution of demographics and risk factors in the DISCOVER trial. To check the comparison, we evaluated whether risk of HIV infection in the shared tenofovir disoproxil fumarate and emtricitabine (TDF/FTC) arms was similar.
Notable differences in demographics and risk factors occurred between trials. After standardization, the difference in risk of HIV infection between the TDF/FTC arms was near zero. The risk of HIV with TAF/FTC was 5.8 percentage points lower (95% confidence interval [CI], −2.0% to −9.6%) or 12.5-fold lower (95% CI, .02 to .31) than placebo standardized to the DISCOVER population.
There was a reduction in HIV infection with TAF/FTC versus placebo across 96 weeks of follow-up.
NCT02842086 and NCT00458393.
Antiretroviral preexposure prophylaxis (PrEP) is an important tool for preventing human immunodeficiency virus (HIV) infection. Tenofovir disoproxil fumarate and emtricitabine (TDF/FTC) combination PrEP has been shown to be effective for a variety of groups [1–4]. Tenofovir alafenamide, an alternative prodrug of tenofovir with greater stability in blood plasma compared to TDF [5–7], and emtricitabine (TAF/FTC) was recently shown to be noninferior to TDF/FTC PrEP among men who have sex with men and transgender women [7, 8]. However, the efficacy of TAF/FTC PrEP relative to placebo remains of clinical and research interest (eg, decisions for PrEP-naive individuals, project benefits of adoption in PrEP naive populations, inputs for simulation or cost-benefit models for HIV transmission under varying PrEP uptake policies).
Transitivity arguments (ie, if A = B and B > C, then A > C) are implicit in the interpretation of noninferiority trial results [9], as the noninferiority of a novel preventative is only meaningful if the active comparator was also effective. While often left implicit, transitive comparisons rely on a number of assumptions to be valid, such as similar populations between trials, comparable definitions of end points, analogous rates of loss to follow-up, and similar adherence levels [10, 11]. When these assumptions are violated, simple transitive comparisons can be misleading as they reflect both differences in the efficacy of treatments as well as underlying differences between trials that may bias the estimated efficacy. Furthermore, transitive comparisons do not immediately lend themselves to the incorporation of uncertainty [ie, estimation of the variance, standard error, confidence intervals [CIs]). Without properly expressing this uncertainty, simple transitive comparisons can lead to overconfidence in results. As noninferiority trials will continue to serve as the basis for evaluation and approval of novel PrEP combinations, accurately contextualizing and interpreting noninferiority trial results is pivotal.
To estimate the efficacy of TAF/FTC relative to placebo for the prevention of HIV among men who have sex with men, we make a multispan bridged treatment comparison using the DISCOVER and iPrEx randomized trials [11–14]. Importantly, bridged treatment comparisons allow for analytical corrections of contextual differences between populations (eg, demographics, risk factors for HIV infection, adherence). Here, differences between trials are accounted for by standardizing the iPrEx trial to the same distribution of contextual factors in the DISCOVER trial using inverse probability weighting. To assess the validity of the contrast across trials, we graphically and statistically compared the shared TDF/FTC arm of the DISCOVER and iPrEx trials.
METHODS
Data Sources
The DISCOVER trial was a phase 3 double-masked, active-controlled noninferiority randomized trial comparing daily TAF/FTC to TDF/FTC for the prevention of HIV infection among men who have sex with men and transgender women (NCT02842086) [7]. Participants were recruited between September 2016 and June 2017 from 11 countries (Austria, Canada, Denmark, France, Germany, Ireland, Italy, Netherlands, Spain, United Kingdom, United States). Eligibility criteria included age (18 years or older), HIV-seronegative status at baseline, and evidence of high risk of HIV infection. High risk of HIV infection was defined based on reported sexual behaviors (condomless anal intercourse with 2 different, HIV-positive or unknown status male partners in the 12 weeks prior to enrollment) or bacterial sexually transmitted infections (diagnosed in the 24 weeks prior to enrollment). Participants were 1:1 block randomized to either daily TAF/FTC (25 mg/200 mg) or daily TDF/FTC (300 mg/200 mg). Study visits were scheduled to occur at 4 and 12 weeks, then every 12 weeks for at least 96 weeks. After all participants completed 96 weeks, DISCOVER transitioned to an open-label phase, where all participants were offered the opportunity to receive daily TAF/FTC.
iPrEx was a phase 3 double-masked, placebo-controlled randomized trial comparing the effect of daily TDF/FTC combination PrEP versus placebo on the prevention of HIV infection among men who have sex with men and transgender women (NCT00458393) [1]. Participants were recruited between July 2007 to December 2009 from study sites in 6 different countries (Brazil, Ecuador, Peru, South Africa, Thailand, United States). Eligibility criteria included age (18 years or older), HIV-seronegative status at baseline, and evidence of high risk of HIV infection. High risk of HIV infection was defined as no or inconsistent condom use during anal intercourse with a HIV-positive or unknown HIV status male partner, anal intercourse with more than 3 partners, commercial sex acts with a male partner, or a sexually transmitted infection in the previous 26 weeks. Participants were 1:1 block randomized to either daily TDF/FTC (300 mg/200 mg) combination PrEP or placebo. Study visits were scheduled every 4 weeks up to 132 weeks.
As the available DISCOVER trial data set was restricted to a subset that only included men who have sex with men, the iPrEx trial data was similarly restricted. To further harmonize the data, all observations were administratively censored at 96 weeks of follow-up. The data set included the following baseline covariates: age (categorized as 18–24, 25–29, 30–34, 35–39, 40+ years), race (white, nonwhite), ethnicity (Hispanic/Latino or non-Hispanic/non-Latino), diagnosed syphilis infection (yes, no), alcohol use (0, 1–4 drinks, 5+ drinks), and unprotected receptive anal intercourse (number of partners in the past 3 months). Race was measured through self-classification with an open-ended category but due to sparsity across trials was collapsed with ethnicity to non-Hispanic white, Hispanic white, and nonwhite. Alcohol use was self-reported alcohol intake on days when drinking. In iPrEx, syphilis was diagnosed via a rapid plasma reagin and confirmatory test, whereas in DISCOVER testing and diagnosis included a screening and confirmatory test and clinical diagnosis by the site investigator, according to local standard of care guidelines (which varied geographically).
The primary outcome of both trials was incident HIV infection. In iPrEx, HIV infection was ascertained at each study visit through 2 different third-generation rapid tests, with reactive rapid test results being established through western blot analysis of serum. Previously collected blood samples (collected every 12 weeks) were further tested for those who seroconverted. Incident HIV infection was defined as first available evidence of infection. In DISCOVER, HIV testing was performed at screening and at each study visit using third- or fourth-generation rapid tests and repeated by central laboratory-instrumented third- or fourth-generation tests; positive results were confirmed with an HIV 1/2 differentiation assay, along with HIV RNA (qualitative and/or quantitative) tests.
The University of North Carolina at Chapel Hill IRB deemed this research to not constitute human subject research and did not require institutional review board approval.
Statistical Analysis
The goal of our analysis was to estimate the risk difference and risk ratio of HIV infection had the DISCOVER trial randomized participants to TAF/FTC or placebo. As the DISCOVER trial was not placebo controlled, data from the iPrEx trial is used instead to stand in for the DISCOVER placebo arm. As a simple transitive comparison to estimate of the efficacy of TAF/FTC relative to placebo, the risk of HIV infection at 96 weeks can be approximated from the reported incidence rate in the TAF/FTC arm of the DISCOVER trial and contrasted with the risk at 96 weeks in the placebo arm of the iPrEx trial. However, this simple comparison is only interpretable as the efficacy if the trial populations are sufficiently similar in terms of demographics, risk factors, adherence, and other contextual features related to HIV infection. There is reason to suspect that this assumption is false based on the characteristics of the trial participants (Table 1 and Supplementary Figure 1) and reported differences in the overall adherence of trial participants [1, 7, 15].
Baseline Characteristics of the Participants of the iPrEx and DISCOVER Trials Included in the Complete-Case Analysis
| Characteristic . | IPrEx (n = 2108) . | DISCOVER (n = 4952) . | ||
|---|---|---|---|---|
| Placebo (n = 1049) . | TDF/FTC (n = 1059) . | TDF/FTC (n = 2488) . | TAF/FTC (n = 2464) . | |
| Age category, y | ||||
| 18–24 | 549 (52) | 498 (47) | 265 (11) | 306 (12) |
| 25–29 | 202 (19) | 228 (22) | 520 (21) | 504 (20) |
| 30–34 | 113 (11) | 132 (12) | 488 (20) | 485 (20) |
| 35–39 | 75 (7) | 75 (7) | 368 (15) | 370 (15) |
| 40+ | 110 (10) | 126 (12) | 847 (34) | 799 (32) |
| Not whitea | 861 (82) | 857 (81) | 363 (15) | 353 (14) |
| Hispanic | 749 (71) | 750 (71) | 606 (24) | 568 (23) |
| BMI, kg/m2 b | 23.7 (3.83) | 24.0 (3.99) | 26.2 (4.98) | 26.3 (5.06) |
| Alcohol usec | ||||
| 0 drinks | 163 (16) | 174 (16) | 190 (8) | 225 (9) |
| 1–4 drinks | 306 (29) | 314 (30) | 1914 (77) | 1857 (75) |
| 5+ drinks | 580 (55) | 571 (54) | 384(15) | 382 (16) |
| Number of partners with URAId | ||||
| 0 | 461 (44) | 482 (46) | 595 (24) | 559 (23) |
| 1 | 191 (18) | 185 (17) | 388 (16) | 373 (15) |
| 2 | 87 (8) | 96 (9) | 529 (21) | 496 (20) |
| 3–4 | 111 (11) | 81 (8) | 509 (20) | 514 (21) |
| 5+ | 199 (19) | 215 (20) | 467 (19) | 522 (21) |
| Syphilise | 128 (12) | 128 (12) | 4 (0) | 7 (0) |
| HIV at 96 wkf | 65 (6) | 29 (3) | 14 (1) | 7 (0) |
| Characteristic . | IPrEx (n = 2108) . | DISCOVER (n = 4952) . | ||
|---|---|---|---|---|
| Placebo (n = 1049) . | TDF/FTC (n = 1059) . | TDF/FTC (n = 2488) . | TAF/FTC (n = 2464) . | |
| Age category, y | ||||
| 18–24 | 549 (52) | 498 (47) | 265 (11) | 306 (12) |
| 25–29 | 202 (19) | 228 (22) | 520 (21) | 504 (20) |
| 30–34 | 113 (11) | 132 (12) | 488 (20) | 485 (20) |
| 35–39 | 75 (7) | 75 (7) | 368 (15) | 370 (15) |
| 40+ | 110 (10) | 126 (12) | 847 (34) | 799 (32) |
| Not whitea | 861 (82) | 857 (81) | 363 (15) | 353 (14) |
| Hispanic | 749 (71) | 750 (71) | 606 (24) | 568 (23) |
| BMI, kg/m2 b | 23.7 (3.83) | 24.0 (3.99) | 26.2 (4.98) | 26.3 (5.06) |
| Alcohol usec | ||||
| 0 drinks | 163 (16) | 174 (16) | 190 (8) | 225 (9) |
| 1–4 drinks | 306 (29) | 314 (30) | 1914 (77) | 1857 (75) |
| 5+ drinks | 580 (55) | 571 (54) | 384(15) | 382 (16) |
| Number of partners with URAId | ||||
| 0 | 461 (44) | 482 (46) | 595 (24) | 559 (23) |
| 1 | 191 (18) | 185 (17) | 388 (16) | 373 (15) |
| 2 | 87 (8) | 96 (9) | 529 (21) | 496 (20) |
| 3–4 | 111 (11) | 81 (8) | 509 (20) | 514 (21) |
| 5+ | 199 (19) | 215 (20) | 467 (19) | 522 (21) |
| Syphilise | 128 (12) | 128 (12) | 4 (0) | 7 (0) |
| HIV at 96 wkf | 65 (6) | 29 (3) | 14 (1) | 7 (0) |
Data are No. (%). To be eligible for the complete-case analysis, trial participants had to have no missing data for the reported variables. Furthermore, participants were restricted to men who have sex with men and a subset of the DISCOVER trial.
Abbreviations: BMI, body mass index; FTC, emtricitabine; HIV, human immunodeficiency virus; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; URAI, unprotected receptive anal intercourse.
aRace was measured through self-classification with an open-ended category (iPrEx, DISCOVER). Due to privacy concerns, data from DISCOVER was collapsed to white versus not white. Therefore, iPrEx was similarly collapsed.
bBMI is presented as mean and standard deviation.
cAlcohol use was defined as self-reported number of drinks on days when drinking (iPrEx) or self-reported alcohol intake in drinks per day (DISCOVER).
dSelf-reported number of unique partners with URAI in the past 84 days (iPrEx) or in the past 90 days (DISCOVER) at enrollment.
eBaseline syphilis diagnosis was determined via rapid plasma reagin and a confirmatory test (iPrEx) or diagnosed per local guidelines (DISCOVER).
fPercentages ignore right censoring.
Baseline Characteristics of the Participants of the iPrEx and DISCOVER Trials Included in the Complete-Case Analysis
| Characteristic . | IPrEx (n = 2108) . | DISCOVER (n = 4952) . | ||
|---|---|---|---|---|
| Placebo (n = 1049) . | TDF/FTC (n = 1059) . | TDF/FTC (n = 2488) . | TAF/FTC (n = 2464) . | |
| Age category, y | ||||
| 18–24 | 549 (52) | 498 (47) | 265 (11) | 306 (12) |
| 25–29 | 202 (19) | 228 (22) | 520 (21) | 504 (20) |
| 30–34 | 113 (11) | 132 (12) | 488 (20) | 485 (20) |
| 35–39 | 75 (7) | 75 (7) | 368 (15) | 370 (15) |
| 40+ | 110 (10) | 126 (12) | 847 (34) | 799 (32) |
| Not whitea | 861 (82) | 857 (81) | 363 (15) | 353 (14) |
| Hispanic | 749 (71) | 750 (71) | 606 (24) | 568 (23) |
| BMI, kg/m2 b | 23.7 (3.83) | 24.0 (3.99) | 26.2 (4.98) | 26.3 (5.06) |
| Alcohol usec | ||||
| 0 drinks | 163 (16) | 174 (16) | 190 (8) | 225 (9) |
| 1–4 drinks | 306 (29) | 314 (30) | 1914 (77) | 1857 (75) |
| 5+ drinks | 580 (55) | 571 (54) | 384(15) | 382 (16) |
| Number of partners with URAId | ||||
| 0 | 461 (44) | 482 (46) | 595 (24) | 559 (23) |
| 1 | 191 (18) | 185 (17) | 388 (16) | 373 (15) |
| 2 | 87 (8) | 96 (9) | 529 (21) | 496 (20) |
| 3–4 | 111 (11) | 81 (8) | 509 (20) | 514 (21) |
| 5+ | 199 (19) | 215 (20) | 467 (19) | 522 (21) |
| Syphilise | 128 (12) | 128 (12) | 4 (0) | 7 (0) |
| HIV at 96 wkf | 65 (6) | 29 (3) | 14 (1) | 7 (0) |
| Characteristic . | IPrEx (n = 2108) . | DISCOVER (n = 4952) . | ||
|---|---|---|---|---|
| Placebo (n = 1049) . | TDF/FTC (n = 1059) . | TDF/FTC (n = 2488) . | TAF/FTC (n = 2464) . | |
| Age category, y | ||||
| 18–24 | 549 (52) | 498 (47) | 265 (11) | 306 (12) |
| 25–29 | 202 (19) | 228 (22) | 520 (21) | 504 (20) |
| 30–34 | 113 (11) | 132 (12) | 488 (20) | 485 (20) |
| 35–39 | 75 (7) | 75 (7) | 368 (15) | 370 (15) |
| 40+ | 110 (10) | 126 (12) | 847 (34) | 799 (32) |
| Not whitea | 861 (82) | 857 (81) | 363 (15) | 353 (14) |
| Hispanic | 749 (71) | 750 (71) | 606 (24) | 568 (23) |
| BMI, kg/m2 b | 23.7 (3.83) | 24.0 (3.99) | 26.2 (4.98) | 26.3 (5.06) |
| Alcohol usec | ||||
| 0 drinks | 163 (16) | 174 (16) | 190 (8) | 225 (9) |
| 1–4 drinks | 306 (29) | 314 (30) | 1914 (77) | 1857 (75) |
| 5+ drinks | 580 (55) | 571 (54) | 384(15) | 382 (16) |
| Number of partners with URAId | ||||
| 0 | 461 (44) | 482 (46) | 595 (24) | 559 (23) |
| 1 | 191 (18) | 185 (17) | 388 (16) | 373 (15) |
| 2 | 87 (8) | 96 (9) | 529 (21) | 496 (20) |
| 3–4 | 111 (11) | 81 (8) | 509 (20) | 514 (21) |
| 5+ | 199 (19) | 215 (20) | 467 (19) | 522 (21) |
| Syphilise | 128 (12) | 128 (12) | 4 (0) | 7 (0) |
| HIV at 96 wkf | 65 (6) | 29 (3) | 14 (1) | 7 (0) |
Data are No. (%). To be eligible for the complete-case analysis, trial participants had to have no missing data for the reported variables. Furthermore, participants were restricted to men who have sex with men and a subset of the DISCOVER trial.
Abbreviations: BMI, body mass index; FTC, emtricitabine; HIV, human immunodeficiency virus; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; URAI, unprotected receptive anal intercourse.
aRace was measured through self-classification with an open-ended category (iPrEx, DISCOVER). Due to privacy concerns, data from DISCOVER was collapsed to white versus not white. Therefore, iPrEx was similarly collapsed.
bBMI is presented as mean and standard deviation.
cAlcohol use was defined as self-reported number of drinks on days when drinking (iPrEx) or self-reported alcohol intake in drinks per day (DISCOVER).
dSelf-reported number of unique partners with URAI in the past 84 days (iPrEx) or in the past 90 days (DISCOVER) at enrollment.
eBaseline syphilis diagnosis was determined via rapid plasma reagin and a confirmatory test (iPrEx) or diagnosed per local guidelines (DISCOVER).
fPercentages ignore right censoring.
To address these contextual differences between trials, we used a bridged treatment comparison [11, 12]. Bridged treatment comparisons operate by comparing differing arms between 2 trials (ie, TAF/FTC and placebo) through a shared arm (ie, TDF/FTC). Importantly, bridged treatment comparisons allow for differences between trials by measured covariates. In other words, bridged treatment comparisons assume that adherence to PrEP and subsequent risk of HIV infection was approximately the same across time and contexts within each unique combination of measured covariates. This assumption is weaker than that needed for simple transitive comparisons, which instead requires adherence and risk of HIV infection to be approximately the same across time and contexts irrespective of any covariates. Both these assumptions can be assessed through the following diagnostic procedure based on the shared trial arms. After accounting for measured covariates, the difference in the risk of HIV infection between the TDF/FTC arms of the trials is expected to be approximately zero. A nonzero difference indicates the assumptions for the bridged treatment comparison are not met (ie, important differences remain that have not been accounted for). Comparisons were made both graphically and via a statistical test [11]. Technical details are provided in the Supplementary Material.
To account for differences by measured covariates between trial populations in the bridged treatment comparison, the iPrEx data were standardized to have the same distribution of baseline variables as the DISCOVER data using inverse odds of sampling weights [16]. Briefly, inverse odds of sampling weights upweight iPrEx participants with covariate patterns that were more common in the DISCOVER trial and down-weight iPrEx participants with covariate patterns that were less commonly observed in the DISCOVER trial. Inverse odds of sampling weights were estimated using a logistic regression model where an indicator of the trial (DISCOVER vs iPrEx) was regressed on a set of variables identified a priori to both differ between trial populations and affect the risk of HIV infection. Variables included age, race/ethnicity, alcohol use, and unprotected receptive anal intercourse (categorized as 0, 1, 2, 3–4, 5+). When the estimated conditional probability of being in DISCOVER is near one for iPrEx participants, their inverse odds of sampling weight become large as the denominator of the weight is equal to one minus the conditional probability of selection. To prevent undue influence on the results from just a few observations, the corresponding conditional probabilities for the sampling weights were truncated to be ≤ 0.95 [17]. This choice of threshold means that a single observation from iPrEx can stand in for at most 19 DISCOVER participants (ie, between 0 and 19 participants).
To account for potentially different rates of loss to follow-up between trials, inverse probability of censoring weights were used [18]. To compute inverse probability of censoring weights, the probability of remaining in each trial was estimated using a Cox proportional hazards model and the Breslow estimator [19–21]. The Cox proportional hazards model was stratified by assigned PrEP and trial, and included the same variables as the selection model. No truncation was performed for the inverse probability of censoring weights.
The trial-arm-specific risks of HIV infection up to 96 weeks were then estimated using the weighted empirical distribution function, where events were reweighted based on the product of the inverse probability of censoring and inverse odds of sampling weights. The risk difference comparing TAF/FTC to placebo was estimated by adding the risk difference comparing TAF/FTC to TDF/FTC in the DISCOVER trial to the risk difference comparing TDF/FTC to placebo for the reweighted iPrEx trial. Similarly, the risk ratio comparing TAF/FTC to placebo was estimated by multiplying the risk ratio comparing TAF/FTC to TDF/FTC in the DISCOVER trial with the risk ratio comparing TDF/FTC to placebo in the reweighted iPrEx trial. Wald-type 95% CIs were computed using a nonparametric bootstrap [11].
Two sensitivity analyses were further conducted. First, sensitivity to the chosen truncation points for the inverse odds of sampling weights was assessed by varying the upper bound on the conditional probabilities. Second, the main analysis was conducted only on participants who were not diagnosed with syphilis at baseline, as we were unable to adjust for syphilis diagnosis with inverse weights due to sparsity.
All analyses were conducted using Python 3.6.8 (Python Software Foundation) with the following open-source libraries: NumPy [22], SciPy [23], pandas [24], statsmodels [25], and matplotlib [26]. Results are presented graphically using twister plots [27].
RESULTS
Data for 7339 participants were available, with 2154 (29%) from iPrEx and 5185 (71%) from DISCOVER. Of the available observations, most had no missing data for all harmonized baseline variables (n = 7060, 96%). Therefore, a complete-case analysis was conducted. As seen in Table 1, notable differences in age, race, ethnicity, alcohol use, and unprotected receptive anal intercourse between the 2 trial populations were observed, suggesting that a simple transitive comparison across trials would be misleading. The problem with the transitive comparison was further corroborated when the risk of HIV infection between the shared TDF/FTC arms was compared. As shown in Figure 1A, the risk in the DISCOVER TDF/FTC arm was lower than the risk in the iPrEx TDF/FTC arm. Therefore, directly comparing the TAF/FTC arm of DISCOVER and the placebo arm of iPrEx is likely to overestimate the protective effect of TAF/FTC. After standardizing the iPrEx data to the DISCOVER trial population, the estimated difference in HIV risk between the TDF/FTC arm of the 2 trials was close to zero (P = .87; Figure 1B), supporting the assumption that standardization accounted for pertinent differences between trial populations.
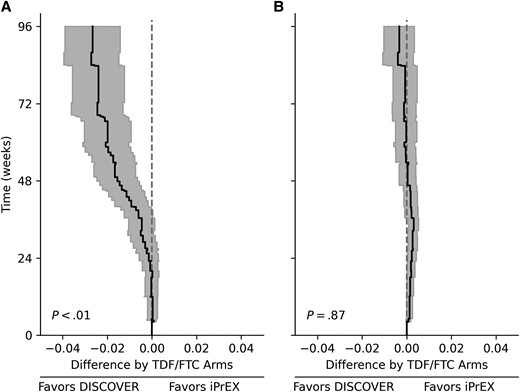
Graphical comparisons of the risk of HIV infection between the DISCOVER and iPrEx TDF/FTC combination preexposure prophylaxis arms prestandardization (A) and poststandardization (B). Lines indicate the estimated risk of HIV infection in the DISCOVER TDF/FTC arm minus the risk of HIV infection in the iPrEx TDF/FTC arm. Shaded regions indicate 95% confidence intervals. P values were calculated with a Wald-type test based on the integrated risk difference over 96 weeks. Abbreviations: HIV, human immunodeficiency virus; TDF/FTC, tenofovir disoproxil fumarate and emtricitabine.
For the bridged treatment comparison, TAF/FTC was protective against HIV infection versus placebo (Figure 2, Figure 3, and Supplementary Figure 2). The 96-week risk difference of HIV infection comparing TAF/FTC to placebo was −5.8% (95% CI, −2.0% to −9.6%), and the risk ratio was 0.08 (95% CI, .02–.31) for men who have sex with men in the DISCOVER source population. The efficacy of TAF/FTC was noticeably larger when making a simple transitive comparison, with an estimated risk difference of −7.8% (and a risk ratio of 0.04). This larger effect was likely due to the contextual differences between trials, as indicated by Figure 1A.
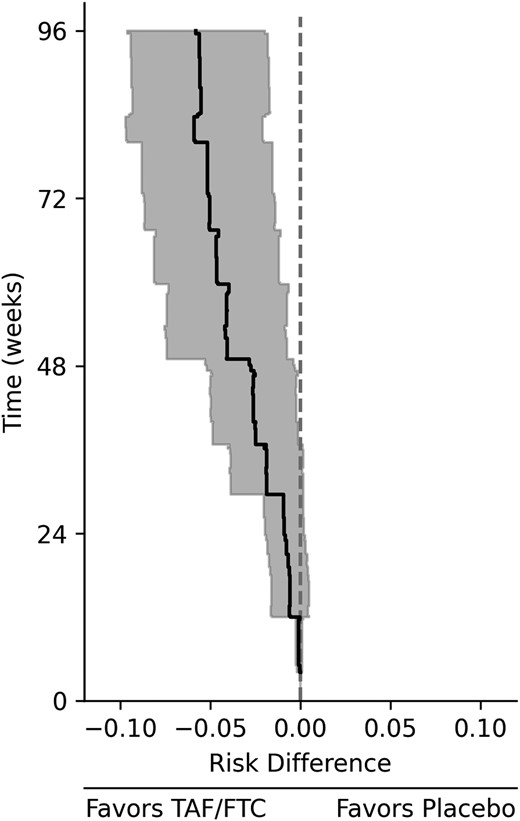
Risk difference of HIV infection comparing TAF/FTC combination preexposure prophylaxis versus placebo for the DISCOVER trial population. The line indicates the estimated risk difference comparing TAF/FTC to placebo among men who have sex with men for the DISCOVER trial population, and the shaded region indicates the 95% confidence interval. Abbreviations: HIV, human immunodeficiency virus; TAF/FTC, tenofovir alafenamide and emtricitabine.
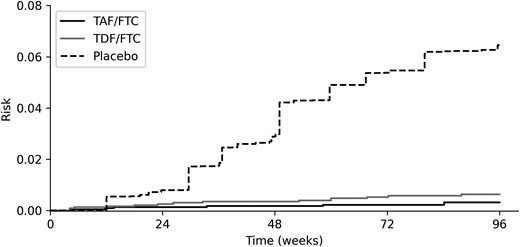
Risk of HIV infection by TAF/FTC PrEP, TDF/FTC PrEP, and placebo standardized to the DISCOVER trial population. Lines indicate the estimated risk of HIV infection. Risk was estimated using the DISCOVER trial data (TAF/FTC, TDF/FTC) and the standardized iPrEx trial data (placebo). Abbreviations: HIV, human immunodeficiency virus; PrEP, preexposure prophylaxis; TAF/FTC, tenofovir alafenamide and emtricitabine; TDF/FTC, tenofovir disoproxil fumarate and emtricitabine.
Results for the bridged treatment comparison were similar for both sensitivity analyses. When relaxing the truncation point for the inverse probability weights, the variance increased, as expected, but point estimates were similar (Supplementary Figures 3–4 and Supplementary Table 1). When restricting to those without a syphilis diagnosis at baseline, there was little change in the results (Supplementary Figures 5–6).
DISCUSSION
By combining trials via a bridged treatment comparison, we were able to quantitatively estimate the protection of TAF/FTC relative to placebo for HIV infection. At 96 weeks, the risk of HIV infection was 5.8% points lower (95% CI, −2.0% to −9.6%) or 0.08 times (95% CI, .02–.31) comparing TAF/FTC to placebo standardized to men who have sex with men in the DISCOVER population. Of note, both efficacy estimates are less pronounced than the simple transitive comparison, which is consistent with the expected results stemming from observed differences in contextual factors and overall adherence differences between the 2 trial populations [7, 15]. This discrepancy highlights the danger of relying on simple transitive comparisons across different trials. Finally, the assumptions underlying our comparisons are supported by the diagnostic comparing the shared TDF/FTC arms across trials. After accounting for the observed differences, we found little to no difference between the TDF/FTC arms across trials, which supports the validity of our TAF/FTC and placebo comparison.
The existence of multiple effective PrEP combinations precludes future placebo-controlled trials [1, 7, 28]. To avoid the corresponding ethical concerns, noninferiority and superiority trials will continue to serve as the basis for the evaluation of new PrEP combinations. However, noninferiority trials and superiority trials are not without challenges [10]. As described above, interpretation of the results of these trials hinges upon the effectiveness of the active comparator over placebo. Another major challenge is the number of participants needed to feasibly meet the corresponding noninferiority or superiority margins. For example, DISCOVER enrolled more than twice as many participants as iPrEx. Recruitment and follow-up of the additional participants can substantially increase costs and lead to delays in the approval of new PrEP combinations. To mitigate these and other challenges in PrEP noninferiority trials, there has been interest in so-called counterfactual placebo methods [29, 30]. These include simple transitivity comparisons, as well as more complex approaches including network meta-analysis [31], cross-sectional recency tests as a stand-in for a placebo arm [32], and priors on adherence-efficacy relationships of the active comparator [33, 34]. Bridged treatment comparisons are another option for counterfactual placebo analyses, which allow for more complex analytical corrections for contextual difference across data sources and provide an intuitive check on the validity of a comparison through the shared arm between trials. Regardless, multiple counterfactual placebo methods with differing assumptions can be leveraged to bolster confidence in comparisons. Here, bridged treatment comparisons and Bayesian adherence-efficacy study results provide reassurance regarding the findings of a protective effect of TAF/FTC versus placebo (but results between these studies are not directly comparable due to differences in the chosen efficacy measure) [33, 34].
Our analysis is premised on the assumption that standardization accounted for all important differences in terms of risk of HIV infection between the trial populations. Despite the reassurances provided by the comparison between the TDF/FTC arm of the trials, potentially important differences could remain. iPrEx was conducted in predominantly middle-income countries, while DISCOVER was conducted in high-income countries. While both trials recruited participants from the United States, there were not enough observed events in iPrEx to restrict by country. HIV testing methods also differed between trials (eg, third- vs fourth-generation tests). Furthermore, variations in HIV epidemiology and prevalence of bacterial sexually transmitted infections likely differed between trial populations, as these 2 trials are separated in time by nearly a decade. Lastly, estimation of the intent-to-treat effect assumed adherence was comparable across trials conditional on age, race/ethnicity, alcohol use, and unprotected receptive anal intercourse [11, 35]. While adherence between trials differed marginally, existing research on PrEP adherence is supportive of the conditional assumption made here, as the factors included in the inverse odds of sampling weight model have been found to be important predictors of adherence [36–39]. Finally, the efficacy of TAF/FTC versus placebo could only be estimated up to 96 weeks postrandomization due to the follow-up period of the DISCOVER trial. As the efficacy of TAF/FTC PrEP is dependent on sustained daily adherence, pill fatigue may result in a declining efficacy of TAF/FTC over longer time periods. Other PrEP combinations that do not necessitate daily adherence, like long-acting injectable cabotegravir [28], are less susceptible to issues of pill fatigue and subsequent adherence issues. As these alternatives pose their own challenges (eg, attending clinic visits for injections every 2 months), the best PrEP option for sustained adherence likely varies between individual patients.
PrEP remains a key individual-level tool to prevent HIV infection. We found a meaningful reduction in HIV risk with TAF/FTC versus placebo, but the estimated reduction was smaller than anticipated based on a simple comparison across trials. The over-estimate from the simple comparison stemmed from underlying differences in the trial populations in terms of demographics, HIV risk factors, and other contextual factors. Pooling trial data with bridged treatment comparisons allows for indirect comparisons across randomized trials, as well as a diagnostic to assess the underlying assumptions. To enhance transitive comparisons, noninferiority and superiority trials should strive for overlapping eligibility criteria with prior trials on the active comparator and collect important predictors of the primary outcome using similar definitions whenever possible.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. S. R. C. conceptualized the research. D. V. G. and M. L. M. curated and provided data for the iPrEx trial. M. D. and Y. S. curated and provided data for the DISCOVER trial. P. N. Z. harmonized the data and conducted the statistical analyses. S. R. C., J. K. E., and B. E. S. provided supervision on the harmonization and statistical analyses. The first draft of the manuscript was written by P. N. Z. All authors reviewed and edited the manuscript and approved the final version. P. N. Z. and S. R. C. made the decision to submit the manuscript for publication.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). The direct funder of this study, the NIH, had no role in study design, data analysis, data interpretation, or writing of the manuscript. Gilead Sciences provided data from the DISCOVER trial but did not directly fund this study. Coauthors employed by Gilead had roles aiding with data interpretation and revising the manuscript.
Financial support. This work was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID; grant numbers R01-AI157758 to P. N. Z., S. R. C., J. K. E., B. E. S., and G. E. M., T32-AI007001 to P. N. Z., and P30-AI50410 to S. R. C., B. E. S., and J. J. E.). The iPrEx trial was supported by the Division of Acquired Immunodeficiency Syndrome, NIAID, NIH (grant number R01-AI062333 and U01-AI64002); and the Bill and Melinda Gates Foundation; with study drugs donated by Gilead Sciences. The DISCOVER trial was funded by Gilead Sciences.
Data availability. Anonymized individual-level data from the iPrEx trial used for this analysis will be made available upon reasonable request to D. G. ([email protected]). Anonymized individual-level data from the DISCOVER trial is available from Gilead Sciences. Gilead Sciences shares anonymized individual patient data with qualified external researchers on request, or as required by law or regulation. Approval of such requests is at the discretion of Gilead Sciences, and is dependent on the nature of the request, merit of the research proposed, availability of the data, and intended use of the data. Data requests should be sent to [email protected].
References
Author notes
Potential conflicts of interest. D. V. G. has accepted funds from Gilead Sciences. M. D., M. L. M., and Y. S. are employees of Gilead Sciences and hold stock interest in the company. A. A. A. has received consulting fees from Merck and Gilead; and research funding to her institution from Merck and Gilead. J. J. E. is an ad hoc consultant to Gilead Sciences and ViiV Healthcare. University of North Carolina at Chapel Hill receives clinical research funding from ViiV Healthcare and Gilead Science for studies on which J. J. E. is an investigator. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




