-
PDF
- Split View
-
Views
-
Cite
Cite
Grant C Paulsen, Robert Frenck, Kay M Tomashek, Rodolfo M Alarcon, Elizabeth Hensel, Ashley Lowe, Rebecca L Brocato, Steve A Kwilas, Matthew D Josleyn, Jay W Hooper, Safety and Immunogenicity of an Andes Virus DNA Vaccine by Needle-Free Injection: A Randomized, Controlled Phase 1 Study, The Journal of Infectious Diseases, Volume 229, Issue 1, 15 January 2024, Pages 30–38, https://doi.org/10.1093/infdis/jiad235
Close - Share Icon Share
Abstract
Andes virus (ANDV), a rodent-borne hantavirus, causes hantavirus pulmonary syndrome (HPS). The safety and immunogenicity of a novel ANDV DNA vaccine was evaluated.
Phase 1, double-blind, dose-escalation trial randomly assigned 48 healthy adults to placebo or ANDV DNA vaccine delivered via needle-free jet injection. Cohorts 1 and 2 received 2 mg of DNA or placebo in a 3-dose (days 1, 29, 169) or 4-dose (days 1, 29, 57, 169) schedule, respectively. Cohorts 3 and 4 received 4 mg of DNA or placebo in the 3-dose and 4-dose schedule, respectively. Subjects were monitored for safety and neutralizing antibodies by pseudovirion neutralization assay (PsVNA50) and plaque reduction neutralization test (PRNT50).
While 98% and 65% of subjects had at least 1 local or systemic solicited adverse event (AE), respectively, most AEs were mild or moderate; no related serious AEs were detected. Cohorts 2, 3, and 4 had higher seroconversion rates than cohort 1 and seropositivity of at least 80% by day 197, sustained through day 337. PsVNA50 geometric mean titers were highest for cohort 4 on and after day 197.
This first-in-human candidate HPS vaccine trial demonstrated that an ANDV DNA vaccine was safe and induced a robust, durable immune response.
Clinical Trials Registration. NCT03682107.
Pathogenic orthohantaviruses are single-stranded RNA viruses that are members of the Hantaviridae family, order Bunyavirales, and can be found worldwide [1, 2]. The primary severe disease manifestations in humans are likely due to inflammation and endothelial cell disruption with increased vascular permeability, resulting in hemorrhagic fever with renal syndrome (HFRS) or hantavirus pulmonary syndrome (HPS) [3]. HPS is caused by region-specific hantavirus strains, predominantly the Andes virus (ANDV) strains in South America [4–6] and Sin Nombre virus in North America [7].
HPS due to ANDV is a zoonotic disease primarily transmitted to humans by inhalation of aerosolized excreta from infected rodents, although human-to-human transmission has been reported [5, 8]. HPS typically presents as a nonspecific viral illness followed by abrupt onset of pulmonary edema and shock [9]. HPS predominantly occurs in South America with over 2900 cases reported since 1993 in Chile, Argentina, and Brazil alone [2, 10]. In the United States and Canada, there have been approximately 950 reported cases and an incidence of 20–45 cases per year since 1993 [11, 12]. HPS remains a serious infection with mortality rates of 35%–40% despite critical care support [3, 9, 13]. There are no Food and Drug Administration (FDA)-licensed HPS vaccines and treatment is supportive with early mechanical ventilation, inotropic support, and extracorporeal membrane oxygenation.
An ANDV DNA vaccine plasmid containing a full-length ANDV M segment that encodes Gn and Gc envelope glycoprotein was recently developed [14–16]. While the human correlate of protection is unknown, Gn and Gc are targets of neutralizing antibodies and passive antibody transfer from species vaccinated with the ANDV DNA vaccine provide robust protection from lethal disease in a HPS Syrian hamster model [7, 14, 17–19]. Currently there are no FDA-licensed DNA vaccines for any pathogen, largely due to difficulties with cellular uptake of DNA resulting in suboptimal immunogenicity [20, 21]. Despite historical concerns with DNA vaccines in general, DNA vaccines for hantaviruses have been in development for years with encouraging safety and immunogenicity results reported against 2 of the HFRS-specific hantaviruses, Hantaan virus (HTNV) and Puumala virus (PUUV) [15, 22]. Other platforms, including inactivated HFRS vaccines have been developed, but continue to face concerns of effectiveness and durability resulting in the need for increased doses or exploration into adjuvants [23, 24]. Nucleic acid/DNA-based molecular vaccines against hantaviruses have become the most active platform under study because a proven M gene-based vaccine could be further optimized as a DNA vaccine, or potentially be delivered by alternative gene-based platforms including mRNA or viral vector [2, 25]. Methods to increase intracellular delivery of DNA have been developed, including electroporation and needle-free jet injection systems (NFIS). Electroporation utilizes an electrical current at the time of vaccination to increase cellular uptake and NFIS delivers the vaccine as a high-pressure stream of liquid that penetrates tissues and deposits the injected DNA intracellularly [14, 26]. Here we report the results of a phase 1, randomized, double-blind, placebo-controlled, dose-escalation trial to assess the safety and immunogenicity of an ANDV DNA vaccine administered at 2 different doses and 2 different vaccination schedules using the spring powered PharmaJet Stratis intramuscular NFIS device.
METHODS
Vaccine
The ANDV DNA vaccine evaluated in this study was developed by the Department of Molecular Virology, US Army Medical Research Institute of Infectious Diseases (USAMRIID; Fort Detrick, Maryland). The ANDV DNA vaccine plasmid, pWRG/AND-M(opt2), was constructed by cloning cDNA representing the ANDV M segment open reading frame (ORF) into the pWRG7077 vector, as described previously for the HTNV and PUUV DNA vaccine plasmids [14–16]. The ANDV ORF was from ANDV Chile-9717869. This ORF encodes the Gn and Gc envelope glycoproteins, and was optimized for Homo sapien codon usage and mRNA stability by Genewiz. The final vaccine product was purified DNA diluted in saline.
PharmaJet Delivery System
The ANDV DNA vaccine was delivered using the PharmaJet Stratis Needle-free Injection System. This is an FDA 510k-cleared NFIS device that delivers a 0.5-mL jet of liquid at high pressure that penetrates the skin into the muscle [14].
Study Subjects
Healthy male and nonpregnant, nonlactating female adult volunteers between the ages of 18 and 49 years were enrolled. All recruiting and consent materials were compliant with current good clinical practice guidelines and approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board. Key exclusions were receipt of a hantavirus vaccine (HTNV, PUUV); known exposure to ANDV or plans to travel to an ANDV endemic area through 6 months after last vaccination; history of severe reactions to vaccines or vaccine products or a severe allergic reaction; current acute illness or unstable chronic medical condition; history of an autoimmune, immunosuppressive, or immunodeficient condition; history of type 1 or 2 diabetes or any chronic neurologic disorder; receipt of any live vaccine within 28 days or inactivated vaccine (except influenza) within 14 days of study vaccination; and receipt of any experimental agent, blood product, or immunoglobulin within 3 months of the first study vaccination.
Clinical Study Design Overview
This phase 1, randomized, double-blind, placebo-controlled, dose-escalation trial (NCT03682107) included 4 cohorts, which varied in either the DNA vaccine dose (2 mg or 4 mg per vaccination) or schedule (3 doses or 4 doses) (Table 1). Cohort 1 and 3 subjects received 2 DNA vaccine or saline placebo injections (1 into each deltoid) on days 1, 29, and 169 and placebo on day 57. The day 57 placebo was used to maintain the study blind between the 3-dose and 4-dose cohorts. Cohort 2 and 4 subjects received 2 DNA vaccine or placebo injections on days 1, 29, 57, and 169. Each cohort had 2 subjects that received placebo on days 1, 29, 57, and 169. All doses were administered using a NFIS device, which provided 1 mg into the left and right deltoid for a total dose of 2 mg for cohort 1 and 2, and 2 mg into the left and right deltoid for a total 4 mg dose for cohort 3 and 4.
| Cohort . | Treatment Arm . | Day 1 . | Day 29 . | Day 57 . | Day 169 . |
|---|---|---|---|---|---|
| Cohort 1 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 2, 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohort 3, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 4, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohorts 1–4 | Placebo (n = 8) | Placebo | Placebo | Placebo | Placebo |
| Total | 48 subjects | ||||
| Cohort . | Treatment Arm . | Day 1 . | Day 29 . | Day 57 . | Day 169 . |
|---|---|---|---|---|---|
| Cohort 1 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 2, 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohort 3, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 4, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohorts 1–4 | Placebo (n = 8) | Placebo | Placebo | Placebo | Placebo |
| Total | 48 subjects | ||||
Abbreviation: ANDV, Andes virus.
aOne mg ANDV DNA administered into the left and right deltoid.
bTwo mg ANDV DNA administered into the right and left deltoid.
| Cohort . | Treatment Arm . | Day 1 . | Day 29 . | Day 57 . | Day 169 . |
|---|---|---|---|---|---|
| Cohort 1 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 2, 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohort 3, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 4, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohorts 1–4 | Placebo (n = 8) | Placebo | Placebo | Placebo | Placebo |
| Total | 48 subjects | ||||
| Cohort . | Treatment Arm . | Day 1 . | Day 29 . | Day 57 . | Day 169 . |
|---|---|---|---|---|---|
| Cohort 1 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 2, 2 mga | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohort 3, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | Placebo | ANDV Vax 3 |
| Cohort 4, 4 mgb | ANDV DNA (n = 10) | ANDV Vax 1 | ANDV Vax 2 | ANDV Vax 3 | ANDV Vax 4 |
| Cohorts 1–4 | Placebo (n = 8) | Placebo | Placebo | Placebo | Placebo |
| Total | 48 subjects | ||||
Abbreviation: ANDV, Andes virus.
aOne mg ANDV DNA administered into the left and right deltoid.
bTwo mg ANDV DNA administered into the right and left deltoid.
Each cohort had 12 subjects, including 1 sentinel subject, 2 placebo, and 9 vaccine recipients (Table 1). Enrollment began in cohort 1 and 2 with sentinel subjects receiving open-label study vaccine. When no halting rules were met, the remaining nonsentinel subjects in cohort 1 and 2 were randomized in a 9:2 ratio to receive either 2 mg of study vaccine or placebo in a 3-dose or 4-dose schedule, respectively. A safety monitoring committee meeting reviewed safety data through 7 days after the second vaccination for cohort 1 and 2. The 24 subjects in cohort 3 and 4 followed the same procedure. All subjects were followed until at least day 337.
Safety Assessments
Safety was assessed by clinical laboratory testing and evaluating local and systemic reactogenicity with the use of a memory aid for 7 days after each injection. Subjects were monitored for adverse events (AE) as defined by the study end points: (1) solicited local and systemic AEs through 7 days after each vaccination; (2) clinical safety laboratory AEs at 7 days after each vaccination; (3) unsolicited AEs through 28 days after the last vaccination; and (4) serious adverse events (SAEs) through 6 months after the last vaccination. Solicited AEs included injection site findings (erythema, swelling/induration, tenderness, pain, bruising, and skin discoloration) and systemic findings (headache, fatigue, malaise, fever, chills/shivering, myalgia, nausea, and dizziness). Safety laboratory tests included white blood cell count, hemoglobin, platelet count, absolute neutrophil count, sodium, potassium, creatinine, total bilirubin, alanine aminotransferase, and blood urea nitrogen.
Pseudovirion Neutralization Assay
Immunogenicity was assessed by detecting the presence of neutralizing antibodies using a pseudovirion neutralization assay (PsVNA50) performed by USAMRIID [14, 15, 18]. Blinded serum specimens from each subject collected prior to administration of study product on day 1 and on days 29, 57, 85, 169, 197, 253, and 337 were evaluated. Briefly, the assay uses a nonreplicating VSVΔG encoding a luciferase reporter. The plasmid, pWRG/AND-M(opt2), used to create pseudovirions was the same as used for the ANDV DNA vaccine in this study. Serum specimens, 5% complement (Cedarlane) and 4000 focus-forming units of pseudovirion were incubated at 2°C–8°C overnight and then added to VERO-76 cells and incubated at 37°C for 18–24 hours. The cells were then lysed and read for luminescence (Promega) the following day. Neutralization 50% titers were interpolated from curves generated from the values for each specimen using GraphPad Prism.
Plaque Reduction Neutralization Test
Blinded serum specimens were also evaluated for neutralizing antibodies using a plaque reduction neutralization test (PRNT50) as described previously [22]. The PRNT50 assay was conducted at USAMRIID using serum specimens collected prior to administration of study product on day 1 and on days 57, 85, 169, and 197. The ANDV Chile 9717869 used in these assays was a P2 stock of twice plaque purified ANDV produced at USAMRIID (lot BB070511). Assays were performed using Vero-E6 monolayers in 6-well plates with 30 or more plaques per well with adequate separation for counting. Samples were initially screened at a 1:20 dilution and positive samples (ie, ≥ 20 PRNT50) were retested in duplicate to determine geometric mean neutralizing antibody titers. PRNT50 titers are the reciprocal of the highest dilution neutralizing 50% of the number of plaques in controls wells.
Statistical Methods
The sample size for this study was primarily chosen to evaluate the safety and immunogenicity of an ANDV DNA vaccine in this first-in-human trial to inform product development pathway including future trials. The sample size was not sufficient to test any formal hypotheses. Subjects who met eligibility criteria and received at least 1 dose of study vaccine were included in the descriptive analysis of safety and reactogenicity outcomes.
Subjects who contributed both pre- and postvaccination samples for which valid results were reported were included in the immunogenicity analyses. The immunogenicity outcome measures included the incidence of seropositivity (ie, an ANDV titer of ≥20) and seroconversion on day 57 (for the 3-dose regimen), day 85 (4-dose regimen), and day 197 (both dosing regimens) as measured by PRNT50 or PsVNA50. Seroconversion was defined as a postvaccination ANDV-specific titer of ≥40 if the baseline (day 1) titer was <20, or a minimum 4-fold rise compared to baseline if the baseline titer was ≥20. Geometric mean titers (GMT) of neutralizing antibodies to ANDV were also assessed on days 1, 57, 85, and 197 and compared to baseline (day 1). To assess agreement between results generated by PsVNA and PRNT, the Spearman rank-order correlation was computed at each common time point as well as using peak titers per subject over the common time points. Exact 95% Clopper-Pearson confidence intervals (CI) were used for safety and immunogenicity summaries.
RESULTS
Clinical Subject Population
Between 19 February 2019 and 4 November 2019, 60 people were screened for enrollment. The mean age of the 48 enrolled subjects was 34.7 (SD 8.6) years and 33 (69%) were female (Supplementary Table 1). Overall, most (77%) of the subjects were white, 17% were African American, 4% were Asian, and 2% were multiracial; 2 (4%) were Hispanic/Latinx.
Vaccination and Safety Assessment
No study-related SAEs were observed. One SAE, acute rhabdomyolysis, occurred 79 days after dose 3. The event was attributed to extreme exertion and atorvastatin use. There were no deaths reported during the study.
Solicited local and systemic AEs were common with 47 out of 48 (98%) subjects reporting at least 1 local AE and 31 (65%) reporting at least 1 systemic AE (Table 2 and Table 3). The most common local AEs were injection-site erythema (90%), induration (90%), tenderness (81%), and pain (67%). A higher proportion of vaccine recipients (70%, 28/40; 95% CI, 53%–83%) reported having at least 1 solicited systemic AE than placebo recipients (38%, 3/8; 95% CI, 9%–76%). The most prevalent systemic symptom was headache (52%), followed by fatigue (48%), and malaise (38%). Mild fever was reported in 3 vaccine recipients (7.5%, 3/40) in the 7 days after vaccination. The majority of all solicited AEs were mild (25%, 12/48) or moderate (58%, 28/48) severity. Six (15%) study vaccine recipients (4 from cohort 1 and 2, and 2 from cohort 3 and 4) experienced a total of 6 related grade 3 symptoms including headache (1), erythema (3), induration (1), and ecchymosis (1). All grade 3 symptoms were improved or resolved after 1 day.
Frequency and Severity of Common Local Solicited Adverse Events Occurring in 5% of Subjects, Overall and by Study Product Received
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Any local solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Erythema | ||||||||
| Any | 19 | (95) | 18 | (90) | 6 | (75) | 43 | (90) |
| Grade 1, mild | 4 | (20) | 12 | (60) | 4 | (50) | 20 | (42) |
| Grade 2, moderate | 12 | (60) | 6 | (30) | 2 | (25) | 20 | (42) |
| Grade 3, severe | 3 | (15) | 0 | (0) | 0 | (0) | 3 | (6) |
| Induration | ||||||||
| Any | 19 | (95) | 19 | (95) | 5 | (63) | 43 | (90) |
| Grade 1, mild | 17 | (85) | 14 | (70) | 5 | (63) | 36 | (75) |
| Grade 2, moderate | 2 | (10) | 4 | (20) | 0 | (0) | 6 | (13) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Tenderness | ||||||||
| Any | 18 | (90) | 17 | (85) | 4 | (50) | 39 | (81) |
| Grade 1, mild | 15 | (75) | 10 | (50) | 4 | (50) | 29 | (60) |
| Grade 2, moderate | 3 | (15) | 7 | (35) | 0 | (0) | 10 | (21) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Pain | ||||||||
| Any | 15 | (75) | 14 | (70) | 3 | (38) | 32 | (67) |
| Grade 1, mild | 12 | (60) | 10 | (50) | 2 | (25) | 24 | (50) |
| Grade 2, moderate | 3 | (15) | 4 | (20) | 1 | (13) | 8 | (17) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bruising | ||||||||
| Any | 9 | (45) | 9 | (45) | 2 | (25) | 20 | (42) |
| Grade 1, mild | 9 | (45) | 6 | (30) | 2 | (25) | 17 | (35) |
| Grade 2, moderate | 0 | (0) | 3 | (15) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Skin discoloration | ||||||||
| Any | 7 | (35) | 6 | (30) | 0 | (0) | 13 | (27) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 0 | (0) | 12 | (25) |
| Grade 2, moderate | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Any local solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Erythema | ||||||||
| Any | 19 | (95) | 18 | (90) | 6 | (75) | 43 | (90) |
| Grade 1, mild | 4 | (20) | 12 | (60) | 4 | (50) | 20 | (42) |
| Grade 2, moderate | 12 | (60) | 6 | (30) | 2 | (25) | 20 | (42) |
| Grade 3, severe | 3 | (15) | 0 | (0) | 0 | (0) | 3 | (6) |
| Induration | ||||||||
| Any | 19 | (95) | 19 | (95) | 5 | (63) | 43 | (90) |
| Grade 1, mild | 17 | (85) | 14 | (70) | 5 | (63) | 36 | (75) |
| Grade 2, moderate | 2 | (10) | 4 | (20) | 0 | (0) | 6 | (13) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Tenderness | ||||||||
| Any | 18 | (90) | 17 | (85) | 4 | (50) | 39 | (81) |
| Grade 1, mild | 15 | (75) | 10 | (50) | 4 | (50) | 29 | (60) |
| Grade 2, moderate | 3 | (15) | 7 | (35) | 0 | (0) | 10 | (21) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Pain | ||||||||
| Any | 15 | (75) | 14 | (70) | 3 | (38) | 32 | (67) |
| Grade 1, mild | 12 | (60) | 10 | (50) | 2 | (25) | 24 | (50) |
| Grade 2, moderate | 3 | (15) | 4 | (20) | 1 | (13) | 8 | (17) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bruising | ||||||||
| Any | 9 | (45) | 9 | (45) | 2 | (25) | 20 | (42) |
| Grade 1, mild | 9 | (45) | 6 | (30) | 2 | (25) | 17 | (35) |
| Grade 2, moderate | 0 | (0) | 3 | (15) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Skin discoloration | ||||||||
| Any | 7 | (35) | 6 | (30) | 0 | (0) | 13 | (27) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 0 | (0) | 12 | (25) |
| Grade 2, moderate | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
AEs in ≥25% of subjects are indicated in bold.
Abbreviations: AE, adverse events; ANDV, Andes virus.
Frequency and Severity of Common Local Solicited Adverse Events Occurring in 5% of Subjects, Overall and by Study Product Received
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Any local solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Erythema | ||||||||
| Any | 19 | (95) | 18 | (90) | 6 | (75) | 43 | (90) |
| Grade 1, mild | 4 | (20) | 12 | (60) | 4 | (50) | 20 | (42) |
| Grade 2, moderate | 12 | (60) | 6 | (30) | 2 | (25) | 20 | (42) |
| Grade 3, severe | 3 | (15) | 0 | (0) | 0 | (0) | 3 | (6) |
| Induration | ||||||||
| Any | 19 | (95) | 19 | (95) | 5 | (63) | 43 | (90) |
| Grade 1, mild | 17 | (85) | 14 | (70) | 5 | (63) | 36 | (75) |
| Grade 2, moderate | 2 | (10) | 4 | (20) | 0 | (0) | 6 | (13) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Tenderness | ||||||||
| Any | 18 | (90) | 17 | (85) | 4 | (50) | 39 | (81) |
| Grade 1, mild | 15 | (75) | 10 | (50) | 4 | (50) | 29 | (60) |
| Grade 2, moderate | 3 | (15) | 7 | (35) | 0 | (0) | 10 | (21) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Pain | ||||||||
| Any | 15 | (75) | 14 | (70) | 3 | (38) | 32 | (67) |
| Grade 1, mild | 12 | (60) | 10 | (50) | 2 | (25) | 24 | (50) |
| Grade 2, moderate | 3 | (15) | 4 | (20) | 1 | (13) | 8 | (17) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bruising | ||||||||
| Any | 9 | (45) | 9 | (45) | 2 | (25) | 20 | (42) |
| Grade 1, mild | 9 | (45) | 6 | (30) | 2 | (25) | 17 | (35) |
| Grade 2, moderate | 0 | (0) | 3 | (15) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Skin discoloration | ||||||||
| Any | 7 | (35) | 6 | (30) | 0 | (0) | 13 | (27) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 0 | (0) | 12 | (25) |
| Grade 2, moderate | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Any local solicited AE | 19 | (95) | 20 | (100) | 8 | (100) | 47 | (98) |
| Erythema | ||||||||
| Any | 19 | (95) | 18 | (90) | 6 | (75) | 43 | (90) |
| Grade 1, mild | 4 | (20) | 12 | (60) | 4 | (50) | 20 | (42) |
| Grade 2, moderate | 12 | (60) | 6 | (30) | 2 | (25) | 20 | (42) |
| Grade 3, severe | 3 | (15) | 0 | (0) | 0 | (0) | 3 | (6) |
| Induration | ||||||||
| Any | 19 | (95) | 19 | (95) | 5 | (63) | 43 | (90) |
| Grade 1, mild | 17 | (85) | 14 | (70) | 5 | (63) | 36 | (75) |
| Grade 2, moderate | 2 | (10) | 4 | (20) | 0 | (0) | 6 | (13) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Tenderness | ||||||||
| Any | 18 | (90) | 17 | (85) | 4 | (50) | 39 | (81) |
| Grade 1, mild | 15 | (75) | 10 | (50) | 4 | (50) | 29 | (60) |
| Grade 2, moderate | 3 | (15) | 7 | (35) | 0 | (0) | 10 | (21) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Pain | ||||||||
| Any | 15 | (75) | 14 | (70) | 3 | (38) | 32 | (67) |
| Grade 1, mild | 12 | (60) | 10 | (50) | 2 | (25) | 24 | (50) |
| Grade 2, moderate | 3 | (15) | 4 | (20) | 1 | (13) | 8 | (17) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Bruising | ||||||||
| Any | 9 | (45) | 9 | (45) | 2 | (25) | 20 | (42) |
| Grade 1, mild | 9 | (45) | 6 | (30) | 2 | (25) | 17 | (35) |
| Grade 2, moderate | 0 | (0) | 3 | (15) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Skin discoloration | ||||||||
| Any | 7 | (35) | 6 | (30) | 0 | (0) | 13 | (27) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 0 | (0) | 12 | (25) |
| Grade 2, moderate | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
AEs in ≥25% of subjects are indicated in bold.
Abbreviations: AE, adverse events; ANDV, Andes virus.
Frequency and Severity of Common Systemic Solicited Adverse Events Occurring in 5% of Subjects, Overall and by Study Product Received
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any systemic solicited AE | 15 | (75) | 13 | (65) | 3 | (38) | 31 | (65) |
| Headache | ||||||||
| Any | 11 | (55) | 11 | (55) | 3 | (38) | 25 | (52) |
| Grade 1, mild | 9 | (45) | 7 | (35) | 3 | (38) | 19 | (40) |
| Grade 2, moderate | 1 | (5) | 4 | (20) | 0 | (0) | 5 | (10) |
| Grade 3, severe | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Fatigue | ||||||||
| Any | 11 | (55) | 10 | (50) | 2 | (25) | 23 | (48) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 2 | (25) | 14 | (29) |
| Grade 2, moderate | 4 | (20) | 5 | (25) | 0 | (0) | 9 | (19) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Malaise | ||||||||
| Any | 9 | (45) | 8 | (40) | 1 | (13) | 18 | (38) |
| Grade 1, mild | 8 | (40) | 5 | (25) | 1 | (13) | 14 | (29) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Fever | ||||||||
| Any | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 1, mild | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 2, moderate | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Chills/shivering | ||||||||
| Any | 5 | (25) | 5 | (25) | 1 | (13) | 11 | (23) |
| Grade 1, mild | 4 | (20) | 2 | (10) | 1 | (13) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Myalgia | ||||||||
| Any | 7 | (35) | 3 | (15) | 0 | (0) | 10 | (21) |
| Grade 1, mild | 5 | (25) | 1 | (5) | 0 | (0) | 6 | (13) |
| Grade 2, moderate | 2 | (10) | 2 | (10) | 0 | (0) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Nausea | ||||||||
| Any | 3 | (15) | 4 | (20) | 2 | (25) | 9 | (19) |
| Grade 1, mild | 1 | (5) | 3 | (15) | 1 | (13) | 5 | (10) |
| Grade 2, moderate | 2 | (10) | 1 | (5) | 1 | (13) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Dizziness | ||||||||
| Any | 3 | (15) | 5 | (25) | 0 | (0) | 8 | (17) |
| Grade 1, mild | 2 | (10) | 5 | (25) | 0 | (0) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any systemic solicited AE | 15 | (75) | 13 | (65) | 3 | (38) | 31 | (65) |
| Headache | ||||||||
| Any | 11 | (55) | 11 | (55) | 3 | (38) | 25 | (52) |
| Grade 1, mild | 9 | (45) | 7 | (35) | 3 | (38) | 19 | (40) |
| Grade 2, moderate | 1 | (5) | 4 | (20) | 0 | (0) | 5 | (10) |
| Grade 3, severe | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Fatigue | ||||||||
| Any | 11 | (55) | 10 | (50) | 2 | (25) | 23 | (48) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 2 | (25) | 14 | (29) |
| Grade 2, moderate | 4 | (20) | 5 | (25) | 0 | (0) | 9 | (19) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Malaise | ||||||||
| Any | 9 | (45) | 8 | (40) | 1 | (13) | 18 | (38) |
| Grade 1, mild | 8 | (40) | 5 | (25) | 1 | (13) | 14 | (29) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Fever | ||||||||
| Any | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 1, mild | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 2, moderate | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Chills/shivering | ||||||||
| Any | 5 | (25) | 5 | (25) | 1 | (13) | 11 | (23) |
| Grade 1, mild | 4 | (20) | 2 | (10) | 1 | (13) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Myalgia | ||||||||
| Any | 7 | (35) | 3 | (15) | 0 | (0) | 10 | (21) |
| Grade 1, mild | 5 | (25) | 1 | (5) | 0 | (0) | 6 | (13) |
| Grade 2, moderate | 2 | (10) | 2 | (10) | 0 | (0) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Nausea | ||||||||
| Any | 3 | (15) | 4 | (20) | 2 | (25) | 9 | (19) |
| Grade 1, mild | 1 | (5) | 3 | (15) | 1 | (13) | 5 | (10) |
| Grade 2, moderate | 2 | (10) | 1 | (5) | 1 | (13) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Dizziness | ||||||||
| Any | 3 | (15) | 5 | (25) | 0 | (0) | 8 | (17) |
| Grade 1, mild | 2 | (10) | 5 | (25) | 0 | (0) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
AEs in ≥25% of subjects are indicated in bold.
Abbreviations: AE, adverse events; ANDV, Andes virus.
Frequency and Severity of Common Systemic Solicited Adverse Events Occurring in 5% of Subjects, Overall and by Study Product Received
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any systemic solicited AE | 15 | (75) | 13 | (65) | 3 | (38) | 31 | (65) |
| Headache | ||||||||
| Any | 11 | (55) | 11 | (55) | 3 | (38) | 25 | (52) |
| Grade 1, mild | 9 | (45) | 7 | (35) | 3 | (38) | 19 | (40) |
| Grade 2, moderate | 1 | (5) | 4 | (20) | 0 | (0) | 5 | (10) |
| Grade 3, severe | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Fatigue | ||||||||
| Any | 11 | (55) | 10 | (50) | 2 | (25) | 23 | (48) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 2 | (25) | 14 | (29) |
| Grade 2, moderate | 4 | (20) | 5 | (25) | 0 | (0) | 9 | (19) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Malaise | ||||||||
| Any | 9 | (45) | 8 | (40) | 1 | (13) | 18 | (38) |
| Grade 1, mild | 8 | (40) | 5 | (25) | 1 | (13) | 14 | (29) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Fever | ||||||||
| Any | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 1, mild | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 2, moderate | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Chills/shivering | ||||||||
| Any | 5 | (25) | 5 | (25) | 1 | (13) | 11 | (23) |
| Grade 1, mild | 4 | (20) | 2 | (10) | 1 | (13) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Myalgia | ||||||||
| Any | 7 | (35) | 3 | (15) | 0 | (0) | 10 | (21) |
| Grade 1, mild | 5 | (25) | 1 | (5) | 0 | (0) | 6 | (13) |
| Grade 2, moderate | 2 | (10) | 2 | (10) | 0 | (0) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Nausea | ||||||||
| Any | 3 | (15) | 4 | (20) | 2 | (25) | 9 | (19) |
| Grade 1, mild | 1 | (5) | 3 | (15) | 1 | (13) | 5 | (10) |
| Grade 2, moderate | 2 | (10) | 1 | (5) | 1 | (13) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Dizziness | ||||||||
| Any | 3 | (15) | 5 | (25) | 0 | (0) | 8 | (17) |
| Grade 1, mild | 2 | (10) | 5 | (25) | 0 | (0) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| AE and Severity . | ANDV DNA 2 mg, No. (%) . | ANDV DNA 4 mg, No. (%) . | Placebo, No. (%) . | All, No. (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 20) . | (n = 20) . | (n = 8) . | (n = 48) . | |||||
| Any systemic solicited AE | 15 | (75) | 13 | (65) | 3 | (38) | 31 | (65) |
| Headache | ||||||||
| Any | 11 | (55) | 11 | (55) | 3 | (38) | 25 | (52) |
| Grade 1, mild | 9 | (45) | 7 | (35) | 3 | (38) | 19 | (40) |
| Grade 2, moderate | 1 | (5) | 4 | (20) | 0 | (0) | 5 | (10) |
| Grade 3, severe | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Fatigue | ||||||||
| Any | 11 | (55) | 10 | (50) | 2 | (25) | 23 | (48) |
| Grade 1, mild | 7 | (35) | 5 | (25) | 2 | (25) | 14 | (29) |
| Grade 2, moderate | 4 | (20) | 5 | (25) | 0 | (0) | 9 | (19) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Malaise | ||||||||
| Any | 9 | (45) | 8 | (40) | 1 | (13) | 18 | (38) |
| Grade 1, mild | 8 | (40) | 5 | (25) | 1 | (13) | 14 | (29) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Fever | ||||||||
| Any | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 1, mild | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 2, moderate | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Chills/shivering | ||||||||
| Any | 5 | (25) | 5 | (25) | 1 | (13) | 11 | (23) |
| Grade 1, mild | 4 | (20) | 2 | (10) | 1 | (13) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 2 | (10) | 0 | (0) | 3 | (6) |
| Grade 3, severe | 0 | (0) | 1 | (5) | 0 | (0) | 1 | (2) |
| Myalgia | ||||||||
| Any | 7 | (35) | 3 | (15) | 0 | (0) | 10 | (21) |
| Grade 1, mild | 5 | (25) | 1 | (5) | 0 | (0) | 6 | (13) |
| Grade 2, moderate | 2 | (10) | 2 | (10) | 0 | (0) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Nausea | ||||||||
| Any | 3 | (15) | 4 | (20) | 2 | (25) | 9 | (19) |
| Grade 1, mild | 1 | (5) | 3 | (15) | 1 | (13) | 5 | (10) |
| Grade 2, moderate | 2 | (10) | 1 | (5) | 1 | (13) | 4 | (8) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Dizziness | ||||||||
| Any | 3 | (15) | 5 | (25) | 0 | (0) | 8 | (17) |
| Grade 1, mild | 2 | (10) | 5 | (25) | 0 | (0) | 7 | (15) |
| Grade 2, moderate | 1 | (5) | 0 | (0) | 0 | (0) | 1 | (2) |
| Grade 3, severe | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
AEs in ≥25% of subjects are indicated in bold.
Abbreviations: AE, adverse events; ANDV, Andes virus.
Unsolicited AEs that were related to the study product or injection procedure were reported in 11 (23%) subjects and included eye pain, injection site eczema, injection site hematoma, injection site pruritis, injection site warmth, hyperbilirubinemia, vomiting, gastroenteritis, upper respiratory tract infection, dizziness, dysgeusia, and breast pain. Two vaccine recipients (4%), 1 in cohort 3 and 1 in cohort 4, had grade 2 neutropenia that was assessed as related to the study product.
Two unanticipated administration events occurred during vaccination. During injection for 2 different subjects, the disposable plastic needle-free syringe holding the study product or placebo cracked. This occurred in 1 placebo recipient and the other in a 2 mg, 3-dose vaccine recipient during the first (out of 2 deltoid) injections at the first vaccination.
Neutralizing Antibody Response
ANDV PsVNA50 seropositivity rates were measured for all cohorts through day 337 (Supplementary Table 2). Two vaccine recipients (5%) were seropositive at baseline. At each time point, the seropositivity rate of vaccine recipients in cohort 1 was numerically lower than the other cohorts, with a maximum of 67% seropositive at day 337. Following the final dose at day 169, seropositivity increased in all cohorts, with cohort 2, 3, and 4 retaining 80% or greater seropositivity at all remaining time points (Figure 1A). At the final day 337 time point, 67% (6/9) of subjects in cohort 1 were seropositive and 88%–90% of those in cohort 2, 3, and 4 were seropositive.
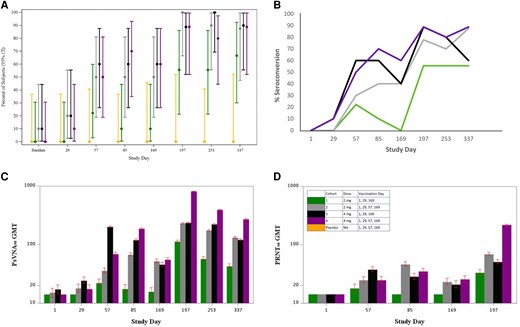
Neutralizing antibody responses by cohort. A, PsVNA50 seropositivity rates, ITT population. Bars are 95% CI. B, PsVNA50 seroconversion by time point and vaccination group, ITT population. C, PsVNA50 geometric mean titers. D, PRNT50 geometric mean titers. C and D, The lower limit of quantitation was 20; values <20 were given a value of 14.1. Bars indicated 95% CI. Abbreviations: CI, confidence interval; GMT, geometric mean titer; ITT, intention to treat; NA, not applicable; PRNT50, 50% plaque reduction neutralization test; PsVNA50, 50% pseudovirion neutralization assay.
Seroconversion rates increased for all cohorts over time, and at day 337 greater than 50% of all participants had seroconverted (Figure 1B). Cohort 2 and 4 had the highest final seroconversion percentages on day 337 at 88% and 89%, respectively.
PsVNA50 GMTs initially peaked in cohort 1 and 3 on day 57, or 28 days after dose 2, and then trended down through day 169 (prior to dose 3) (Figure 1C). GMTs increased in cohort 2 and 4 through day 85, followed by a downward trend through day 169 (prior to dose 4). All 4 cohorts, regardless of dose or schedule, had a boost in titer after day 169 with peak GMTs that occurred on day 197, or 28 days after the last vaccination. The highest GMT was 808.2 (95% CI, 168.2–3882.4) in cohort 4 on day 197. Cohort 4 also had the highest GMT at day 337.
PRNT50 GMTs followed a similar pattern to PsVNA50 with an initial peak occurring 28 days after dose 2 in the 3-dose cohorts and 28 days after dose 3 in the 4-dose cohorts, irrespective of vaccine dose (Figure 1D). Similarly, following the final vaccination on day 169, PRNT50 GMTs on day 197 increased for all cohorts with the highest GMT of 217.7 (95% CI, 71.7–660.9) in cohort 4. PsVNA50 and PRNT50 titers for individual subjects by group are shown in Figure 2 and Supplementary Figures 1–6.
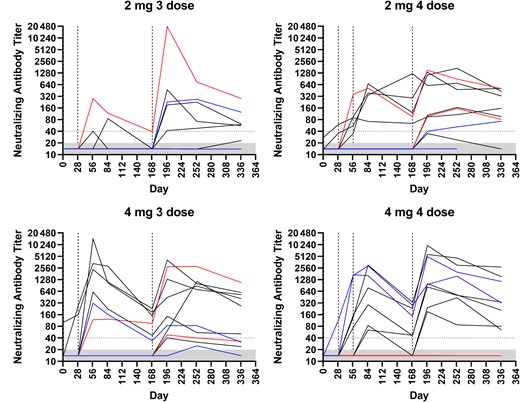
PsVNA50 titers for individual subjects. Dashed lines indicate day of vaccination. Dotted line at 40 represents the threshold for seroconversion for subjects with titers <20 prevaccination. The lower limit of the assay is a titer of 20, grey-shaded area. Abbreviation: PsVNA50, 50% pseudovirion neutralization assay
Neutralizing antibody results obtained by PsVNA50 and PRNT50 were comparable with relatively strong correlation coefficients of greater than 0.80 in cohort 1 and 2 and coefficients of greater than 0.60 in cohort 3 and 4 at each time point (Figure 3 and Supplementary Table 3).
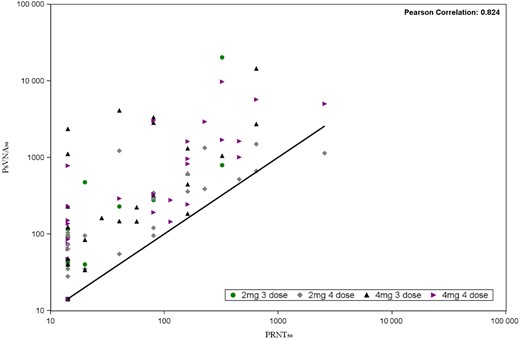
Scatter plot of PRNT50 and PsVNA50 pairs. The black line represents the ideal linear function of perfect correlation. Abbreviations: PRNT50, 50% plaque reduction neutralization test; PsVNA50, 50% pseudovirion neutralization assay.
DISCUSSION
This first-in-human clinical trial of an ANDV DNA vaccine found that the vaccine is both safe and immunogenic. In fact, this is the first HPS vaccine of any kind that has advanced to human clinical trials. While local and systemic AEs were common among subjects in both dose groups, the majority of events were mild to moderate in severity. There were no related SAEs and the very few severe reactions that did occur were improved or resolved after a single day.
The ANDV DNA vaccine elicited a strong and durable immune response that varied by dose and vaccination schedule. There were noticeable trends with the lowest seropositivity and seroconversion rates consistently seen in cohort 1. While cohort 1 had an increase in seropositivity rates following the final vaccination on day 169, cohort 2, 3, and 4 had the greatest increase and highest proportion of seropositive subjects, a rate that was sustained through day 337. Rates of seroconversion were similar among cohort 2, 3, and 4 with the 4-dose cohorts having the highest seroconversion rates at day 337.
PsVNA50 GMTs also varied by dose and vaccination schedule. The absolute highest GMTs were most consistently seen in cohort 4. However, even though cohort 4 had the highest peak GMT (28 days after the fourth dose) and demonstrated the strongest durability on day 337, GMTs were similar at the final 3 time points for cohort 2, 3, and 4. While there is no human correlate of protection, passive antibody transfer studies in animals have been conducted. One study found PRNT50 titers of 320–1280 to be 100% protective after passive antibody transfer from ANDV-vaccinated macaques to a lethal hamster challenge model. A similar HTNV postvaccine antibody transfer into a hamster challenge model found a PsVNA50 titer of >100 was predictive of a greater than 80% chance of protection from infection [17, 27].
Two neutralizing antibody assays were used in this study. While the PRNT50 is the historical comparator, the PsVNA50 has been used in more recent hantavirus (HTNV and PUUV) DNA vaccine trials and serves as the primary comparator [14, 15, 18]. The differences in the absolute PRNT50 and PsVNA50 are likely due to differences in what each assay measures; the PsVNA50 measures the ability of antibodies to prevent viral entry into cells, while PRNT50 measures both the prevention of viral entry, egress, and inhibition of viral spread. Results from the 2 assays were highly correlated across all cohorts. The GMT responses as measured by PsVNA50 in this study compare favorably with published results of a phase 2a HTNV/PUUV combination DNA vaccine delivered by electroporation [15]. In that study, using the same vaccine schedule, peak PsVNA50 GMTs at day 196, 28 days after the fourth vaccination of 1 mg or 2 mg combined HTNV/PUUV DNA vaccine, were 456 for HTNV and 223 for PUUV. Seropositive rates for HTNV and PUUV at the same day 197 time point were 92.3% and 88.5%, respectively.
Our study demonstrated a clear booster response to the final vaccination on day 169, with all cohorts reaching peak GMT after that vaccination. This booster response at 6 months was also reported in the phase 2a clinical trial of a combined HTNV/PUUV DNA vaccine referenced above [15].
While the cohort 4 (4 mg, 4-dose) regimen was safe, with similar reactogenicity compared to the other cohorts and induced the highest GMTs, the optimal dose and regimen would likely depend on the intended use and target population. In the setting of an ANDV outbreak or planned travel to an endemic area, a rapid response may be a priority over maximum GMT. Conversely, cohort 2 (2 mg, 4-dose regimen) had a similar response to the day 169 booster with durability and GMTs comparable to the 4 mg regimens. This lower dose regimen may be relevant when considering development of a multivalent hantavirus vaccine comprised of ANDV and Sin Nombre virus DNA to protect against HPS in the Americas.
Limitations of this study include the fact that the sample size per treatment arm was relatively small, with 10 subjects receiving the ANDV DNA vaccine per cohort. Furthermore, as a first-in-human, dose escalation trial with 4 treatment arms, the study was not powered to detect statistical differences. A strength of this study was the long duration of follow-up which allowed assessment of safety and immunogenicity for 11 months after the first vaccination.
In conclusion, we demonstrated that an ANDV DNA vaccine administered by NFIS in healthy adult volunteers is safe and induces a robust and durable immune response. Moreover, our study provides the first evidence that the full-length ANDV M genome segment ORF delivered by a vaccine platform, in this case plasmid DNA delivered by jet injection, can effectively produce an immune response in humans. Our finding that the vaccine elicited robust and durable neutralizing antibody titers is encouraging because anti-ANDV neutralizing antibodies have been shown to be sufficient to protect in animal models against lethal HPS and are therefore a likely correlate of protection [7, 14, 17–19]. In addition, it is notable that this vaccine was not formulated (eg, as lipid nanoparticles) and did not include an adjuvant. Rather, it was purified DNA diluted in saline. How formulation or the inclusion of adjuvant might increase the potency of this HPS vaccine remains an area of active research and future considerations to improve immunogenicity could include other platforms such as mRNA or viral vectors. This study provides foundational evidence for further large-scale testing of this vaccine candidate.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the participants who volunteered for this study and Aldevron and PharmaJet for study vaccine product and jet-injector device support, respectively, throughout. We also appreciate the efforts of USAMRIID assay technicians Lucia Principe and Brandon Somerville, Study Coordinator Margo Moore, Monica McNeal, MS, and the Laboratory for Specialized Clinical Studies, as well as the Gamble Vaccine Research Center Pharmacy.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Opinions, interpretations, conclusions, and recommendations are those of the authors and not necessarily endorsed by the US Army or the Department of Defense.
Financial support. This work was supported by the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (grant number HHSN272201300016I); the Infectious Diseases Clinical Research Consortium through the NIAID, NIH (grant number UM1AI148684); and the Military Infectious Disease Program, Program Area T.
References
Author notes
Presented in part: NSV 2022, Braga Portugal, 12-17 June 2022. Abstract P-132 “Hantavirus nucleic acid vaccines delivered by needle-free jet injection: from hamsters to humans.”
Potential conflicts of interest. J. W. H. is listed as an inventor on a patent that includes the ANDV DNA vaccine; the patent is assigned to the United States Government. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




