-
PDF
- Split View
-
Views
-
Cite
Cite
Christy A Comeaux, Stephan Bart, Arangassery Rosemary Bastian, Vladislav Klyashtornyy, Els De Paepe, Edmund Omoruyi, Leslie van der Fits, Roy van Heesbeen, Esther Heijnen, Benoit Callendret, Jerald Sadoff, Safety, Immunogenicity, and Regimen Selection of Ad26.RSV.preF–Based Vaccine Combinations: A Randomized, Double-blind, Placebo-Controlled, Phase 1/2a Study, The Journal of Infectious Diseases, Volume 229, Issue 1, 15 January 2024, Pages 19–29, https://doi.org/10.1093/infdis/jiad220
Close - Share Icon Share
Abstract
Ad26.RSV.preF is an adenovirus serotype 26 vector–based respiratory syncytial virus (RSV) vaccine encoding a prefusion conformation-stabilized RSV fusion protein (preF) that demonstrated robust humoral and cellular immunogenicity and showed promising efficacy in a human challenge study in younger adults. Addition of recombinant RSV preF protein might enhance RSV-specific humoral immune responses, especially in older populations.
This randomized, double-blind, placebo-controlled, phase 1/2a study compared the safety and immunogenicity of Ad26.RSV.preF alone and varying doses of Ad26.RSV.preF–RSV preF protein combinations in adults aged ≥60 years. This report includes data from cohort 1 (initial safety, n = 64) and cohort 2 (regimen selection, n = 288). Primary immunogenicity and safety analyses were performed 28 days postvaccination (cohort 2) for regimen selection.
All vaccine regimens were well tolerated, with similar reactogenicity profiles among them. Combination regimens induced greater humoral immune responses (virus-neutralizing and preF-specific binding antibodies) and similar cellular ones (RSV-F–specific T cells) as compared with Ad26.RSV.preF alone. Vaccine-induced immune responses remained above baseline up to 1.5 years postvaccination.
All Ad26.RSV.preF–based regimens were well tolerated. A combination regimen comprising Ad26.RSV.preF, which elicits strong humoral and cellular responses, and RSV preF protein, which increases humoral responses, was selected for further development.
Clinical Trials Registration. NCT03502707.
Respiratory syncytial virus (RSV) can cause serious lower respiratory tract disease in older adults [1, 2]. Among adults aged ≥60 years, RSV has an estimated fatality rate of 8.18% [3] and causes approximately 5.2 million acute respiratory infections, 470 000 hospitalizations, and 33 000 in-hospital deaths annually in industrialized countries [4]. Waning immunity with age may exacerbate RSV in older adults [5]. Among those at high risk, RSV carries disease burden comparable to influenza, including hospitalizations, intensive care unit admissions, and deaths [6–8].
During natural RSV infection, human RSV neutralizing antibodies (nAbs) primarily target the prefusion conformation of the surface RSV fusion (F) glycoprotein (RSV preF protein) [9, 10]. Immunization with RSV preF protein induces robust nAb responses [11, 12], which correlate with reduced infection risk [13]. However, RSV reinfections are frequent, despite existing nAbs [14]. In addition to nAbs, evidence suggests that cell-mediated immunity plays a protective role against RSV. In murine models, T cells promote viral clearance and protective immunity [15]. In a human challenge study, airway-resident RSV-specific T cells correlated with reduced symptom severity and viral load [16]. Yet, natural RSV infections do not always induce robust cell-mediated responses [15]. Thus, a successful RSV vaccine candidate will likely need to induce nAb and T-cell responses [15, 17]. To that end, clinical studies of adenoviral-vectored vaccines have shown robust T helper 1 (Th1)–dominated T-cell responses [18, 19].
Ad26.RSV.preF is a recombinant, replication-incompetent, adenovirus 26 (Ad26)–vectored vaccine encoding conformation-stabilized RSV preF protein. Single-dose administration of Ad26.RSV.preF was well tolerated and showed durable humoral and cellular immunogenicity (up to 2 years) in adults aged ≥60 years [20]. In a human challenge study, Ad26.RSV.preF induced robust nAb responses and reduced RSV infection, viral loads, and disease severity [21]. In the same study, high nAb titers correlated with reduced infection risk; however, some participants with high nAb responses developed RSV infections, suggesting that vaccine-induced cellular immune responses contribute to protection [21]. In preclinical studies, combination regimens containing Ad26.RSV.preF and recombinant RSV preF protein showed greater humoral and cellular immunogenicity and improved protection vs either component alone [22].
We hypothesized that adding RSV preF protein to Ad26.RSV.preF would enhance humoral immunogenicity while maintaining cellular responses elicited by Ad26.RSV.preF in humans, similar to preclinical observations [22]. This phase 1/2a first-in-human study assessed the safety and immunogenicity of Ad26.RSV.preF alone and in combination with recombinant RSV preF protein to support optimal vaccine regimen selection for adults aged ≥60 years.
METHODS
Study Design
This multicenter study was based on a randomized, double-blind, placebo-controlled design (NCT03502707). Trial enrollment began on 9 July 2018; the cutoff date for this analysis was 30 June 2022. The study protocol and amendments were approved by institutional review boards at participating centers. The trial was designed and overseen by the sponsor (Janssen Vaccines & Prevention BV) and conducted in accordance with the Declaration of Helsinki and principles of Good Clinical Practice.
This report includes data from cohort 1 (initial safety cohort) and cohort 2 (regimen selection cohort). The primary objective in cohort 1 was to assess the safety and reactogenicity of intramuscularly administered vaccine regimens; primary end points in cohort 1 were serious adverse events (SAEs) from first vaccination until cutoff, solicited local and systemic adverse events (AEs) for 7 days postvaccination, and unsolicited AEs up to 28 days postvaccination. Primary objectives in cohort 2 were to assess the safety and reactogenicity of intramuscularly administered vaccine regimens and RSV nAb levels induced by Ad26.RSV.preF–RSV preF protein combinations when compared with Ad26.RSV.preF alone. Primary end points in cohort 2 included SAEs from first vaccination until cutoff, solicited local and systemic AEs for 7 days postvaccination, unsolicited AEs up to 28 days postvaccination, and RSV A2 nAb levels on day 29.
Participants and Inclusion/Exclusion Criteria
Adults ≥60 years of age in good or stable health per investigators’ clinical judgment and laboratory tests at screening were eligible. Prior to enrollment, participants provided written informed consent indicating that they understood the study and were willing and able to participate, attend all scheduled visits, and comply with all procedures. For detailed eligibility criteria, see the Supplementary Material.
Study Procedures
The study comprised a 28-day screening period, vaccination with a 1- or 2-dose regimen (day 1 vs days 1 and 57), a booster at month 12 (cohort 1 only), a follow-up period for solicited and unsolicited AEs after each vaccination (for 7 and 28 days postvaccination, respectively), and a 2-year follow-up period after the first vaccinations (Figure 1). Participants in groups 14 and 15 were followed up to day 1095 for extended safety and immunogenicity analyses. All Ad26.RSV.preF and recombinant RSV preF protein injections were administered as one 1-mL injection into the deltoid muscle (herein, combination regimens), with 1 group receiving Ad26.RSV.preF and RSV preF protein as separate injections in opposite arms (1 mL each). Participants receiving Ad26.RSV.preF or an Ad26.RSV.preF–RSV preF protein combination also received a placebo injection (1 mL) in the opposite arm to maintain blinding.
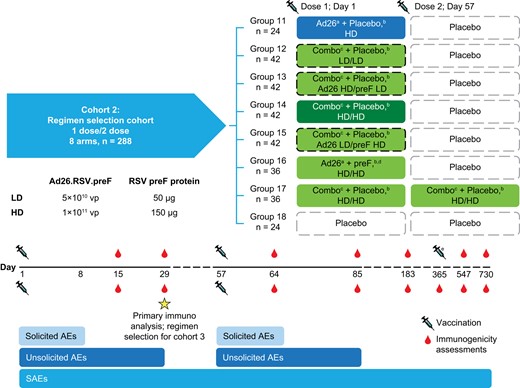
Study schematic: cohort 2. Ad26, adenovector 26; AE, adverse event; HD, high dose; LD, low dose; preF, prefusion conformation-stabilized RSV F protein; RSV, respiratory syncytial virus; SAE, serious adverse event; vp, viral particle. aAd26.RSV.preF. bInjections given in opposite arms. cA combination injection of Ad26.RSV.preF and recombinant RSV preF protein at the specified dosages. dRecombinant RSV preF protein. eCohort 1 received an additional dose at day 365.
Participants, site personnel, and investigators were blinded to vaccine allocation, except for a pharmacist or qualified staff member primarily responsible for vaccine preparation and dispensing. This individual could administer vaccinations but had no other study functions thereafter.
Participants in cohort 1 (initial safety cohort) were randomly assigned into 4 subcohorts, with safety checks after enrollment of each subcohort before enrolling the next subcohort. Within subcohorts, participants were randomly assigned to treatment groups. Randomization procedures in cohort 1 are available in the Supplementary Material. Ten groups were enrolled by dosing regimen (Supplementary Figure 1): participants received low- or high-dose (LD or HD) Ad26.RSV.preF (5 × 1010 or 1 × 1011 viral particles, respectively), LD or HD recombinant RSV preF protein (50 or 150 µg, respectively), combinations of HD and LD Ad26.RSV.preF–recombinant RSV preF protein, or placebo. Cohort 1 participants received the same dose regimens at days 1 and 57 (except for group 10, which received HD Ad26.RSV.preF and HD RSV preF protein as separate injections at day 1 and placebo at day 57). The schedule of study activities in cohort 1 is available in Supplementary Figure 1.
Participants in cohort 2 (regimen selection cohort) were randomly assigned into 8 groups based on dosing regimen (Supplementary Table 1): participants received HD Ad26.RSV.preF, combination regimens consisting of Ad26.RSV.preF (LD or HD) and recombinant RSV preF protein (LD or HD), HD Ad26.RSV.preF and HD RSV preF protein administered separately, or placebo. Cohort 2 participants received active vaccination regimens or placebo on day 1, followed by placebo on day 57 (except for group 17, which received a second administration of the HD-HD combination regimen on day 57). For cohort 2, immunogenicity analyses were performed 28 days post–dose 1 for regimen selection, and safety analyses were performed 28 days post–dose 1. Final study visits occurred at 36 months post–first vaccination; immunogenicity evaluations up to day 547 are described herein.
Study Assessments
Participants recorded solicited local AEs (injection-site pain/tenderness, swelling/induration, or erythema) and systemic AEs (fatigue, headache, myalgia, arthralgia, nausea, or chills) and body temperatures (to assess pyrexia) for 7 days postvaccination. All participants recorded solicited local AEs separately for each arm. Unsolicited AEs were recorded through 28 days postvaccination. SAEs and AEs leading to study discontinuation were recorded through completion of final study procedures. Blood was collected for safety assessments at screening, on day 1 (prevaccination), and on day 8.
Humoral and cellular immunogenicity assays included neutralization assays against RSV A and B strains and the Ad26 vector, RSV preF protein antibody enzyme-linked immunosorbent assay (ELISA), RSV post–fusion F (postF) binding antibody ELISA, and RSV-F–specific interferon (IFN)-γ T-cell frequency enzyme-linked immunosorbent spot (ELISpot) measurements. Blood samples for immunogenicity analyses were collected on day 1 (prevaccination) and days 15, 29, 57, 85, 183, 365, 547, and 730. For assay details, see the Supplementary Material.
Statistical Analysis
Safety analyses were performed on the full analysis set (ie, all participants who were randomly assigned and received ≥1 dose of vaccine), regardless of protocol deviations or type of vaccine. Final safety analysis results are reported here, including safety data until study end. All immunogenicity analyses were based on the per-protocol immunogenicity set (ie, all participants who were randomly assigned and received the complete first dose with available immunogenicity data), with samples excluded if a participant (1) experienced a major protocol deviation expected to affect immunogenicity outcomes, (2) had a natural RSV infection (based on reverse transcriptase–polymerase chain reaction or other source), or (3) missed ≥1 active dose.
No formal hypothesis for immunogenicity was tested. Descriptive statistics were reported for ELISA and nAb data (geometric mean titers [GMTs], geometric mean fold increases, and 95% CIs) and medians and quartiles for IFN-γ ELISpot measurements. For humoral assays, geometric mean fold increases from baseline and 95% CIs were reported. GMT ratios of RSV A2 nAb titers were calculated for 1-dose combination regimens (groups 12–16) on day 29 and the 2-dose regimen (group 17) on day 85 vs the 1-dose Ad26.RSV.preF regimen (group 11) on day 29. For this analysis, immunogenicity data from groups 9 and 10 of cohort 1 were combined with groups 16 and 17 of cohort 2. A regression model was fitted with log2-transformed RSV A2 nAb titers as the dependent variable and with regimens and baseline levels as covariates. The Satterthwaite method was used to calculate degrees of freedom, and the estimate and confidence interval obtained were back-transformed to a GMT ratio and corresponding 95% CI.
A similar analysis was performed to obtain ratios of log10-transformed RSV-F–specific IFN-γ ELISpot measurements for combination regimens vs Ad26.RSV.preF alone.
RESULTS
Participant Demographic and Baseline Characteristics
Of 114 participants screened for inclusion in cohort 1, 64 were randomly assigned and vaccinated and 55 completed study treatment (Supplementary Figure 2). Of 444 participants screened in cohort 2, 288 were randomly assigned and vaccinated and 273 completed study treatment (Supplementary Figure 3). Forty-one participants are undergoing long-term follow-up for safety and immunogenicity. The primary reason for screening failure was attributed to eligibility criteria. Most participants were White and female. The median (range) age was 64.5 (60–79) and 67.0 (60–89) years in cohorts 1 and 2, respectively (Table 1). Demographic and baseline characteristics were similar across vaccination groups; for full participant demographics in cohorts 1 and 2, see Supplementary Tables 2 and 3, respectively.
| . | Median (Range) or No. (%) . | |
|---|---|---|
| Characteristic . | Cohort 1 (n = 64) . | Cohort 2 (n = 288) . |
| Age, y | 64.5 (60-79) | 67.0 (60-89) |
| 60–64 | 32 (50.0) | 99 (34.4) |
| 65–74 | 23 (35.9) | 130 (45.1) |
| 75–84 | 9 (14.1) | 55 (19.1) |
| ≥85 | 0 (0.0) | 4 (1.4) |
| Sex | ||
| Female | 35 (54.7) | 180 (62.5) |
| Male | 29 (45.3) | 108 (37.5) |
| Racea | ||
| White | 45 (70.3) | 257 (89.5) |
| Black or African American | 13 (20.3) | 22 (7.7) |
| Asian | 4 (6.3) | 4 (1.4) |
| American Indian or Alaska Native | 1 (1.6) | 2 (0.7) |
| Hawaiian or Pacific Islander | 1 (1.6) | 0 (0.0) |
| Multiple | 0 (0.0) | 2 (0.7) |
| Body mass index, kg/m2 | 28.05 (19.1-39.1) | 28.35 (16.8-52.1) |
| . | Median (Range) or No. (%) . | |
|---|---|---|
| Characteristic . | Cohort 1 (n = 64) . | Cohort 2 (n = 288) . |
| Age, y | 64.5 (60-79) | 67.0 (60-89) |
| 60–64 | 32 (50.0) | 99 (34.4) |
| 65–74 | 23 (35.9) | 130 (45.1) |
| 75–84 | 9 (14.1) | 55 (19.1) |
| ≥85 | 0 (0.0) | 4 (1.4) |
| Sex | ||
| Female | 35 (54.7) | 180 (62.5) |
| Male | 29 (45.3) | 108 (37.5) |
| Racea | ||
| White | 45 (70.3) | 257 (89.5) |
| Black or African American | 13 (20.3) | 22 (7.7) |
| Asian | 4 (6.3) | 4 (1.4) |
| American Indian or Alaska Native | 1 (1.6) | 2 (0.7) |
| Hawaiian or Pacific Islander | 1 (1.6) | 0 (0.0) |
| Multiple | 0 (0.0) | 2 (0.7) |
| Body mass index, kg/m2 | 28.05 (19.1-39.1) | 28.35 (16.8-52.1) |
aCohort 2: n = 287.
| . | Median (Range) or No. (%) . | |
|---|---|---|
| Characteristic . | Cohort 1 (n = 64) . | Cohort 2 (n = 288) . |
| Age, y | 64.5 (60-79) | 67.0 (60-89) |
| 60–64 | 32 (50.0) | 99 (34.4) |
| 65–74 | 23 (35.9) | 130 (45.1) |
| 75–84 | 9 (14.1) | 55 (19.1) |
| ≥85 | 0 (0.0) | 4 (1.4) |
| Sex | ||
| Female | 35 (54.7) | 180 (62.5) |
| Male | 29 (45.3) | 108 (37.5) |
| Racea | ||
| White | 45 (70.3) | 257 (89.5) |
| Black or African American | 13 (20.3) | 22 (7.7) |
| Asian | 4 (6.3) | 4 (1.4) |
| American Indian or Alaska Native | 1 (1.6) | 2 (0.7) |
| Hawaiian or Pacific Islander | 1 (1.6) | 0 (0.0) |
| Multiple | 0 (0.0) | 2 (0.7) |
| Body mass index, kg/m2 | 28.05 (19.1-39.1) | 28.35 (16.8-52.1) |
| . | Median (Range) or No. (%) . | |
|---|---|---|
| Characteristic . | Cohort 1 (n = 64) . | Cohort 2 (n = 288) . |
| Age, y | 64.5 (60-79) | 67.0 (60-89) |
| 60–64 | 32 (50.0) | 99 (34.4) |
| 65–74 | 23 (35.9) | 130 (45.1) |
| 75–84 | 9 (14.1) | 55 (19.1) |
| ≥85 | 0 (0.0) | 4 (1.4) |
| Sex | ||
| Female | 35 (54.7) | 180 (62.5) |
| Male | 29 (45.3) | 108 (37.5) |
| Racea | ||
| White | 45 (70.3) | 257 (89.5) |
| Black or African American | 13 (20.3) | 22 (7.7) |
| Asian | 4 (6.3) | 4 (1.4) |
| American Indian or Alaska Native | 1 (1.6) | 2 (0.7) |
| Hawaiian or Pacific Islander | 1 (1.6) | 0 (0.0) |
| Multiple | 0 (0.0) | 2 (0.7) |
| Body mass index, kg/m2 | 28.05 (19.1-39.1) | 28.35 (16.8-52.1) |
aCohort 2: n = 287.
Safety Analyses
All vaccination regimens were well tolerated, with no substantial differences across combination regimens or between combination regimens and Ad26.RSV.preF (Table 2; Supplementary Tables 4 and 5). Eight participants in cohort 1 and 33 in cohort 2 reported SAEs; none were considered related to the study vaccine. Four participants in each cohort reported an AE leading to vaccine discontinuation:
Cohort 1: (1) aortic aneurysm; (2) melena; (3) atrial fibrillation and prostate cancer; and (4) cardiac arrest, coronary artery disease, distributive shock, and hyperlipidemia
Cohort 2: (1) respiratory tract infection, (2) thrombocytopenia, (3) chronic inflammatory demyelinating polyradiculoneuropathy, and (4) arthralgia
| . | Ad26a + Placebob . | Comboc + Placebob . | Ad26a + preFb,d . | 2-Dose Comboc + Placebob,e . | Placebo + Placebob . | |||
|---|---|---|---|---|---|---|---|---|
| Ad26a/preFd Dosage . | HD/− (n = 24) . | LD/LD (n = 42) . | HD/LD (n = 42) . | HD/HD (n = 42) . | LD/HD (n = 42) . | HD/HD (n = 36) . | HD/HD (n = 36) . | −/− (n = 24) . |
| Solicited local AEsf | 13 (54.2) | 23 (54.8) | 24 (57.1) | 25 (59.5) | 24 (57.1) | 20 (55.6)/ 10 (27.8)g | 22 (61.1) | 5 (20.8) |
| Grade ≥3 | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0)/0 (0.0)g | 2 (5.6) | 0 (0.0) |
| Solicited systemic AEsf | 11 (45.8) | 23 (54.8) | 20 (47.6) | 22 (52.4) | 21 (50.0) | 20 (55.6) | 20 (55.6) | 11 (45.8) |
| Grade ≥3 | 3 (12.5) | 2 (4.8) | 2 (4.8) | 3 (7.1) | 1 (2.4) | 0 (0.0) | 3 (8.3) | 1 (4.2) |
| SAEsh | 0 (0.0) | 5 (11.9) | 5 (11.9) | 4 (9.5) | 5 (11.9) | 7 (19.4) | 3 (8.3) | 4 (16.7) |
| AEs leading to vaccine discontinuationh | 0 (0.0) | 0 (0.0) | 2 (4.8) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 1 (2.8) | 0 (0.0) |
| . | Ad26a + Placebob . | Comboc + Placebob . | Ad26a + preFb,d . | 2-Dose Comboc + Placebob,e . | Placebo + Placebob . | |||
|---|---|---|---|---|---|---|---|---|
| Ad26a/preFd Dosage . | HD/− (n = 24) . | LD/LD (n = 42) . | HD/LD (n = 42) . | HD/HD (n = 42) . | LD/HD (n = 42) . | HD/HD (n = 36) . | HD/HD (n = 36) . | −/− (n = 24) . |
| Solicited local AEsf | 13 (54.2) | 23 (54.8) | 24 (57.1) | 25 (59.5) | 24 (57.1) | 20 (55.6)/ 10 (27.8)g | 22 (61.1) | 5 (20.8) |
| Grade ≥3 | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0)/0 (0.0)g | 2 (5.6) | 0 (0.0) |
| Solicited systemic AEsf | 11 (45.8) | 23 (54.8) | 20 (47.6) | 22 (52.4) | 21 (50.0) | 20 (55.6) | 20 (55.6) | 11 (45.8) |
| Grade ≥3 | 3 (12.5) | 2 (4.8) | 2 (4.8) | 3 (7.1) | 1 (2.4) | 0 (0.0) | 3 (8.3) | 1 (4.2) |
| SAEsh | 0 (0.0) | 5 (11.9) | 5 (11.9) | 4 (9.5) | 5 (11.9) | 7 (19.4) | 3 (8.3) | 4 (16.7) |
| AEs leading to vaccine discontinuationh | 0 (0.0) | 0 (0.0) | 2 (4.8) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 1 (2.8) | 0 (0.0) |
Data are presented as No. (%).
Abbreviations: Ad26, adenovector 26; AE, adverse event; HD, high dose; LD, low dose; preF, prefusion conformation-stabilized RSV F protein; RSV, respiratory syncytial virus; SAE, serious adverse event.
aAd26.RSV.preF.
bInjections were given in opposite arms.
cA combination injection of Ad26.RSV.preF and recombinant RSV preF protein at the specified dosages.
dRecombinant RSV preF protein.
eParticipants in this group received a combination of Ad26.RSV.preF HD and recombinant RSV preF protein HD at days 1 and 57.
fSolicited local and systemic AEs are reported through 7 days postvaccination.
gSolicited local AEs for the Ad26.RSV.preF and RSV preF protein arms are reported separately.
hSAEs and AEs leading to vaccine discontinuation are reported through the end of the study.
| . | Ad26a + Placebob . | Comboc + Placebob . | Ad26a + preFb,d . | 2-Dose Comboc + Placebob,e . | Placebo + Placebob . | |||
|---|---|---|---|---|---|---|---|---|
| Ad26a/preFd Dosage . | HD/− (n = 24) . | LD/LD (n = 42) . | HD/LD (n = 42) . | HD/HD (n = 42) . | LD/HD (n = 42) . | HD/HD (n = 36) . | HD/HD (n = 36) . | −/− (n = 24) . |
| Solicited local AEsf | 13 (54.2) | 23 (54.8) | 24 (57.1) | 25 (59.5) | 24 (57.1) | 20 (55.6)/ 10 (27.8)g | 22 (61.1) | 5 (20.8) |
| Grade ≥3 | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0)/0 (0.0)g | 2 (5.6) | 0 (0.0) |
| Solicited systemic AEsf | 11 (45.8) | 23 (54.8) | 20 (47.6) | 22 (52.4) | 21 (50.0) | 20 (55.6) | 20 (55.6) | 11 (45.8) |
| Grade ≥3 | 3 (12.5) | 2 (4.8) | 2 (4.8) | 3 (7.1) | 1 (2.4) | 0 (0.0) | 3 (8.3) | 1 (4.2) |
| SAEsh | 0 (0.0) | 5 (11.9) | 5 (11.9) | 4 (9.5) | 5 (11.9) | 7 (19.4) | 3 (8.3) | 4 (16.7) |
| AEs leading to vaccine discontinuationh | 0 (0.0) | 0 (0.0) | 2 (4.8) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 1 (2.8) | 0 (0.0) |
| . | Ad26a + Placebob . | Comboc + Placebob . | Ad26a + preFb,d . | 2-Dose Comboc + Placebob,e . | Placebo + Placebob . | |||
|---|---|---|---|---|---|---|---|---|
| Ad26a/preFd Dosage . | HD/− (n = 24) . | LD/LD (n = 42) . | HD/LD (n = 42) . | HD/HD (n = 42) . | LD/HD (n = 42) . | HD/HD (n = 36) . | HD/HD (n = 36) . | −/− (n = 24) . |
| Solicited local AEsf | 13 (54.2) | 23 (54.8) | 24 (57.1) | 25 (59.5) | 24 (57.1) | 20 (55.6)/ 10 (27.8)g | 22 (61.1) | 5 (20.8) |
| Grade ≥3 | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0)/0 (0.0)g | 2 (5.6) | 0 (0.0) |
| Solicited systemic AEsf | 11 (45.8) | 23 (54.8) | 20 (47.6) | 22 (52.4) | 21 (50.0) | 20 (55.6) | 20 (55.6) | 11 (45.8) |
| Grade ≥3 | 3 (12.5) | 2 (4.8) | 2 (4.8) | 3 (7.1) | 1 (2.4) | 0 (0.0) | 3 (8.3) | 1 (4.2) |
| SAEsh | 0 (0.0) | 5 (11.9) | 5 (11.9) | 4 (9.5) | 5 (11.9) | 7 (19.4) | 3 (8.3) | 4 (16.7) |
| AEs leading to vaccine discontinuationh | 0 (0.0) | 0 (0.0) | 2 (4.8) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 1 (2.8) | 0 (0.0) |
Data are presented as No. (%).
Abbreviations: Ad26, adenovector 26; AE, adverse event; HD, high dose; LD, low dose; preF, prefusion conformation-stabilized RSV F protein; RSV, respiratory syncytial virus; SAE, serious adverse event.
aAd26.RSV.preF.
bInjections were given in opposite arms.
cA combination injection of Ad26.RSV.preF and recombinant RSV preF protein at the specified dosages.
dRecombinant RSV preF protein.
eParticipants in this group received a combination of Ad26.RSV.preF HD and recombinant RSV preF protein HD at days 1 and 57.
fSolicited local and systemic AEs are reported through 7 days postvaccination.
gSolicited local AEs for the Ad26.RSV.preF and RSV preF protein arms are reported separately.
hSAEs and AEs leading to vaccine discontinuation are reported through the end of the study.
Of these, only the event of thrombocytopenia was considered related to the study vaccine. The event of thrombocytopenia, which occurred after the first vaccination in the HD-LD combination group, was grade 3 in severity, was identified in routine day 8 postvaccination safety laboratory assessments, and persisted for 40 days before returning to the normal range. The participant remained asymptomatic and had normal physical examination results (no bruising or petechiae). No AEs with fatal outcomes were considered related to the study vaccine.
Among active vaccine recipients in cohort 2, 54.2% to 61.1% reported solicited local AEs, as compared with 20.8% in the placebo group. Most solicited local AEs among active vaccination recipients in cohort 2 were grade 1 or 2 in severity except for 4 participants who indicated grade 3 pain/tenderness (1 in the HD-HD combination regimen group and 3 in the 2-dose HD-HD combination regimen group) and 2 participants who had grade 3 swelling (1 in the HD-LD combination regimen group and 1 in the 2-dose HD-HD combination regimen group). Post–dose 1, median onset times for solicited local AEs ranged from 1 to 3 days postvaccination across the active vaccine groups and from 1 to 1.5 days in participants receiving placebo (post–dose 2: active vaccine groups, 1–2 days). Post–dose 1, the median duration of solicited local AEs ranged from 1 to 4 days for pain/tenderness, 1 to 5.5 days for swelling, and 1.5 to 14 days for erythema of any grade (post–dose 2: pain/tenderness, 1–4 days; swelling, 1–4 days; erythema, none) across the active vaccine groups.
Solicited systemic AEs were reported by 45.8% to 55.6% of participants receiving active vaccine regimens in cohort 2, as compared with 45.8% who received placebo. Grade 3 systemic AEs were indicated in up to 12.5% of participants in the active vaccination groups and in 4.2% receiving placebo; there were no grade 4 systemic AEs. Grade 3 solicited systemic AEs were reported in 14 participants receiving the study vaccine; the most common grade 3 AEs were chills, fatigue, headache, and myalgia, in ≤3 participants per group. One participant in the HD-HD combination group reported 5 grade 3 solicited systemic AEs (arthralgia, chills, fatigue, headache, and myalgia), all of which were considered related to the study vaccine. Post–dose 1, median onset times for solicited systemic AEs ranged from 1 to 3 days across the active vaccine groups and 1 to 3.5 days in the placebo group (post–dose 2: vaccine groups, 1–3 days; placebo group, 1 day). Post–dose 1, the median duration of solicited systemic AEs ranged from 1 to 4.5 days in the active vaccine groups and 1 to 4 days in the placebo group (post–dose 2: vaccine groups, 1–8 days; placebo group, 1–2 days).
For additional safety data in cohorts 1 and 2, see Supplementary Tables 4 and 5, respectively.
Immunogenicity Analyses
In cohort 2, all vaccine regimens elicited robust humoral and cellular immune responses (Figure 2). RSV preF immunoglobulin G (IgG) serum antibody titers peaked at day 15, with an increase of 2.7-fold from baseline in participants receiving Ad26.RSV.preF alone and 10.9- to 21.7-fold in those receiving combination regimens. At day 29, RSV preF serum IgG antibodies were increased by 2.1-fold for Ad26.RSV.preF alone and by 8.0- to 13.9-fold for combination regimens (Figure 2A). RSV A2 nAb titers also peaked at day 15, with an increase of 2.8-fold from baseline for Ad26.RSV.preF alone and 7.3- to 13.6-fold for combination regimens. At day 29, RSV A2 nAb titers were increased by 2.7-fold from baseline for Ad26.RSV.preF alone and by 5.4- to 10.5-fold for combination regimens (Figure 2B). Baseline median RSV-F–specific IFN-γ ELISpot responses were comparable among all groups, ranging from 30 to 50 spot-forming cells (SFCs) per 106 peripheral blood mononuclear cells (PBMCs). Among active vaccine recipients, median RSV-F–specific IFN-γ ELISpot responses ranged from 278 to 497 SFCs/106 PBMCs at day 15 and from 290 to 424 SFCs/106 PBMCs at day 29 (Figure 2C). RSV-F–specific IFN-γ ELISpot responses peaked at day 15 for most groups, except Ad26.RSV.preF alone and LD/HD combination, which showed peak responses at day 29. All humoral and cellular immune responses remained well above baseline for 1.5 years. Combination regimens showed higher humoral responses when compared with Ad26.RSV.preF alone at 1.5 years. The 2-dose combination regimen did not substantially increase immune responses after the second dose at day 57. No relevant changes in immune responses were observed in the placebo group.
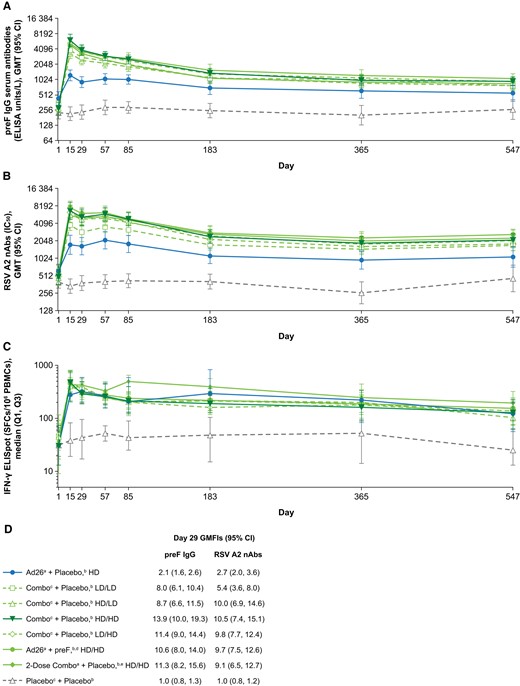
Immunogenicity analyses. A, RSV preF IgG serum antibody titers, B, RSV A2 nAb titers, and C, RSV-F–specific IFN-γ ELISpot values measured at days 1, 15, 29, 57, 85, 183, 365, and 547 for all regimens. D, Day 29 GMFIs for RSV preF IgG serum antibody titers and RSV A2 nAb titers. Ad26, adenovector 26; ELISA, enzyme-linked immunosorbent assay; ELISpot, enzyme-linked immune absorbent spot; GMFI, geometric mean fold increase; GMT, geometric mean titer; HD, high dose; IC50, half-maximum inhibitory concentration; IFN-γ, interferon-γ; IgG, immunoglobulin G; LD, low dose; nAb, neutralizing antibody; PBMC, peripheral blood mononuclear cell; preF, prefusion conformation-stabilized RSV F protein; Q, quartile; RSV, respiratory syncytial virus; SFC, spot-forming cell. aAd26.RSV.preF. bInjections given in opposite arms. cA combination injection of Ad26.RSV.preF and recombinant RSV preF protein at the specified dosages. dRecombinant RSV preF protein. eParticipants in this group received the HD/HD combination regimen at days 1 and 57.
At day 29, RSV A2 nAb titers were greater for combination regimens when compared with HD Ad26.RSV.preF alone (Figure 3A). For the LD-LD combination regimen, day 29 RSV A2 nAbs were 1.83-fold higher than HD Ad26.RSV.preF alone; day 29 RSV A2 nAbs for the other combination regimens were 2.79- to 3.45-fold higher (Supplementary Table 6). Among combination regimens, the LD-LD combination showed the lowest humoral response; all other combination regimens produced similar humoral responses (Supplementary Figure 4). Ad26.RSV.preF alone and combination regimens produced similar median day 29 RSV-F–specific IFN-γ ELISpot responses (Figure 3B). The geometric mean ratio of fold changes in RSV-F binding to RSV nAbs was 0.8 for Ad26.RSV.preF alone and 0.5 to 1.1 for combination regimens (Figure 3C).
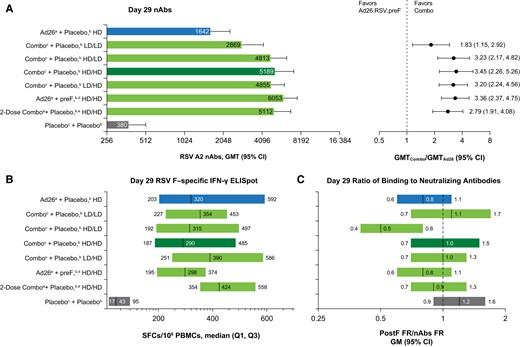
Comparison of combination regimens vs Ad26.RSV.preF. A, Day 29 nAbs for all vaccine regimens (left) and GMT ratios for combination regimens vs Ad26.RSV.preF alone (right). B, Day 29 RSV-F–specific IFN-γ ELISpot values (left) and the proportion of participants with >3-fold increase in ELISpot values (right). C, Day 29 ratio of fold increases in postF-binding antibodies to nAbs in all vaccine regimens. Ad26, adenovector 26; ELISpot, enzyme-linked immune absorbent spot; FR, fold rise; GM, geometric mean; GMFI, geometric mean fold increase; GMT, geometric mean titer; HD, high dose; IFN-γ, interferon-γ; LD, low dose; nAb, neutralizing antibody; PBMC, peripheral blood mononuclear cell; postF, postfusion conformation RSV F protein; preF, prefusion conformation-stabilized RSV F protein; Q, quartile; RSV, respiratory syncytial virus; SFC, spot-forming cell. aAd26.RSV.preF. bInjections given in opposite arms. cA combination injection of Ad26.RSV.preF and recombinant RSV preF protein at the specified dosages. dRecombinant RSV preF protein. eParticipants in this group received the HD/HD combination regimen at days 1 and 57.
To explore the protective potential of the Ad26.RSV.preF–RSV preF protein combination vaccine against circulating RSV A and B strains, serum-neutralizing capacity against 12 RSV clinical isolates from several RSV seasons (2011–2018) was evaluated at day 29 in participants vaccinated with the HD-HD combination regimen (Figure 4). Robust nAb responses were observed against all 12 strains, with comparable fold increases from baseline for RSV strains A (14.2- to 19.1-fold) and B (10.7- to 16.7-fold).

nAbs against RSV clinical isolates. GMFIs are shown from baseline to day 29 in nAbs against RSV A and B clinical isolates for participants receiving the HD/HD combination regimen (n = 24). For each isolate, A and B denote the RSV subtype, and the 2-digit number afterward denotes the year from which each strain was isolated. Ad26, adenovector 26; GMFI, geometric mean fold increase; HD, high dose; nAb, neutralizing antibody; preF, prefusion conformation-stabilized RSV F protein; RSV, respiratory syncytial virus. aA combination injection of Ad26.RSV.preF and recombinant RSV preF protein at the specified dosages. bInjections given in opposite arms.
Study participants had an average baseline Ad26 seropositivity of 13.5%. Limited data suggested no correlation between preexisting Ad26 nAb titers and vaccine-induced humoral and cellular immune responses (Supplementary Figure 5).
DISCUSSION
Addition of recombinant RSV preF protein to Ad26.RSV.preF in combination regimens increased humoral immunogenicity when compared with Ad26.RSV.preF alone, without affecting Ad26.RSV.preF–induced cellular immune responses, as observed in prior preclinical studies [22]. All combination regimens elicited durable humoral and cellular immune responses that remained well above baseline for 1.5 years. Among the regimens tested, the combination of Ad26.RSV.preF (1 × 1011 viral particles) and RSV preF protein (150 µg; HD-HD) was selected for further development.
In this study, combination regimens increased RSV A2 nAbs by 5.4-fold (LD-LD combination regimen) to 10.5-fold (selected HD-HD combination regimen) above baseline at day 29 and by 1.83- to 3.45-fold, respectively, when compared with Ad26.RSV.preF alone. Notably, the selected regimen induced similarly robust nAb responses against 12 RSV A and B clinical isolates from several RSV seasons, consistent with results of preclinical studies [23]. Although there is no clear immune correlate of protection identified for RSV, literature suggests that vaccine-induced increases in nAbs correlate with protection [13]. A correlation between RSV A2 nAbs and protection from RSV infection was also observed in a recent human challenge study of Ad26.RSV.preF; however, only a partial correlation was observed, suggesting that additional immune functions contribute to the protection conferred by Ad26.RSV.preF [21]. Previous work suggests that Th1-dominated T-cell responses are also protective [17]. In the current study, Ad26.RSV.preF alone and Ad26.RSV.preF–RSV preF protein combinations induced comparable RSV-F–specific IFN-γ (ie, Th1) cellular responses that remained above baseline up to day 547.
Antibodies that can bind RSV but not neutralize it may provide limited protection [24, 25]. Moreover, a nonneutralizing humoral immune response was implicated in enhanced respiratory disease associated with a formalin-inactivated RSV vaccine for children in the 1960s [26, 27]. In infants, antibodies against RSV preF are the most potent nAbs, with most exhibiting medium to high neutralizing activity [24], whereas in adults, the majority of RSV nAbs are cross-reactive and bind RSV preF and postF [28]. In this study, Ad26.RSV.preF alone and Ad26.RSV.preF–RSV preF protein combinations elicited favorable ratios of RSV postF binding to RSV nAbs, which is promising for the potential of the vaccine to induce protective immunity in adults without priming for enhanced disease. Our study also suggests that vaccine-induced humoral and cellular immune responses are not affected by preexisting Ad26 nAbs, which were present in 13.5% of participants at baseline, confirming previous observations with other Ad26-based vaccines [29–31].
In this study, Ad26.RSV.preF alone and in combination with RSV preF protein was well tolerated, with mostly mild to moderate solicited local and systemic AEs of short duration. Although 1 event of thrombocytopenia occurred that was considered related to the study vaccine, the participant remained asymptomatic, and no thrombosis was observed. Events of vaccine-induced immune thrombotic thrombocytopenia (VITT) have been rarely observed following vaccination with the Janssen Ad26.COV2.S COVID-19 vaccine. As a mechanism for development of VITT has not been confirmed and the Ad26.RSV.preF–RSV preF protein and Ad26.COV2.S vaccines are Ad26 based, there is a theoretical concern for the occurrence of VITT after vaccination with Ad26.RSV.preF–RSV preF protein. Thus, to closely monitor for potential VITT cases, all thrombotic and/or thrombocytopenia events are defined as potential AEs of special interest (AESIs) for 6 months following vaccination in ongoing and newly initiated studies utilizing Ad26.RSV.preF–RSV preF protein. Additional standardized prospective and retrospective reporting, data collection, and review procedures, including assessments by external hematologic experts, were implemented to follow up on these events. For studies utilizing Ad26.RSV.preF–RSV preF protein that completed dosing >6 months before the prospective collection process of potential AESIs was implemented, including this study, a retrospective analysis was performed to identify AEs meeting the definition of a potential AESI. In this study, there were no cases of thrombosis concurrent with thrombocytopenia and no cases of VITT identified. To date, with an overall exposure of >290 000 recipients, no cases of VITT have been identified in studies with Ad26.RSV.preF–RSV preF protein or in other Janssen (non–COVID-19) Ad26-vectored vaccines [20, 21, 30–41].
Additionally, 1 event of chronic inflammatory demyelinating polyradiculoneuropathy, a Guillain-Barré syndrome–like AE, occurred in the vaccine group in this study; notably, no other such events have been identified in clinical studies of Ad26.RSV.preF–RSV preF protein. However, given potential associations between Ad26.COV2.S and Guillain-Barré syndrome [42], additional data are needed to exclude the possibility of any association with Ad26.RSV.preF–RSV preF protein.
Our data support further development of the Ad26.RSV.preF–RSV preF protein combination regimen for prophylactic vaccination against RSV in older adults.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all the investigators, participants and their families, and site personnel involved with this study. We thank Karin Weber for her contributions to the manuscript. Medical writing support was provided by William J. Kelley, PhD, and Elizabeth A. Ohneck, PhD (Lumanity Communications Inc.).
Financial support. This work was supported by Janssen Vaccines & Prevention B.V. during all stages of the trial and its analysis and the development and publishing of the manuscript, including scientific writing assistance and statistical analyses.
References
Author notes
Presented in part: 7th European Scientific Working Group on Influenza (7 December 2021; Valencia, Spain; abstract 223) and 32nd European Congress of Clinical Microbiology and Infectious Diseases (26 April 2022; Lisbon, Portugal; abstract 00682).
Potential conflicts of interests. C. A. C and E. H. are former employees of Janssen Vaccines & Prevention B.V. A. R. B., L.v. d. F., R. v. H., B. C., and J. S. are employees of Janssen Vaccines & Prevention B.V. V. K. is an employee of Janssen Infectious Diseases. E. D. P. and E. O. are former employees of Janssen Infectious Diseases. S. B. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




