-
PDF
- Split View
-
Views
-
Cite
Cite
Sofía Scévola, Jordi Niubó, Pere Domingo, Guillermo Verdejo, Adrian Curran, Vicens Diaz-Brito, Judith Peñafiel, Juan Tiraboschi, Sandra Morenilla, Benito Garcia, Irene Soriano, Daniel Podzamczer, Arkaitz Imaz, Decay of HIV RNA in Seminal Plasma and Rectal Fluid in Treatment-Naive Adults Starting Antiretroviral Therapy With Dolutegravir Plus Lamivudine or Bictegravir/Emtricitabine/Tenofovir Alafenamide, The Journal of Infectious Diseases, Volume 228, Issue 7, 1 October 2023, Pages 919–925, https://doi.org/10.1093/infdis/jiad304
Close - Share Icon Share
Abstract
Decay of HIV in seminal plasma (SP) and rectal fluid (RF) has not yet been described for the antiretroviral combination of dolutegravir (DTG) + lamivudine (3TC).
In this randomized multicenter pilot trial, males who were antiretroviral naive were randomized (2:1) to DTG + 3TC or bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF). HIV-1 RNA was measured in blood plasma (BP), SP, and RF at baseline; days 3, 7, 14, and 28; and weeks 12 and 24.
Of 25 individuals enrolled, 24 completed the study (DTG + 3TC, n = 16; BIC/FTC/TAF, n = 8). No significant differences were observed between groups for median decline in HIV-1 RNA from baseline at each time point or median time to achieve HIV-1 RNA <20 copies/mL in BP and SP and <20 copies/swab in RF. HIV-1 RNA decay patterns were compared in individuals receiving DTG + 3TC. Despite significantly higher percentages for changes from baseline in BP, median (IQR) times to HIV-1 RNA suppression were shorter in SP (7 days; 0–8.75) and RF (10.5 days; 3–17.5) than in BP (28 days; 14–84; P < .001).
Comparable HIV-1 RNA decay in BP, SP, and RF was observed between DTG + 3TC and BIC/FTC/TAF. As shown with triple-drug integrase inhibitor–based regimens, rapid HIV-1 RNA suppression in SP and RF is achieved with DTG + 3TC, despite decay patterns differing from those of BP.
EudraCT 2019-004109-28.
Sexual transmission of HIV can be prevented if undetectable HIV plasma viral load is maintained [1, 2]. However, HIV decay kinetics differs between blood plasma (BP) and genital and rectal fluids and is dependent on the antiretroviral regimen [3, 4]. Therefore, knowledge of HIV decay kinetics in genital and rectal fluids with different antiretroviral regimens can be of interest for predicting the risk of sexual transmission during the first weeks after initiating antiretroviral therapy (ART), when plasma HIV-1 RNA is not yet suppressed [5]. Current treatment guidelines recommend integrase strand transfer inhibitor (InSTI)–based regimens as preferred initial ART for people living with HIV (PLWH) owing to their favorable efficacy and safety profile [6–8]. These recommended first-line regimens also include the dual combination of dolutegravir (DTG) plus lamivudine (3TC). HIV-1 RNA decay in BP is similar in PLWH starting ART with DTG + 3TC and in those receiving DTG + emtricitabine/tenofovir disoproxil fumarate [9]. Yet, data have not been reported on HIV-1 RNA decay kinetics in genital and rectal fluids in ART-naive individuals treated with DTG + 3TC.
This study aimed to compare viral decay in seminal plasma (SP) and rectal fluid (RF) between DTG + 3TC and the triple antiretroviral combination bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF).
METHODS
Study Design and Population
We performed a phase IV multicenter pilot trial based on a randomized open-label design between January 2020 and October 2021. Eligible participants were cisgender male adults (≥18 years) who were ART naive and HIV-1 infected, who had plasma HIV-1 RNA viral load >1000 and <500 000 copies/mL, and whose CD4 cell count was >200 cells/μL at screening. The exclusion criteria were acute HIV infection, mutations associated with resistance to any of the drugs included in the study treatments, hepatitis B virus infection, moderate or severe hepatic impairment, estimated glomerular filtration rate <50 mL/min, and active opportunistic infections or active malignancies.
Participants were randomized 2:1 to initiate first-line ART with DTG (50 mg) + 3TC (300 mg) administered once daily as separate pills or BIC/FTC/TAF (50/200/25 mg, respectively) coformulated in a fixed-dose tablet once daily. Randomization was stratified by a plasma HIV-1 RNA viral load below or above 100 000 copies/mL and a CD4 count below or above 350 cells/μL.
The main objective of this study was to compare the decline in HIV-1 RNA in SP and RF over 24 weeks in adults initiating ART with the dual combination DTG + 3TC and those initiating with the 3-drug regimen BIC/FTC/TAF. The secondary objective was to compare decay in HIV-1 RNA in SP, RF, and BP in individuals receiving DTG + 3TC.
Procedures and Assessments
All study procedures were carried out at the coordinating center. The other centers were involved in the recruitment, preselection visits, and follow-up visits. The study visits were scheduled at baseline; days 3, 7, 14, and 28; and weeks 12 and 24. HIV-1 RNA was measured in BP, SP, and RF at all visits. Blood samples were obtained by peripheral venous puncture, semen samples by self-collection, and RF samples through a proctoscope with Weck-Cel sponges. Participants were requested to abstain from sexual activity for at least 72 hours and from using intrarectal products for at least 24 hours before the study visits. CD4 + lymphocyte count, hematology, and biochemistry tests (liver function, renal function, electrolytes, and lipids) were performed at baseline, day 28, and weeks 12 and 24. Clinical events and drug-related adverse events were recorded at each study visit.
Laboratory Methods
Sample Processing
Blood and semen samples were processed within 2 hours of collection. Blood samples were collected in EDTA-containing tubes, and plasma samples were separated by centrifugation (2600 rpm for 15 minutes at 4 °C) and transferred to cryogenic vials. SP samples were left to liquefy at room temperature for 30 minutes. SP was separated by centrifugation (20 minutes at 2000–2600 rpm in a conical tube at 4 °C) and transferred to cryogenic vials. RF samples were placed in cryogenic vials directly. SP and RF samples were stored at −70 °C within 2 hours of collection until analysis.
Determination of HIV-1 RNA
HIV-1 RNA was determined in BP, SP, and RF by using the Alinity m HIV-1 AMP assay (Abbott Molecular) following the manufacturer's protocol (limit of quantification, 20 copies/mL). At the time of the analysis, RF samples were thawed and diluted to 1 mL with sterile saline, which was thoroughly mixed by vortexing (1 minute at 2500 rpm) and centrifuged (10 minutes at 2300 relative centrifugal force). The results were calculated by reversing the initial dilution and expressed as copies per swab.
Statistical Methods
Categorical variables are presented as number of cases and percentages. Continuous variables are presented as mean and SD or median and IQR. The Kruskal-Wallis test was used to compare the differences in HIV-1 RNA (log10) from baseline between the groups at each time point. The chi-square or Fisher exact test was used to examine differences between the percentages of participants with HIV-1 RNA <20 copies/mL (or swab) in each compartment according to the treatment group. A mixed linear regression model with a cluster of patients was constructed to examine changes in the differences in HIV-1 RNA (log10) from baseline according to the treatment groups in BP, SP, and RF. Baseline HIV-1 RNA, time, and treatment group were used as independent variables. The interaction between time and treatment group was also assessed. To compare decay in HIV-1 RNA in the different compartments among participants receiving DTG + 3TC, changes in standardized HIV-1 RNA (log10) from baseline were studied in each compartment, and a mixed linear regression model with a cluster of patients was constructed. Baseline, time, and compartment were used as independent variables. The interaction between time and compartment was also assessed. Time to undetectable HIV-1 RNA was compared between treatment groups via the Kaplan-Meier method with interval censoring and the asymptotic log-rank test. All analyses were performed with a 2-sided significance level of .05 through R version 4.1.0 (R Foundation for Statistical Computing).
Ethics
This study was conducted following the principles of good clinical practice, the provisions of the Declaration of Helsinki, and the requirements of the Spanish regulatory authorities. The study protocol was approved by the Institutional Review Board at Bellvitge University Hospital. Written informed consent was provided by all participants before any study procedures were performed. This study was registered at the EU Clinical Trials Registry (DOLLARS Study, EudraCT 2019-004109-28).
RESULTS
Baseline Characteristics
A total of 25 individuals were screened and enrolled. After randomization (n = 16, DTG + 3TC; n = 9, BIC/FTC/TAF), 24 completed the study (1 participant in the BIC/FTC/TAF arm was excluded at day 14 because of a protocol violation). The participants’ characteristics at baseline are summarized in Table 1. Median (range) HIV-1 RNA at baseline was higher in BP (log10 copies/mL, 4.56 [3.09–5.65]) than in SP (log10 copies/mL, 2.38 [1.30–5.0]) and RF (log10 copies/swab, 3.20 [1.30–4.36]). At baseline, 9 and 1 adults had HIV-1 RNA below the limit of detection in SP (6/16, DTG + 3TC; 3/8, BIC/FTC/TAF) and RF (1/16, DTG + 3TC). At baseline, none of the participants had other sexually transmitted infections.
| Characteristic . | DTG + 3TC (n = 16) . | BIC/FTC/TAF (n = 8) . |
|---|---|---|
| Age, y | 32 [26.75–38] | 31 [24.5–39] |
| CD4 count, cells/μL | 436.5 [270.5–597.25] | 392 [345–442.25] |
| HIV-1 RNA | ||
| Blood plasmaa | 4.56 [4.22–4.86] | 4.48 [4.15–5.04] |
| Seminal plasmaa | 2.38 [1.30–2.95] | 2.71 [1.30–3.90] |
| Rectal fluidb | 2.92 [2.36–3.73] | 3.55 [3.05–4.13] |
| Characteristic . | DTG + 3TC (n = 16) . | BIC/FTC/TAF (n = 8) . |
|---|---|---|
| Age, y | 32 [26.75–38] | 31 [24.5–39] |
| CD4 count, cells/μL | 436.5 [270.5–597.25] | 392 [345–442.25] |
| HIV-1 RNA | ||
| Blood plasmaa | 4.56 [4.22–4.86] | 4.48 [4.15–5.04] |
| Seminal plasmaa | 2.38 [1.30–2.95] | 2.71 [1.30–3.90] |
| Rectal fluidb | 2.92 [2.36–3.73] | 3.55 [3.05–4.13] |
Data are presented as median [IQR].
Abbreviations: 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
Log10 copies/mL.
Log10 copies/swab.
| Characteristic . | DTG + 3TC (n = 16) . | BIC/FTC/TAF (n = 8) . |
|---|---|---|
| Age, y | 32 [26.75–38] | 31 [24.5–39] |
| CD4 count, cells/μL | 436.5 [270.5–597.25] | 392 [345–442.25] |
| HIV-1 RNA | ||
| Blood plasmaa | 4.56 [4.22–4.86] | 4.48 [4.15–5.04] |
| Seminal plasmaa | 2.38 [1.30–2.95] | 2.71 [1.30–3.90] |
| Rectal fluidb | 2.92 [2.36–3.73] | 3.55 [3.05–4.13] |
| Characteristic . | DTG + 3TC (n = 16) . | BIC/FTC/TAF (n = 8) . |
|---|---|---|
| Age, y | 32 [26.75–38] | 31 [24.5–39] |
| CD4 count, cells/μL | 436.5 [270.5–597.25] | 392 [345–442.25] |
| HIV-1 RNA | ||
| Blood plasmaa | 4.56 [4.22–4.86] | 4.48 [4.15–5.04] |
| Seminal plasmaa | 2.38 [1.30–2.95] | 2.71 [1.30–3.90] |
| Rectal fluidb | 2.92 [2.36–3.73] | 3.55 [3.05–4.13] |
Data are presented as median [IQR].
Abbreviations: 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
Log10 copies/mL.
Log10 copies/swab.
Decline in HIV-1 RNA in BP, SP, and RF
No statistically significant differences in median HIV-1 RNA were observed between the DTG + 3TC and BIC/FTC/TAF groups in BP, SP, and RF at any time points (supplementary material). When HIV-1 RNA decline from baseline was evaluated, differences were not statistically significant between treatment groups at each time point in any of the compartments analyzed (Table 2, Figure 1).
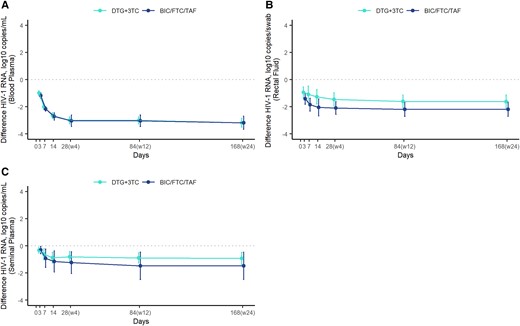
Differences in the decline in HIV-1 RNA from baseline. Observed mean and 95% CI for the difference in HIV-1 RNA (log10 copies/mL or copies/swab) from baseline at each time point by treatment group. A, Blood plasma. B, Rectal fluid. C, Seminal plasma. 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
| . | HIV-1 RNA Decline From Baseline,a Median [IQR] . | |||||
|---|---|---|---|---|---|---|
| Treatment Groupb . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.13, −0.88] | −2.05 [−2.14, −1.71] | −2.72 [−2.98, −2.36] | −3.08 [−3.39, −2.79] | −3.08 [−3.39, −2.79] | −3.20 [−3.56, −2.89] |
| BIC/FTC/TAF (n = 8) | −1.12 [−1.28, −0.95] | −2.21 [−2.23, −2.13] | −2.61 [−3.01, −2.49] | −3.07 [−3.13, −2.85] | −3.07 [−3.13, −2.85] | −3.18 [−3.50, −2.85] |
| P value | .098 | .107 | .951 | .903 | .903 | >.99 |
| Seminal plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.64, −0.14] | −1.04 [−2.29, −0.18] | −1.35 [−2.17, −0.51] | −1.55 [−2.23, −0.84] | −1.62 [−2.26, −1.06] | −1.62 [−2.43, −1.06] |
| BIC/FTC/TAF (n = 8) | −1.27 [−1.78, −1.00] | −1.91 [−2.18, −1.22] | −2.23 [−2.71, −1.71] | −2.17 [−2.56, −1.75] | −2.25 [−2.83, −1.75] | −2.25 [−2.82, −1.75] |
| P value | .221 | .159 | .076 | .111 | .159 | .159 |
| Rectal fluid | ||||||
| DTG + 3TC (n = 16) | −0.11 [−0.61, 0.00] | −0.83 [−1.12, 0.00] | −0.97 [−1.65, 0.00] | −0.95 [−1.64, 0.00] | −1.08 [−1.61, 0.00] | −1.08 [−1.65, 0.00] |
| BIC/FTC/TAF (n = 8) | −0.17 [−0.47, 0.00] | −0.90 [−1.22, 0.00] | −1.07 [−1.98, 0.00] | −1.41 [−2.01, 0.00] | −1.41 [−2.60, 0.00] | −1.41 [−2.60, 0.00] |
| P value | .801 | .717 | .529 | .248 | .314 | .414 |
| . | HIV-1 RNA Decline From Baseline,a Median [IQR] . | |||||
|---|---|---|---|---|---|---|
| Treatment Groupb . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.13, −0.88] | −2.05 [−2.14, −1.71] | −2.72 [−2.98, −2.36] | −3.08 [−3.39, −2.79] | −3.08 [−3.39, −2.79] | −3.20 [−3.56, −2.89] |
| BIC/FTC/TAF (n = 8) | −1.12 [−1.28, −0.95] | −2.21 [−2.23, −2.13] | −2.61 [−3.01, −2.49] | −3.07 [−3.13, −2.85] | −3.07 [−3.13, −2.85] | −3.18 [−3.50, −2.85] |
| P value | .098 | .107 | .951 | .903 | .903 | >.99 |
| Seminal plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.64, −0.14] | −1.04 [−2.29, −0.18] | −1.35 [−2.17, −0.51] | −1.55 [−2.23, −0.84] | −1.62 [−2.26, −1.06] | −1.62 [−2.43, −1.06] |
| BIC/FTC/TAF (n = 8) | −1.27 [−1.78, −1.00] | −1.91 [−2.18, −1.22] | −2.23 [−2.71, −1.71] | −2.17 [−2.56, −1.75] | −2.25 [−2.83, −1.75] | −2.25 [−2.82, −1.75] |
| P value | .221 | .159 | .076 | .111 | .159 | .159 |
| Rectal fluid | ||||||
| DTG + 3TC (n = 16) | −0.11 [−0.61, 0.00] | −0.83 [−1.12, 0.00] | −0.97 [−1.65, 0.00] | −0.95 [−1.64, 0.00] | −1.08 [−1.61, 0.00] | −1.08 [−1.65, 0.00] |
| BIC/FTC/TAF (n = 8) | −0.17 [−0.47, 0.00] | −0.90 [−1.22, 0.00] | −1.07 [−1.98, 0.00] | −1.41 [−2.01, 0.00] | −1.41 [−2.60, 0.00] | −1.41 [−2.60, 0.00] |
| P value | .801 | .717 | .529 | .248 | .314 | .414 |
Abbreviations: 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
Log10 copies/mL for blood and seminal plasma and log10 copies/swab for rectal fluid.
P values are based on differences between groups by time within compartment type.
| . | HIV-1 RNA Decline From Baseline,a Median [IQR] . | |||||
|---|---|---|---|---|---|---|
| Treatment Groupb . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.13, −0.88] | −2.05 [−2.14, −1.71] | −2.72 [−2.98, −2.36] | −3.08 [−3.39, −2.79] | −3.08 [−3.39, −2.79] | −3.20 [−3.56, −2.89] |
| BIC/FTC/TAF (n = 8) | −1.12 [−1.28, −0.95] | −2.21 [−2.23, −2.13] | −2.61 [−3.01, −2.49] | −3.07 [−3.13, −2.85] | −3.07 [−3.13, −2.85] | −3.18 [−3.50, −2.85] |
| P value | .098 | .107 | .951 | .903 | .903 | >.99 |
| Seminal plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.64, −0.14] | −1.04 [−2.29, −0.18] | −1.35 [−2.17, −0.51] | −1.55 [−2.23, −0.84] | −1.62 [−2.26, −1.06] | −1.62 [−2.43, −1.06] |
| BIC/FTC/TAF (n = 8) | −1.27 [−1.78, −1.00] | −1.91 [−2.18, −1.22] | −2.23 [−2.71, −1.71] | −2.17 [−2.56, −1.75] | −2.25 [−2.83, −1.75] | −2.25 [−2.82, −1.75] |
| P value | .221 | .159 | .076 | .111 | .159 | .159 |
| Rectal fluid | ||||||
| DTG + 3TC (n = 16) | −0.11 [−0.61, 0.00] | −0.83 [−1.12, 0.00] | −0.97 [−1.65, 0.00] | −0.95 [−1.64, 0.00] | −1.08 [−1.61, 0.00] | −1.08 [−1.65, 0.00] |
| BIC/FTC/TAF (n = 8) | −0.17 [−0.47, 0.00] | −0.90 [−1.22, 0.00] | −1.07 [−1.98, 0.00] | −1.41 [−2.01, 0.00] | −1.41 [−2.60, 0.00] | −1.41 [−2.60, 0.00] |
| P value | .801 | .717 | .529 | .248 | .314 | .414 |
| . | HIV-1 RNA Decline From Baseline,a Median [IQR] . | |||||
|---|---|---|---|---|---|---|
| Treatment Groupb . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.13, −0.88] | −2.05 [−2.14, −1.71] | −2.72 [−2.98, −2.36] | −3.08 [−3.39, −2.79] | −3.08 [−3.39, −2.79] | −3.20 [−3.56, −2.89] |
| BIC/FTC/TAF (n = 8) | −1.12 [−1.28, −0.95] | −2.21 [−2.23, −2.13] | −2.61 [−3.01, −2.49] | −3.07 [−3.13, −2.85] | −3.07 [−3.13, −2.85] | −3.18 [−3.50, −2.85] |
| P value | .098 | .107 | .951 | .903 | .903 | >.99 |
| Seminal plasma | ||||||
| DTG + 3TC (n = 16) | −0.96 [−1.64, −0.14] | −1.04 [−2.29, −0.18] | −1.35 [−2.17, −0.51] | −1.55 [−2.23, −0.84] | −1.62 [−2.26, −1.06] | −1.62 [−2.43, −1.06] |
| BIC/FTC/TAF (n = 8) | −1.27 [−1.78, −1.00] | −1.91 [−2.18, −1.22] | −2.23 [−2.71, −1.71] | −2.17 [−2.56, −1.75] | −2.25 [−2.83, −1.75] | −2.25 [−2.82, −1.75] |
| P value | .221 | .159 | .076 | .111 | .159 | .159 |
| Rectal fluid | ||||||
| DTG + 3TC (n = 16) | −0.11 [−0.61, 0.00] | −0.83 [−1.12, 0.00] | −0.97 [−1.65, 0.00] | −0.95 [−1.64, 0.00] | −1.08 [−1.61, 0.00] | −1.08 [−1.65, 0.00] |
| BIC/FTC/TAF (n = 8) | −0.17 [−0.47, 0.00] | −0.90 [−1.22, 0.00] | −1.07 [−1.98, 0.00] | −1.41 [−2.01, 0.00] | −1.41 [−2.60, 0.00] | −1.41 [−2.60, 0.00] |
| P value | .801 | .717 | .529 | .248 | .314 | .414 |
Abbreviations: 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
Log10 copies/mL for blood and seminal plasma and log10 copies/swab for rectal fluid.
P values are based on differences between groups by time within compartment type.
Mixed linear regression models showed a significant association between the HIV-1 RNA value at baseline and the decline in HIV-1 RNA from baseline through week 24 in BP, SP, and RF. However, no significant association was observed between decline in HIV-1 RNA and treatment arm. In the interaction model, no significant interactions were detected between treatment arm and time (Supplementary Tables 1–3).
No statistically significant differences were observed between the DTG + 3TC and BIC/FTC/TAF groups in the percentage of individuals with HIV-1 RNA <20 copies/mL (or swab) in BP, SP, and RF at each study time point (Table 3). In addition, the Kaplan-Meier analysis did not show differences between treatment arms in median (IQR) days to HIV-1 RNA below the limit of detection in BP (28 [14–84] vs 28 [24.5–42], P = .677), SP (7 [0–8.75] vs 5 [0–8.75], P = .677), or RF (10.5 [3–17.5] vs 10.5 [3–28], P = .489; Figure 2).
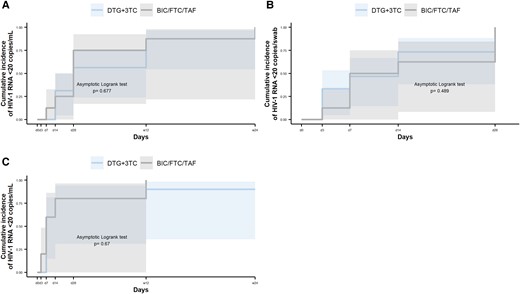
Time to HIV-1 RNA suppression <20 copies/mL or copies/swab by treatment group. Data are presented as cumulative incidence and 95% CI (shading). A, Blood plasma. B, Rectal fluid. C, Seminal plasma. 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
| . | HIV-1 RNA <20 Copies/mL,a No. (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Treatment Groupb . | Baseline . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | |||||||
| DTG + 3TC (n = 16) | 0 | 0 | 0 | 5 (31) | 9 (56) | 14 (87) | 15 (94) |
| BIC/FTC/TAF (n = 8) | 0 | 0 | 1 (12) | 2 (25) | 6 (75) | 7 (87) | 7 (87) |
| P value | … | … | .333 | >.99 | .371 | >.99 | .602 |
| Seminal plasma | |||||||
| DTG + 3TC (n = 16) | 6 (37) | 6 (37) | 11 (69) | 14 (87) | 13 (81) | 15 (94) | 14 (87) |
| BIC/FTC/TAF (n = 8) | 3 (37) | 4 (50) | 6 (75) | 7 (87) | 7 (87) | 8 (100) | 8 (100) |
| P value | >.99 | .673 | .751 | >.99 | .699 | .470 | .296 |
| Rectal fluid | |||||||
| DTG + 3TC (n = 16) | 1 (6) | 6 (37) | 6 (37) | 10 (62) | 13 (81) | 15 (94) | 16 (100) |
| BIC/FTC/TAF (n = 8) | 0 | 1 (12) | 4 (50) | 4 (50) | 7 (87) | 8 (100) | 7 (87) |
| P value | >.99 | .352 | .673 | .673 | .699 | .470 | .149 |
| . | HIV-1 RNA <20 Copies/mL,a No. (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Treatment Groupb . | Baseline . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | |||||||
| DTG + 3TC (n = 16) | 0 | 0 | 0 | 5 (31) | 9 (56) | 14 (87) | 15 (94) |
| BIC/FTC/TAF (n = 8) | 0 | 0 | 1 (12) | 2 (25) | 6 (75) | 7 (87) | 7 (87) |
| P value | … | … | .333 | >.99 | .371 | >.99 | .602 |
| Seminal plasma | |||||||
| DTG + 3TC (n = 16) | 6 (37) | 6 (37) | 11 (69) | 14 (87) | 13 (81) | 15 (94) | 14 (87) |
| BIC/FTC/TAF (n = 8) | 3 (37) | 4 (50) | 6 (75) | 7 (87) | 7 (87) | 8 (100) | 8 (100) |
| P value | >.99 | .673 | .751 | >.99 | .699 | .470 | .296 |
| Rectal fluid | |||||||
| DTG + 3TC (n = 16) | 1 (6) | 6 (37) | 6 (37) | 10 (62) | 13 (81) | 15 (94) | 16 (100) |
| BIC/FTC/TAF (n = 8) | 0 | 1 (12) | 4 (50) | 4 (50) | 7 (87) | 8 (100) | 7 (87) |
| P value | >.99 | .352 | .673 | .673 | .699 | .470 | .149 |
Abbreviations: 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
In the case of rectal fluid, <20 copies/swab.
P values are based on differences between groups by time within compartment type.
| . | HIV-1 RNA <20 Copies/mL,a No. (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Treatment Groupb . | Baseline . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | |||||||
| DTG + 3TC (n = 16) | 0 | 0 | 0 | 5 (31) | 9 (56) | 14 (87) | 15 (94) |
| BIC/FTC/TAF (n = 8) | 0 | 0 | 1 (12) | 2 (25) | 6 (75) | 7 (87) | 7 (87) |
| P value | … | … | .333 | >.99 | .371 | >.99 | .602 |
| Seminal plasma | |||||||
| DTG + 3TC (n = 16) | 6 (37) | 6 (37) | 11 (69) | 14 (87) | 13 (81) | 15 (94) | 14 (87) |
| BIC/FTC/TAF (n = 8) | 3 (37) | 4 (50) | 6 (75) | 7 (87) | 7 (87) | 8 (100) | 8 (100) |
| P value | >.99 | .673 | .751 | >.99 | .699 | .470 | .296 |
| Rectal fluid | |||||||
| DTG + 3TC (n = 16) | 1 (6) | 6 (37) | 6 (37) | 10 (62) | 13 (81) | 15 (94) | 16 (100) |
| BIC/FTC/TAF (n = 8) | 0 | 1 (12) | 4 (50) | 4 (50) | 7 (87) | 8 (100) | 7 (87) |
| P value | >.99 | .352 | .673 | .673 | .699 | .470 | .149 |
| . | HIV-1 RNA <20 Copies/mL,a No. (%) . | ||||||
|---|---|---|---|---|---|---|---|
| Treatment Groupb . | Baseline . | Day 3 . | Day 7 . | Day 14 . | Day 28 . | Wk 12 . | Wk 24 . |
| Blood plasma | |||||||
| DTG + 3TC (n = 16) | 0 | 0 | 0 | 5 (31) | 9 (56) | 14 (87) | 15 (94) |
| BIC/FTC/TAF (n = 8) | 0 | 0 | 1 (12) | 2 (25) | 6 (75) | 7 (87) | 7 (87) |
| P value | … | … | .333 | >.99 | .371 | >.99 | .602 |
| Seminal plasma | |||||||
| DTG + 3TC (n = 16) | 6 (37) | 6 (37) | 11 (69) | 14 (87) | 13 (81) | 15 (94) | 14 (87) |
| BIC/FTC/TAF (n = 8) | 3 (37) | 4 (50) | 6 (75) | 7 (87) | 7 (87) | 8 (100) | 8 (100) |
| P value | >.99 | .673 | .751 | >.99 | .699 | .470 | .296 |
| Rectal fluid | |||||||
| DTG + 3TC (n = 16) | 1 (6) | 6 (37) | 6 (37) | 10 (62) | 13 (81) | 15 (94) | 16 (100) |
| BIC/FTC/TAF (n = 8) | 0 | 1 (12) | 4 (50) | 4 (50) | 7 (87) | 8 (100) | 7 (87) |
| P value | >.99 | .352 | .673 | .673 | .699 | .470 | .149 |
Abbreviations: 3TC, lamivudine; BIC, bictegravir; DTG, dolutegravir; FTC, emtricitabine; TAF, tenofovir alafenamide.
In the case of rectal fluid, <20 copies/swab.
P values are based on differences between groups by time within compartment type.
At week 24, 15 of 16 and 7 of 8 individuals in the DTG + 3TC and BIC/FTC/TAF groups achieved HIV-1 RNA <20 copies/mL in BP. In the DTG + 3TC group, all had undetectable HIV-1 RNA in RF at week 24, and 2 of 16 had low but detectable HIV-1 RNA in SP (32 and 82 copies/mL); viral load was <20 copies/mL in BP. In the BIC/FTC/TAF group, 100% achieved undetectable HIV-1 RNA in SP, and 1 of 8 had detectable HIV-1 RNA in RF (21 copies/swab). HIV-1 RNA was <20 copies/mL in BP.
Decline in HIV-1 RNA in SP and RF vs BP in Individuals Receiving DTG + 3TC
Different viral decay patterns were observed in BP, SP, and RF. To compare decay in HIV-1 RNA among compartments through week 24, the differences in standardized HIV-1 RNA values from baseline at each time point were analyzed (Figure 3). The linear mixed effects models showed a significant interaction between the compartment and the magnitude of decline, which was more pronounced in BP than in SP and RF. Specifically, the percentage change in HIV-1 RNA from baseline was significantly greater in BP than in SP at all time points and RF at all time points except at day 3. Regarding differences between SP and RF, the mean percentage change in HIV-1 RNA from baseline was significantly greater in RF at all time points except days 7 and 14 (supplementary material). The median (IQR) time to HIV-1 RNA <20 copies/mL (or swab) was shorter in SP (7 days [0–8.75]) and RF (10.5 days [3–17.5]) than in BP (28 days [14–84]; P < .001).
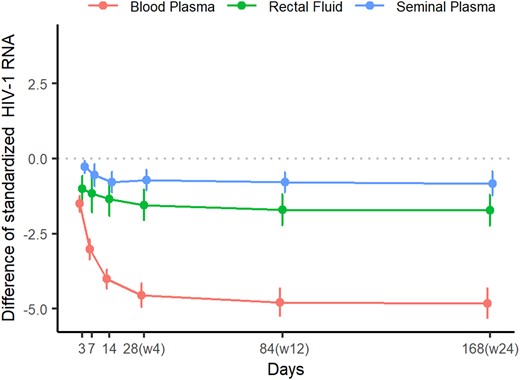
Differences in standardized HIV-1 RNA values from baseline at each time point in blood plasma, seminal plasma, and rectal fluid.
During the study period, 2 of 24 participants reported sexually transmitted infections. One experienced 2 episodes of proctitis caused by Chlamydia trachomatis between weeks 4 and 12, and the other had urethritis caused by Neisseria gonorrhoeae between weeks 12 and 24. Both maintained undetectable HIV-1 RNA in SP and RF. These episodes were not diagnosed at the time of sample collection and were reported retrospectively. The participants had been appropriately treated and were asymptomatic at the time of the visit. No severe adverse effects were reported, and there were no toxicity-related treatment withdrawals.
DISCUSSION
The dual combination DTG + 3TC is recommended in current treatment guidelines as a preferred initial ART option for treatment-naive individuals [6–8]. The efficacy of DTG + 3TC has proven noninferior to DTG + 3TC/tenofovir disoproxil fumarate in phase III randomized clinical trials [9]. Moreover, viral decay was similar with DTG + 3TC and the triple combination at all time points studied. However, viral decay dynamics in SP and RF in PLWH treated with DTG + 3TC have not yet been evaluated. Considering that penetration of drugs in genital and rectal tissues is limited by physiologic barriers [10], potential differences in viral decay kinetics between dual and triple antiretroviral drug combinations during the first weeks of ART might give cause for concern.
In the present study, no differences were observed in the decline in HIV-1 RNA in BP, SP, and RF through 24 weeks between ART-naive individuals starting treatment with DTG + 3TC and those starting with BIC/FTC/TAF. Accordingly, no differences were observed in either the median time to or the percentage of HIV-1 RNA <20 copies/mL (or swab) at each study time point in BP, SP, or RF. Of note, both regimens achieved rapid suppression of HIV-1 RNA in SP and RF, and this was even faster than in BP. The finding can be explained by the lower viral load in SP and RF at baseline as compared with BP. Despite the low distribution of DTG and BIC in genital fluids and the rectum [3, 11, 12], both drugs have a very high protein-free (active drug fraction) inhibitory quotient in these compartments [3, 12]. The various antiretroviral drug classes have a different impact on HIV decay kinetics in semen, and viral suppression in semen is achieved earlier with InSTI-based regimens than with a boosted protease inhibitor–based combination [4]. However, our study shows similar HIV decline and time to viral suppression in SP and RF with a 2-drug regimen and a 3-drug InSTI-based regimen.
Our findings are relevant for PLWH who initiate ART. Sexual transmission of HIV is prevented if plasma viral load is undetectable. The approach that we followed provides insight into predicting the risk of sexual transmission during the first weeks or months after initiating ART while plasma HIV-1 RNA is not yet suppressed. Our study shows that viral suppression in semen and the rectum is similar with DTG + 3TC and the triple combination BIC/FTC/TAF. Moreover, this study has implications for PLWH with suppressed plasma viral load. Optimal suppression of HIV replication in the genital tract and rectum is necessary because of the role that these sanctuaries play in the latent HIV reservoir in limiting residual replication and replenishment [13–15]. The activity of DTG + 3TC in these sanctuaries does not seem different from that of the triple combination BIC/FTC/TAF [3].
Some individuals in both treatment groups had detectable HIV-1 RNA in SP or RF, although this was undetectable in the other compartments at week 24. However, it is notable that in all cases, the HIV-1 RNA value was low. These findings are concordant with compartmentalized HIV-1 RNA dynamics in the genital tract and rectum, as described elsewhere [3, 4, 12].
Our study is subject to a series of limitations. Among 24 individuals at baseline, HIV-1 RNA was undetectable in 9 and 1 in SP and RF, respectively. This finding may limit the virologic analyses. Nevertheless, our comprehensive approach for the assessment of HIV-1 RNA decay kinetics provides interesting information about the activity of DTG + 3TC in the seminal and rectal compartments. In addition, our relatively small sample (only 24 participants) leaves our results more prone to influence from inter- and intraindividual variability. However, it is comparable to previously published studies of antiretroviral decay in genital fluids and the rectum [3, 4, 12]. Although we did not measure drug concentrations in BP, SP, or RF, concentrations of DTG and BIC in SP and RF have been assessed elsewhere, and the results are useful when interpreting our findings [3, 11, 12]. Finally, the study included only cisgender men, although the results can probably be extrapolated to other population groups, such as transgender women and nonbinary individuals.
In summary, no significant differences were observed between DTG + 3TC and BIC/FTC/TAF in either decline in HIV-1 RNA from baseline or the percentages of individuals with undetectable HIV-1 RNA in SP and RF at each study time point. Although the HIV-1 RNA decay patterns observed in SP and RF differed from those in BP in ART-naive PLWH treated with DTG + 3TC, rapid HIV-1 RNA suppression was achieved in both compartments, as reported with triple-drug InSTI-based regimens.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. I. and D. P. designed the study. S. S., A. I., J. T., P. D., G. V., A. C., and V. D.-B. recruited participants. S. S., S. M., B. G., I. S., and A. I. conducted the study visits. B. G., I. S., and J. N. processed the samples. J. N. performed the microbiological procedures. S. M. and S. S. assisted in data collection and study coordination. S. S., J. P., and A. I. analyzed and interpreted the results. S. S. drafted the manuscript and J. T., J. P., D. P., and A. I. reviewed it. All the authors revised the manuscript for important intellectual content and contributed to the final version.
Acknowledgments. We thank all the individuals who participated in this study and the CERCA Programme/Generalitat de Catalunya for institutional support. Conference on Retroviruses and Opportunistic Infections (CROI) 2022, Virtual, from 12-16 February, 2022, Abstract 495; and Conference on Retroviruses and Opportunistic Infections (CROI) 2023, Seattle, Washington, from 19-22 February, 2023, Abstract 537.
Data availability. The data used in this study are not publicly available, for reasons of confidentiality. Data-sharing requests will be considered by the trial management group upon written request to the corresponding author and after careful consideration. Only anonymized data with a low risk of reidentification will be made available.
Financial support. This work was supported in part by the Spanish Network for AIDS Research through the Instituto de Salud Carlos III–Red Temática de Investigación Cooperativa en Sida (RD16/0025/0003) as part of the Plan Nacional R + D + I and by ISCIII Subdirección General de Evaluación and the European Regional Development Fund.
References
Author notes
Potential conflicts of interest. S. S. has received financial compensation for educational activities, as well as training courses, funds for research, travel grants, and nonfinancial support from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. P. D. has received financial compensation for lectures, consultancy work, and educational activities, from Gilead Sciences, GSK, Ferrer International, Janssen-Cilag, Merck Sharp & Dohme, Roche, Thera Technologies, and ViiV Healthcare. A. C. has received financial compensation for lectures, consultancy work, and educational activities, as well as funding for research, travel grants, and nonfinancial support from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, Thera Technologies, and ViiV Healthcare. J. T. has received financial compensation for lectures, consultancies, and educational activities as well as research funding from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. D. P. has received research grants and/or honoraria for advisory boards and/or conferences from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. A. I. has received financial compensation for lectures, consultancy work, and educational activities, as well as funding for research, travel grants, and nonfinancial support from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, Thera Technologies, and ViiV Healthcare. All other authors report no potential conflicts.




