-
PDF
- Split View
-
Views
-
Cite
Cite
Selin Somersan-Karakaya, Eleftherios Mylonakis, Vidya P Menon, Jason C Wells, Shazia Ali, Sumathi Sivapalasingam, Yiping Sun, Rafia Bhore, Jingning Mei, Jutta Miller, Lisa Cupelli, Eduardo Forleo-Neto, Andrea T Hooper, Jennifer D Hamilton, Cynthia Pan, Viet Pham, Yuming Zhao, Romana Hosain, Adnan Mahmood, John D Davis, Kenneth C Turner, Yunji Kim, Amanda Cook, Bari Kowal, Yuhwen Soo, A Thomas DiCioccio, Gregory P Geba, Neil Stahl, Leah Lipsich, Ned Braunstein, Gary A Herman, George D Yancopoulos, David M Weinreich, for the COVID-19 Phase 2/3 Hospitalized Trial Team, Casirivimab and Imdevimab for the Treatment of Hospitalized Patients With COVID-19, The Journal of Infectious Diseases, Volume 227, Issue 1, 1 January 2023, Pages 23–34, https://doi.org/10.1093/infdis/jiac320
Close - Share Icon Share
Abstract
The open-label RECOVERY study reported improved survival in hospitalized, SARS-CoV-2 seronegative patients treated with casirivimab and imdevimab (CAS + IMD).
In this phase 1/2/3, double-blind, placebo-controlled trial conducted prior to widespread circulation of Delta and Omicron, hospitalized COVID-19 patients were randomized (1:1:1) to 2.4 g or 8.0 g CAS + IMD or placebo, and characterized at baseline for viral load and SARS-CoV-2 serostatus.
In total, 1336 patients on low-flow or no supplemental (low-flow/no) oxygen were treated. The primary endpoint was met in seronegative patients, the least-squares mean difference (CAS + IMD versus placebo) for time-weighted average change from baseline in viral load through day 7 was −0.28 log10 copies/mL (95% confidence interval [CI], −.51 to −.05; P = .0172). The primary clinical analysis of death or mechanical ventilation from day 6 to 29 in patients with high viral load had a strong positive trend but did not reach significance. CAS + IMD numerically reduced all-cause mortality in seronegative patients through day 29 (relative risk reduction, 55.6%; 95% CI, 24.2%–74.0%). No safety concerns were noted.
In hospitalized COVID-19 patients on low-flow/no oxygen, CAS + IMD reduced viral load and likely improves clinical outcomes in the overall population, with the benefit driven by seronegative patients, and no harm observed in seropositive patients.
NCT04426695.
Lay Summary
Lay Summary. Monoclonal antibody therapies that block the virus that causes COVID-19 (SARS-CoV-2) can prevent patients from being hospitalized. We hypothesized that these antibodies may also benefit patients who are already hospitalized with COVID-19. Therefore, we performed a study to determine if the monoclonal antibody combination of casirivimab and imdevimab (CAS + IMD) can decrease the amount of virus in the nose of hospitalized patients and prevent the disease from becoming more severe. The study, conducted from June 2020 to April 2021, found that CAS + IMD treatment reduced the amount of virus in these patients, and may reduce their chance of dying or needing a ventilator (a machine that helps patients breathe). Patients were examined in 2 groups: those whose immune systems, at the start of the study, had not produced their own antibodies to fight SARS-CoV-2 (seronegative patients); or those that had already produced their own antibodies (seropositive patients) at the start of the study. Seronegative patients benefited the most from CAS + IMD. No safety concerns related to CAS + IMD were observed. These results demonstrate that monoclonal antibody therapy can help hospitalized patients with COVID-19 and may decrease their chances of needing assistance to breathe or dying.
Progression of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is highly variable; while many cases manifest with relatively mild symptoms, others progress to severe respiratory failure requiring supplemental oxygen and/or mechanical ventilation [1–4]. Casirivimab and imdevimab (CAS + IMD) is a monoclonal antibody combination that binds nonoverlapping epitopes of the SARS-CoV-2 spike protein receptor binding domain [5, 6]. CAS + IMD was previously authorized for treatment and postexposure prophylaxis of COVID-19 in certain settings in the United States for susceptible strains, and for treatment and/or prevention of COVID-19 in other jurisdictions [7–9]. Preclinical data show that CAS + IMD exhibits diminished neutralization against Omicron-lineage variants [10], but retains neuralization potency against all other historical variants of concern.
Studies conducted prior to widespread circulation of the Omicron variant showed that CAS + IMD reduced hospitalization or all-cause death, reduced viral load, and shortened symptom duration in outpatients with COVID-19 [11–13]. Data have also shown that CAS + IMD is highly effective in preventing asymptomatic as well as symptomatic COVID-19 among recently exposed and asymptomatic individuals [14]. In an open-label platform trial of hospitalized patients with COVID-19 in the United Kingdom (RECOVERY), CAS + IMD improved overall survival in patients who had not mounted their own immune response at baseline (seronegative) by 21%, and also increased the probability of being discharged alive within 28 days [15]. Although efficacy of CAS + IMD was seen throughout the spectrum of disease, evidence suggests that the benefit is greatest when treatment is administered early [16].
Based on the potent antiviral activity of CAS + IMD, it was prospectively hypothesized that reducing viral burden as early as possible would also decrease morbidity and mortality associated with SARS-CoV-2 infection in hospitalized patients. Here, we describe the final efficacy and safety results from a phase 1/2/3 double-blind placebo-controlled trial of CAS + IMD in hospitalized patients with COVID-19, with a focus on those on low-flow or no supplemental oxygen.
METHODS
Trial Design
This adaptive, phase 1/2/3, double-blinded, placebo-controlled trial evaluated the efficacy, safety, and tolerability of CAS + IMD in hospitalized adult patients with COVID-19. The study was conducted at 103 sites in the United States, Brazil, Chile, Mexico, Moldova, and Romania between 10 June 2020 and 9 April 2021 (NCT04426695).
Patients were enrolled in 1 of 4 cohorts based on disease severity: no supplemental oxygen (cohort 1A), low-flow oxygen (cohort 1), high-intensity oxygen (cohort 2), or mechanical ventilation (cohort 3; Supplementary Figure 1). The trial proceeded through phase 2 for patients requiring no supplemental oxygen (cohort 1A) and phase 3 for patients requiring low-flow oxygen (cohort 1); together, these patients are the subject of this article. The definition of low-flow oxygen was based on the device requirement and not by the amount of flow. As phase 1/2 data from patients on low-flow oxygen were previously unblinded for an interim analysis, they were not included in the current analysis.
For patients requiring high-intensity oxygen (cohort 2) or mechanical ventilation (cohort 3), enrollment was paused early (30 October 2020) per recommendation of the independent data monitoring committee (IDMC), which observed an imbalance in mortality (see “Trial Adaptations” section in the Supplementary Material). Data from these cohorts were subsequently unblinded in an interim analysis, and mortality data are presented in Supplementary Table 1 (cohort 2) and Supplementary Table 2 (cohort 3). Due to very low sample size, patients from cohorts 2 and 3 were not included in analyses with patients from cohorts 1 and 1A, for whom the trial proceeded per IDMC recommendation until premature termination by the sponsor due to low enrollment on 9 April 2021.
Enrolled patients were randomized 1:1:1 to a single intravenous dose of 2.4 g CAS + IMD (1.2 g casirivimab and 1.2 g imdevimab), 8.0 g CAS + IMD (4.0 g casirivimab and 4.0 g imdevimab), or placebo. Within each cohort, randomization was stratified by standard-of-care treatment (antiviral therapies, nonantiviral therapies; phase 1/2/3) and country (phases 2/3 only). The trial included a screening/baseline period (days −1 to 1), a hospitalization/postdischarge period, a monthly follow-up period, and an end-of-study visit (phase 1 day 169, phase 2/3 day 57; Supplementary Figure 1).
Patients
The study included patients who were ≥ 18 years of age and hospitalized with confirmed SARS-CoV-2 ≤ 72 hours, with symptom onset ≤ 10 days from randomization. Standard-of-care treatments for COVID-19 were permitted. While COVID-19 vaccination was not prohibited, the study was conducted prior to widespread use of COVID-19 vaccines. All participants provided written informed consent. Full inclusion and exclusion criteria are in the Supplementary Material.
SARS-CoV-2 Serostatus Determination
All patients were assessed prior to dosing for baseline viral load and anti–SARS-CoV-2 antibodies: anti-spike (S1) immunoglobulin (Ig) A (EUROIMMUN), anti-S1 IgG (EUROIMMUN), and anti-nucleocapsid IgG (Abbott) using the cutoffs for negative, positive, or borderline as defined per the manufacturer’s instructions for use. All serology assays at baseline were run at a central laboratory (ICON Central Laboratories, Farmingdale, NY). Because serology results were not immediately available, patients underwent randomization regardless of their baseline serostatus, and were later grouped for analyses as seronegative (if all antibody tests were negative), seropositive (if any antibody test was positive), borderline (if any test was borderline and other tests were negative), or other (missing, not determined, pending, or inconclusive results).
Outcome Measures
The primary virologic efficacy endpoint was the time-weighted average daily change from baseline (day 1) in viral load (nasopharyngeal samples) through day 7 in the seronegative population [13]. The primary clinical efficacy endpoint was the proportion of patients who died or required mechanical ventilation from days 6 to 29 and days 1 to 29 for the high viral load, seronegative, and overall populations, tested in a statistical hierarchy (Supplementary Table 3). Clinical efficacy from days 6 to 29 was included as part of the hierarchical testing strategy because several days of viral suppression in this severe population may be required before clinical impact is observed. The high viral load population was selected for the first clinical efficacy endpoint in the hierarchy based on previous experience with treatment in the outpatient setting [11, 13].
Secondary efficacy endpoints examined all-cause mortality and hospital discharge/readmission. Safety endpoints included the proportion of patients with treatment-emergent serious adverse events (SAEs) and adverse events of special interest (AESIs): infusion-related reactions through day 4, and grade ≥ 2 hypersensitivity reactions through day 29.
Statistical Analysis
The statistical analysis plan was finalized prior to database lock and unblinding; all analyses were prespecified in the protocol and statistical analysis plan before database lock. The full analysis set (FAS) was used for safety analyses and includes all randomized patients who received any amount of study drug. The modified FAS (mFAS) was used for efficacy analyses and excludes patients with negative central laboratory SARS-CoV-2 quantitative reverse transcriptase polymerase chain reaction at baseline.
The primary virologic endpoint was analyzed using the analysis of covariance model; primary clinical endpoints were analyzed using either the exact method for binomial distribution or asymptotic normal approximation method, as predefined in the statistical analysis plan (also see Supplementary Material). Sample size for this adaptive study was estimated separately by phase, as detailed in the statistical analysis plan. However, because the trial was stopped earlier than planned (due to low enrollment prior to the surge associated with the Delta variant), the sample size was smaller than anticipated and it was elected to combine the CAS + IMD dose groups and pool patients on no supplemental oxygen (phase 2) and low-flow oxygen (phase 3) for efficacy measures to determine if the observed treatment effect exceeds the minimal significant effect in relative risk reduction (also see “Trial Adaptations” section in the Supplementary Material). The multiplicity adjustment approach, a hierarchical procedure, was used to control the overall type-1 error rate at 0.05 for the primary virologic and clinical outcome endpoints (Supplementary Table 3). If an endpoint in the hierarchy did not reach statistical significance the subsequent data were reported descriptively. Other analyses, including all-cause mortality, were reported descriptively.
Safety was assessed in separate analyses for patients receiving no supplemental oxygen (phase 2) and low-flow oxygen (phase 1/2/3). Prespecified subgroup analyses using baseline serostatus and viral load were selected based on previous results [13]. Sample size calculations and missing data handling are described in the Supplementary Methods.
RESULTS
Demographics and Baseline Characteristics
A total of 1364 patients on low-flow or no supplemental oxygen were randomized between 10 June 2020 and 9 April 2021; 1336 were treated. Of those, 1197 (89.6%) tested positive centrally for SARS-CoV-2 (constituting the mFAS) with 406, 398, and 393 in the CAS + IMD 2.4 g, 8.0 g, and placebo groups, respectively (Figure 1 and Supplementary Figure 2).

Flow diagram for the phase 2/3 population receiving low-flow or no supplemental oxygen (cohorts 1 and 1A). The flow diagram depicts patients randomized, treated, and discontinued for patients receiving either 2.4 or 8.0 g of CAS + IMD, or placebo. aThe FAS included all randomized patients who received at least 1 dose (full or partial) of the study drug. Analysis of the FAS population was done according to the treatment allocated (as randomized). The FAS was the same as the SAF for this study. bThe mFAS included all FAS patients with a positive SARS-CoV-2 RT-qPCR conducted in the central laboratory in nasopharyngeal swab samples at randomization, and analysis was based on the treatment allocated (as randomized). cThe seronegative mFAS was defined as all patients in mFAS with documented seronegative status at baseline. Abbreviations: EOS, end of study; FAS, full analysis set; IV, intravenous; mFAS, modified full analysis set; RT-qPCR, quantitative reverse transcription polymerase chain reaction; SAF, safety analysis set; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Baseline demographics were well balanced. The median age was 62 years, 54.1% were male, mean body mass index was 31.1 kg/m2, 12.1% identified as Black/African American, and 30.1% identified as Hispanic/Latino (Table 1). COVID-19 characteristics were similar except for a higher proportion of seropositive patients in the placebo (51.1%) versus the combined CAS + IMD group (45.9%; Table 1). Demographics and baseline characteristics by serostatus are presented in Supplementary Table 4.
| Characteristic . | Placebo (n = 393) . | CAS + IMD 2.4 g IV (n = 406) . | CAS + IMD 8.0 g IV (n = 398) . | CAS + IMD Combined Doses (n = 804) . | Total (N = 1197) . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median (range) | 64.0 (24–100) | 60.0 (20–97) | 62.0 (20–98) | 61.0 (20–98) | 62.0 (20–100) |
| ≥65 | 191 (48.6) | 164 (40.4) | 170 (42.7) | 334 (41.5) | 525 (43.9) |
| Male sex | 210 (53.4) | 221 (54.4) | 216 (54.3) | 437 (54.4) | 647 (54.1) |
| Race | |||||
| White | 239 (60.8) | 246 (60.6) | 264 (66.3) | 510 (63.4) | 749 (62.6) |
| Black or African American | 46 (11.7) | 57 (14.0) | 42 (10.6) | 99 (12.3) | 145 (12.1) |
| Asian | 16 (4.1) | 17 (4.2) | 14 (3.5) | 31 (3.9) | 47 (3.9) |
| American Indian or Alaska Native | 9 (2.3) | 9 (2.2) | 13 (3.3) | 22 (2.7) | 31 (2.6) |
| Native Hawaiian or Pacific Islander | 0 (0) | 1 (0.2) | 2 (0.5) | 3 (0.4) | 3 (0.3) |
| Unknown | 26 (6.6) | 28 (6.9) | 22 (5.5) | 50 (6.2) | 76 (6.3) |
| Not reported | 57 (14.5) | 48 (11.8) | 41 (10.3) | 89 (11.1) | 146 (12.2) |
| Ethnicity | |||||
| Hispanic or Latino | 115 (29.3) | 137 (33.7) | 108 (27.1) | 245 (30.5) | 360 (30.1) |
| Not Hispanic or Latino | 260 (66.2) | 251 (61.8) | 269 (67.6) | 520 (64.7) | 780 (65.2) |
| Not reported | 18 (4.6) | 18 (4.4) | 21 (5.3) | 39 (4.9) | 57 (4.8) |
| Mean weight, kg | 87.0 ± 23.4 | 89.0 ± 24.9 | 89.0 ± 24.6 | 89.0 ± 24.7 | 88.3 ± 24.3 |
| Body-mass indexb | |||||
| Mean | 30.8 ± 7.5 | 31.2 ± 7.9 | 31.2 ± 8.2 | 31.2 ± 8.1 | 31.1 ± 7.9 |
| ≥30 | 186 (47.3) | 192 (47.3) | 190 (47.7) | 382 (47.5) | 568 (47.5) |
| Median days COVID-19 illness prior to baseline (Q1–Q3) | 5.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) |
| Baseline viral load | |||||
| Median (Q1–Q3), log10 copies/mL | 6.3 (5.0–7.6) | 6.4 (5.1–7.6) | 6.5 (5.3–7.8) | 6.4 (5.1–7.7) | 6.4 (5.1–7.7) |
| >104 copies/mL | 356 (90.6) | 366 (90.1) | 359 (90.2) | 725 (90.2) | 1081 (90.3) |
| >106 copies/mL | 229 (58.3) | 231 (56.9) | 236 (59.3) | 467 (58.1) | 696 (58.1) |
| Baseline serology status | |||||
| Negative | 160 (40.7) | 172 (42.4) | 188 (47.2) | 360 (44.8) | 520 (43.4) |
| Positive | 201 (51.1) | 191 (47.0) | 178 (44.7) | 369 (45.9) | 570 (47.6) |
| Other, not determined, borderline | 32 (8.1) | 43 (10.6) | 32 (8.0) | 75 (9.3) | 107 (8.9) |
| Presence of neutralizing antibodies for seropositive patients, n/N (%) | |||||
| Positive | 140/201 (69.7) | 140/191 (73.3) | 129/178 (72.5) | 269/369 (72.9) | 409/570 (71.8) |
| Negative | 35/201 (17.4) | 30/191 (15.7) | 31/178 (17.4) | 61/369 (16.5) | 96/570 (16.8) |
| Borderline | 15/201 (7.5) | 10/191 (5.2) | 8/178 (4.5) | 18/369 (4.9) | 33/570 (5.8) |
| Unknown/missing/indeterminate | 11/201 (5.5) | 11/191 (5.8) | 10/178 (5.6) | 21/369 (5.7) | 32/570 (5.6) |
| Mean C-reactive protein, mg/L | 75.1 ± 68.6 | 73.9 ± 96.7 | 71.1 ± 84.5 | 72.5 ± 91.0 | 73.4 ± 84.3 |
| Mean neutrophil-lymphocyte ratio | 5.9 ± 5.7 | 2.3 ± 2.1 | 8.0 ± 4.3 | 5.6 ± 4.4 | 5.7 ± 4.9 |
| Concomitant medications | |||||
| Remdesivir | 220 (56.0) | 212 (52.2) | 225 (56.5) | 437 (54.4) | 657 (54.9) |
| Systemic corticosteroids | 294 (74.8) | 294 (72.4) | 307 (77.1) | 601 (74.8) | 895 (74.8) |
| Use of supplemental oxygen | 226 (57.5) | 223 (54.9) | 223 (56.0) | 446 (55.5) | 672 (56.1) |
| Noninvasive ventilation or high-flow oxygen devices | 1 (0.4) | 0 | 0 | 0 | 1 (0.1) |
| Supplemental oxygenc | 225 (99.6) | 223 (100) | 223 (100) | 446 (100) | 671 (99.9) |
| Immunocompromised | 85 (21.6) | 87 (21.4) | 85 (21.4) | 172 (21.4) | 257 (21.5) |
| Characteristic . | Placebo (n = 393) . | CAS + IMD 2.4 g IV (n = 406) . | CAS + IMD 8.0 g IV (n = 398) . | CAS + IMD Combined Doses (n = 804) . | Total (N = 1197) . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median (range) | 64.0 (24–100) | 60.0 (20–97) | 62.0 (20–98) | 61.0 (20–98) | 62.0 (20–100) |
| ≥65 | 191 (48.6) | 164 (40.4) | 170 (42.7) | 334 (41.5) | 525 (43.9) |
| Male sex | 210 (53.4) | 221 (54.4) | 216 (54.3) | 437 (54.4) | 647 (54.1) |
| Race | |||||
| White | 239 (60.8) | 246 (60.6) | 264 (66.3) | 510 (63.4) | 749 (62.6) |
| Black or African American | 46 (11.7) | 57 (14.0) | 42 (10.6) | 99 (12.3) | 145 (12.1) |
| Asian | 16 (4.1) | 17 (4.2) | 14 (3.5) | 31 (3.9) | 47 (3.9) |
| American Indian or Alaska Native | 9 (2.3) | 9 (2.2) | 13 (3.3) | 22 (2.7) | 31 (2.6) |
| Native Hawaiian or Pacific Islander | 0 (0) | 1 (0.2) | 2 (0.5) | 3 (0.4) | 3 (0.3) |
| Unknown | 26 (6.6) | 28 (6.9) | 22 (5.5) | 50 (6.2) | 76 (6.3) |
| Not reported | 57 (14.5) | 48 (11.8) | 41 (10.3) | 89 (11.1) | 146 (12.2) |
| Ethnicity | |||||
| Hispanic or Latino | 115 (29.3) | 137 (33.7) | 108 (27.1) | 245 (30.5) | 360 (30.1) |
| Not Hispanic or Latino | 260 (66.2) | 251 (61.8) | 269 (67.6) | 520 (64.7) | 780 (65.2) |
| Not reported | 18 (4.6) | 18 (4.4) | 21 (5.3) | 39 (4.9) | 57 (4.8) |
| Mean weight, kg | 87.0 ± 23.4 | 89.0 ± 24.9 | 89.0 ± 24.6 | 89.0 ± 24.7 | 88.3 ± 24.3 |
| Body-mass indexb | |||||
| Mean | 30.8 ± 7.5 | 31.2 ± 7.9 | 31.2 ± 8.2 | 31.2 ± 8.1 | 31.1 ± 7.9 |
| ≥30 | 186 (47.3) | 192 (47.3) | 190 (47.7) | 382 (47.5) | 568 (47.5) |
| Median days COVID-19 illness prior to baseline (Q1–Q3) | 5.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) |
| Baseline viral load | |||||
| Median (Q1–Q3), log10 copies/mL | 6.3 (5.0–7.6) | 6.4 (5.1–7.6) | 6.5 (5.3–7.8) | 6.4 (5.1–7.7) | 6.4 (5.1–7.7) |
| >104 copies/mL | 356 (90.6) | 366 (90.1) | 359 (90.2) | 725 (90.2) | 1081 (90.3) |
| >106 copies/mL | 229 (58.3) | 231 (56.9) | 236 (59.3) | 467 (58.1) | 696 (58.1) |
| Baseline serology status | |||||
| Negative | 160 (40.7) | 172 (42.4) | 188 (47.2) | 360 (44.8) | 520 (43.4) |
| Positive | 201 (51.1) | 191 (47.0) | 178 (44.7) | 369 (45.9) | 570 (47.6) |
| Other, not determined, borderline | 32 (8.1) | 43 (10.6) | 32 (8.0) | 75 (9.3) | 107 (8.9) |
| Presence of neutralizing antibodies for seropositive patients, n/N (%) | |||||
| Positive | 140/201 (69.7) | 140/191 (73.3) | 129/178 (72.5) | 269/369 (72.9) | 409/570 (71.8) |
| Negative | 35/201 (17.4) | 30/191 (15.7) | 31/178 (17.4) | 61/369 (16.5) | 96/570 (16.8) |
| Borderline | 15/201 (7.5) | 10/191 (5.2) | 8/178 (4.5) | 18/369 (4.9) | 33/570 (5.8) |
| Unknown/missing/indeterminate | 11/201 (5.5) | 11/191 (5.8) | 10/178 (5.6) | 21/369 (5.7) | 32/570 (5.6) |
| Mean C-reactive protein, mg/L | 75.1 ± 68.6 | 73.9 ± 96.7 | 71.1 ± 84.5 | 72.5 ± 91.0 | 73.4 ± 84.3 |
| Mean neutrophil-lymphocyte ratio | 5.9 ± 5.7 | 2.3 ± 2.1 | 8.0 ± 4.3 | 5.6 ± 4.4 | 5.7 ± 4.9 |
| Concomitant medications | |||||
| Remdesivir | 220 (56.0) | 212 (52.2) | 225 (56.5) | 437 (54.4) | 657 (54.9) |
| Systemic corticosteroids | 294 (74.8) | 294 (72.4) | 307 (77.1) | 601 (74.8) | 895 (74.8) |
| Use of supplemental oxygen | 226 (57.5) | 223 (54.9) | 223 (56.0) | 446 (55.5) | 672 (56.1) |
| Noninvasive ventilation or high-flow oxygen devices | 1 (0.4) | 0 | 0 | 0 | 1 (0.1) |
| Supplemental oxygenc | 225 (99.6) | 223 (100) | 223 (100) | 446 (100) | 671 (99.9) |
| Immunocompromised | 85 (21.6) | 87 (21.4) | 85 (21.4) | 172 (21.4) | 257 (21.5) |
Data are n (%), mean ± SD, or median (range).
Abbreviations: CAS + IMD, casirivimab and imdevimab; COVID-19, coronavirus disease 2019; IV, intravenous.
Modified full analysis set presented for pooled phase 3 cohort 1 and phase 2 cohort 1A.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Not requiring high-flow oxygen devices.
| Characteristic . | Placebo (n = 393) . | CAS + IMD 2.4 g IV (n = 406) . | CAS + IMD 8.0 g IV (n = 398) . | CAS + IMD Combined Doses (n = 804) . | Total (N = 1197) . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median (range) | 64.0 (24–100) | 60.0 (20–97) | 62.0 (20–98) | 61.0 (20–98) | 62.0 (20–100) |
| ≥65 | 191 (48.6) | 164 (40.4) | 170 (42.7) | 334 (41.5) | 525 (43.9) |
| Male sex | 210 (53.4) | 221 (54.4) | 216 (54.3) | 437 (54.4) | 647 (54.1) |
| Race | |||||
| White | 239 (60.8) | 246 (60.6) | 264 (66.3) | 510 (63.4) | 749 (62.6) |
| Black or African American | 46 (11.7) | 57 (14.0) | 42 (10.6) | 99 (12.3) | 145 (12.1) |
| Asian | 16 (4.1) | 17 (4.2) | 14 (3.5) | 31 (3.9) | 47 (3.9) |
| American Indian or Alaska Native | 9 (2.3) | 9 (2.2) | 13 (3.3) | 22 (2.7) | 31 (2.6) |
| Native Hawaiian or Pacific Islander | 0 (0) | 1 (0.2) | 2 (0.5) | 3 (0.4) | 3 (0.3) |
| Unknown | 26 (6.6) | 28 (6.9) | 22 (5.5) | 50 (6.2) | 76 (6.3) |
| Not reported | 57 (14.5) | 48 (11.8) | 41 (10.3) | 89 (11.1) | 146 (12.2) |
| Ethnicity | |||||
| Hispanic or Latino | 115 (29.3) | 137 (33.7) | 108 (27.1) | 245 (30.5) | 360 (30.1) |
| Not Hispanic or Latino | 260 (66.2) | 251 (61.8) | 269 (67.6) | 520 (64.7) | 780 (65.2) |
| Not reported | 18 (4.6) | 18 (4.4) | 21 (5.3) | 39 (4.9) | 57 (4.8) |
| Mean weight, kg | 87.0 ± 23.4 | 89.0 ± 24.9 | 89.0 ± 24.6 | 89.0 ± 24.7 | 88.3 ± 24.3 |
| Body-mass indexb | |||||
| Mean | 30.8 ± 7.5 | 31.2 ± 7.9 | 31.2 ± 8.2 | 31.2 ± 8.1 | 31.1 ± 7.9 |
| ≥30 | 186 (47.3) | 192 (47.3) | 190 (47.7) | 382 (47.5) | 568 (47.5) |
| Median days COVID-19 illness prior to baseline (Q1–Q3) | 5.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) |
| Baseline viral load | |||||
| Median (Q1–Q3), log10 copies/mL | 6.3 (5.0–7.6) | 6.4 (5.1–7.6) | 6.5 (5.3–7.8) | 6.4 (5.1–7.7) | 6.4 (5.1–7.7) |
| >104 copies/mL | 356 (90.6) | 366 (90.1) | 359 (90.2) | 725 (90.2) | 1081 (90.3) |
| >106 copies/mL | 229 (58.3) | 231 (56.9) | 236 (59.3) | 467 (58.1) | 696 (58.1) |
| Baseline serology status | |||||
| Negative | 160 (40.7) | 172 (42.4) | 188 (47.2) | 360 (44.8) | 520 (43.4) |
| Positive | 201 (51.1) | 191 (47.0) | 178 (44.7) | 369 (45.9) | 570 (47.6) |
| Other, not determined, borderline | 32 (8.1) | 43 (10.6) | 32 (8.0) | 75 (9.3) | 107 (8.9) |
| Presence of neutralizing antibodies for seropositive patients, n/N (%) | |||||
| Positive | 140/201 (69.7) | 140/191 (73.3) | 129/178 (72.5) | 269/369 (72.9) | 409/570 (71.8) |
| Negative | 35/201 (17.4) | 30/191 (15.7) | 31/178 (17.4) | 61/369 (16.5) | 96/570 (16.8) |
| Borderline | 15/201 (7.5) | 10/191 (5.2) | 8/178 (4.5) | 18/369 (4.9) | 33/570 (5.8) |
| Unknown/missing/indeterminate | 11/201 (5.5) | 11/191 (5.8) | 10/178 (5.6) | 21/369 (5.7) | 32/570 (5.6) |
| Mean C-reactive protein, mg/L | 75.1 ± 68.6 | 73.9 ± 96.7 | 71.1 ± 84.5 | 72.5 ± 91.0 | 73.4 ± 84.3 |
| Mean neutrophil-lymphocyte ratio | 5.9 ± 5.7 | 2.3 ± 2.1 | 8.0 ± 4.3 | 5.6 ± 4.4 | 5.7 ± 4.9 |
| Concomitant medications | |||||
| Remdesivir | 220 (56.0) | 212 (52.2) | 225 (56.5) | 437 (54.4) | 657 (54.9) |
| Systemic corticosteroids | 294 (74.8) | 294 (72.4) | 307 (77.1) | 601 (74.8) | 895 (74.8) |
| Use of supplemental oxygen | 226 (57.5) | 223 (54.9) | 223 (56.0) | 446 (55.5) | 672 (56.1) |
| Noninvasive ventilation or high-flow oxygen devices | 1 (0.4) | 0 | 0 | 0 | 1 (0.1) |
| Supplemental oxygenc | 225 (99.6) | 223 (100) | 223 (100) | 446 (100) | 671 (99.9) |
| Immunocompromised | 85 (21.6) | 87 (21.4) | 85 (21.4) | 172 (21.4) | 257 (21.5) |
| Characteristic . | Placebo (n = 393) . | CAS + IMD 2.4 g IV (n = 406) . | CAS + IMD 8.0 g IV (n = 398) . | CAS + IMD Combined Doses (n = 804) . | Total (N = 1197) . |
|---|---|---|---|---|---|
| Age, y | |||||
| Median (range) | 64.0 (24–100) | 60.0 (20–97) | 62.0 (20–98) | 61.0 (20–98) | 62.0 (20–100) |
| ≥65 | 191 (48.6) | 164 (40.4) | 170 (42.7) | 334 (41.5) | 525 (43.9) |
| Male sex | 210 (53.4) | 221 (54.4) | 216 (54.3) | 437 (54.4) | 647 (54.1) |
| Race | |||||
| White | 239 (60.8) | 246 (60.6) | 264 (66.3) | 510 (63.4) | 749 (62.6) |
| Black or African American | 46 (11.7) | 57 (14.0) | 42 (10.6) | 99 (12.3) | 145 (12.1) |
| Asian | 16 (4.1) | 17 (4.2) | 14 (3.5) | 31 (3.9) | 47 (3.9) |
| American Indian or Alaska Native | 9 (2.3) | 9 (2.2) | 13 (3.3) | 22 (2.7) | 31 (2.6) |
| Native Hawaiian or Pacific Islander | 0 (0) | 1 (0.2) | 2 (0.5) | 3 (0.4) | 3 (0.3) |
| Unknown | 26 (6.6) | 28 (6.9) | 22 (5.5) | 50 (6.2) | 76 (6.3) |
| Not reported | 57 (14.5) | 48 (11.8) | 41 (10.3) | 89 (11.1) | 146 (12.2) |
| Ethnicity | |||||
| Hispanic or Latino | 115 (29.3) | 137 (33.7) | 108 (27.1) | 245 (30.5) | 360 (30.1) |
| Not Hispanic or Latino | 260 (66.2) | 251 (61.8) | 269 (67.6) | 520 (64.7) | 780 (65.2) |
| Not reported | 18 (4.6) | 18 (4.4) | 21 (5.3) | 39 (4.9) | 57 (4.8) |
| Mean weight, kg | 87.0 ± 23.4 | 89.0 ± 24.9 | 89.0 ± 24.6 | 89.0 ± 24.7 | 88.3 ± 24.3 |
| Body-mass indexb | |||||
| Mean | 30.8 ± 7.5 | 31.2 ± 7.9 | 31.2 ± 8.2 | 31.2 ± 8.1 | 31.1 ± 7.9 |
| ≥30 | 186 (47.3) | 192 (47.3) | 190 (47.7) | 382 (47.5) | 568 (47.5) |
| Median days COVID-19 illness prior to baseline (Q1–Q3) | 5.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) |
| Baseline viral load | |||||
| Median (Q1–Q3), log10 copies/mL | 6.3 (5.0–7.6) | 6.4 (5.1–7.6) | 6.5 (5.3–7.8) | 6.4 (5.1–7.7) | 6.4 (5.1–7.7) |
| >104 copies/mL | 356 (90.6) | 366 (90.1) | 359 (90.2) | 725 (90.2) | 1081 (90.3) |
| >106 copies/mL | 229 (58.3) | 231 (56.9) | 236 (59.3) | 467 (58.1) | 696 (58.1) |
| Baseline serology status | |||||
| Negative | 160 (40.7) | 172 (42.4) | 188 (47.2) | 360 (44.8) | 520 (43.4) |
| Positive | 201 (51.1) | 191 (47.0) | 178 (44.7) | 369 (45.9) | 570 (47.6) |
| Other, not determined, borderline | 32 (8.1) | 43 (10.6) | 32 (8.0) | 75 (9.3) | 107 (8.9) |
| Presence of neutralizing antibodies for seropositive patients, n/N (%) | |||||
| Positive | 140/201 (69.7) | 140/191 (73.3) | 129/178 (72.5) | 269/369 (72.9) | 409/570 (71.8) |
| Negative | 35/201 (17.4) | 30/191 (15.7) | 31/178 (17.4) | 61/369 (16.5) | 96/570 (16.8) |
| Borderline | 15/201 (7.5) | 10/191 (5.2) | 8/178 (4.5) | 18/369 (4.9) | 33/570 (5.8) |
| Unknown/missing/indeterminate | 11/201 (5.5) | 11/191 (5.8) | 10/178 (5.6) | 21/369 (5.7) | 32/570 (5.6) |
| Mean C-reactive protein, mg/L | 75.1 ± 68.6 | 73.9 ± 96.7 | 71.1 ± 84.5 | 72.5 ± 91.0 | 73.4 ± 84.3 |
| Mean neutrophil-lymphocyte ratio | 5.9 ± 5.7 | 2.3 ± 2.1 | 8.0 ± 4.3 | 5.6 ± 4.4 | 5.7 ± 4.9 |
| Concomitant medications | |||||
| Remdesivir | 220 (56.0) | 212 (52.2) | 225 (56.5) | 437 (54.4) | 657 (54.9) |
| Systemic corticosteroids | 294 (74.8) | 294 (72.4) | 307 (77.1) | 601 (74.8) | 895 (74.8) |
| Use of supplemental oxygen | 226 (57.5) | 223 (54.9) | 223 (56.0) | 446 (55.5) | 672 (56.1) |
| Noninvasive ventilation or high-flow oxygen devices | 1 (0.4) | 0 | 0 | 0 | 1 (0.1) |
| Supplemental oxygenc | 225 (99.6) | 223 (100) | 223 (100) | 446 (100) | 671 (99.9) |
| Immunocompromised | 85 (21.6) | 87 (21.4) | 85 (21.4) | 172 (21.4) | 257 (21.5) |
Data are n (%), mean ± SD, or median (range).
Abbreviations: CAS + IMD, casirivimab and imdevimab; COVID-19, coronavirus disease 2019; IV, intravenous.
Modified full analysis set presented for pooled phase 3 cohort 1 and phase 2 cohort 1A.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Not requiring high-flow oxygen devices.
Virologic Efficacy
CAS + IMD significantly reduced viral load in seronegative patients on low-flow or no supplemental oxygen; the least-squares mean (95% confidence interval [CI]) time-weighted average daily change in viral load from baseline through day 7 was −1.03 log10 copies/mL (95% CI, −1.22 to −.84) in the placebo group versus −1.31 log10 copies/mL (95% CI, −1.43 to −1.18) in the CAS + IMD group, with an least-squares mean difference versus placebo of −0.28 log10 copies/mL (95% CI, −.51 to −.05; P = .0172; Table 2).
| Hierarchy . | Endpoint . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|---|
| Primary virologic outcomea,b | |||||
| 1. | Time-weighted average change in viral load from baseline (day 1) to day 7 in seronegative mFAS | ||||
| Patients, n | 131 | 150 | 160 | 310 | |
| LS mean change (SE), log10 copies/mL | —1.03 (0.10) | 1.28 (0.09) | 1.34 (0.09) | 1.31 (0.06) | |
| 95% CI | 1.22 to .84 | 1.46 to 1.10 | 1.51 to 1.16 | 1.43 to 1.18 | |
| Difference versus placebo at day 7, log10 copies/mL | |||||
| LS mean (SE) | … | 0.25 (0.13) | 0.31 (0.13) | 0.28 (0.12) | |
| 95% CI | … | .51 to .02 | .57 to .05 | .51 to .05 | |
| P value | … | .0663 | .0204 | .0172 | |
| Primary clinical outcomea,c,d | |||||
| 2. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 28/211 (13.3) | 16/220 (7.3) | 28/225 (12.4) | 44/445 (9.9) | |
| Relative risk reduction, % | … | 45.2 | 6.2 | 25.5 | |
| 95% CI, % | … | 1.7 to 69.5 | −52.9 to 42.5 | −16.2 to 52.2 | |
| P value | … | .0431 | .7975 | .2048 | |
| 3. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 22/147 (15.0) | 8/162 (4.9) | 19/179 (10.6) | 27/341 (7.9) | |
| Relative risk reduction, % | … | 67.0 | 29.1 | 47.1 | |
| 95% CI, % | … | 28.2 to 84.8 | −25.9 to 60.0 | 10.2 to 68.8 | |
| 4. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in overall mFAS | ||||
| N/total n (%) | 39/367 (10.6) | 21/387 (5.4) | 41/383 (10.7) | 62/770 (8.1) | |
| Relative risk reduction, % | … | 48.9 | −0.7 | 24.2 | |
| 95% CI, % | … | 14.9 to 69.4 | −52.5 to 33.4 | −10.9 to 48.2 | |
| 5. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 43/229 (18.8) | 23/231 (10.0) | 34/236 (14.4) | 57/467 (12.2) | |
| Relative risk reduction, % | … | 47.0 | 23.3 | 35.0 | |
| 95% CI, % | … | 15.0 to 66.9 | −15.8 to 49.2 | 6.6–54.8 | |
| 6. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 31/160 (19.4) | 14/172 (8.1) | 23/188 (12.2) | 37/360 (10.3) | |
| Relative risk reduction, % | … | 58.0 | 36.9 | 47.0 | |
| 95% CI, % | … | 24.0 to 76.8 | −3.7 to 61.6 | 17.7 to 65.8 | |
| 7. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in overall mFAS | ||||
| N/total n (%) | 58/393 (14.8) | 32/406 (7.9) | 50/398 (12.6) | 82/804 (10.2) | |
| Relative risk reduction, % | … | 46.6 | 14.9 | 30.9 | |
| 95% CI, % | … | 19.6 to 64.5 | −21.0 to 40.1 | 5.4 to 49.5 | |
| Hierarchy . | Endpoint . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|---|
| Primary virologic outcomea,b | |||||
| 1. | Time-weighted average change in viral load from baseline (day 1) to day 7 in seronegative mFAS | ||||
| Patients, n | 131 | 150 | 160 | 310 | |
| LS mean change (SE), log10 copies/mL | —1.03 (0.10) | 1.28 (0.09) | 1.34 (0.09) | 1.31 (0.06) | |
| 95% CI | 1.22 to .84 | 1.46 to 1.10 | 1.51 to 1.16 | 1.43 to 1.18 | |
| Difference versus placebo at day 7, log10 copies/mL | |||||
| LS mean (SE) | … | 0.25 (0.13) | 0.31 (0.13) | 0.28 (0.12) | |
| 95% CI | … | .51 to .02 | .57 to .05 | .51 to .05 | |
| P value | … | .0663 | .0204 | .0172 | |
| Primary clinical outcomea,c,d | |||||
| 2. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 28/211 (13.3) | 16/220 (7.3) | 28/225 (12.4) | 44/445 (9.9) | |
| Relative risk reduction, % | … | 45.2 | 6.2 | 25.5 | |
| 95% CI, % | … | 1.7 to 69.5 | −52.9 to 42.5 | −16.2 to 52.2 | |
| P value | … | .0431 | .7975 | .2048 | |
| 3. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 22/147 (15.0) | 8/162 (4.9) | 19/179 (10.6) | 27/341 (7.9) | |
| Relative risk reduction, % | … | 67.0 | 29.1 | 47.1 | |
| 95% CI, % | … | 28.2 to 84.8 | −25.9 to 60.0 | 10.2 to 68.8 | |
| 4. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in overall mFAS | ||||
| N/total n (%) | 39/367 (10.6) | 21/387 (5.4) | 41/383 (10.7) | 62/770 (8.1) | |
| Relative risk reduction, % | … | 48.9 | −0.7 | 24.2 | |
| 95% CI, % | … | 14.9 to 69.4 | −52.5 to 33.4 | −10.9 to 48.2 | |
| 5. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 43/229 (18.8) | 23/231 (10.0) | 34/236 (14.4) | 57/467 (12.2) | |
| Relative risk reduction, % | … | 47.0 | 23.3 | 35.0 | |
| 95% CI, % | … | 15.0 to 66.9 | −15.8 to 49.2 | 6.6–54.8 | |
| 6. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 31/160 (19.4) | 14/172 (8.1) | 23/188 (12.2) | 37/360 (10.3) | |
| Relative risk reduction, % | … | 58.0 | 36.9 | 47.0 | |
| 95% CI, % | … | 24.0 to 76.8 | −3.7 to 61.6 | 17.7 to 65.8 | |
| 7. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in overall mFAS | ||||
| N/total n (%) | 58/393 (14.8) | 32/406 (7.9) | 50/398 (12.6) | 82/804 (10.2) | |
| Relative risk reduction, % | … | 46.6 | 14.9 | 30.9 | |
| 95% CI, % | … | 19.6 to 64.5 | −21.0 to 40.1 | 5.4 to 49.5 | |
Abbreviations: CAS + IMD, casirivimab and imdevimab; CI, confidence interval; IV, intravenous; LS, least-squares; mFAS, modified full analysis set.
Pooled phase 3 cohort 1 and phase 2 cohort 1A.
LS mean, 95% CI, and P value for change from baseline on log scale for each treatment group is based on the analysis of covariance model with treatment group and the type of background standard of care (antiviral therapies and non-antiviral therapies) as fixed effects, and baseline viral load and treatment baseline as covariates. Negative changes imply improvement in viral load.
The 95% CI for the relative risk and relative risk reduction (1 − relative risk) uses the Farrington-Manning method.
P value is derived from the Cochran-Mantel-Haenszel test stratified by the type of background standard of care (antiviral therapies and non-antiviral therapies). If np ≤ 5 or n (1 − p) ≤ 5 in any treatment group, P value is based on Fisher exact test.
| Hierarchy . | Endpoint . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|---|
| Primary virologic outcomea,b | |||||
| 1. | Time-weighted average change in viral load from baseline (day 1) to day 7 in seronegative mFAS | ||||
| Patients, n | 131 | 150 | 160 | 310 | |
| LS mean change (SE), log10 copies/mL | —1.03 (0.10) | 1.28 (0.09) | 1.34 (0.09) | 1.31 (0.06) | |
| 95% CI | 1.22 to .84 | 1.46 to 1.10 | 1.51 to 1.16 | 1.43 to 1.18 | |
| Difference versus placebo at day 7, log10 copies/mL | |||||
| LS mean (SE) | … | 0.25 (0.13) | 0.31 (0.13) | 0.28 (0.12) | |
| 95% CI | … | .51 to .02 | .57 to .05 | .51 to .05 | |
| P value | … | .0663 | .0204 | .0172 | |
| Primary clinical outcomea,c,d | |||||
| 2. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 28/211 (13.3) | 16/220 (7.3) | 28/225 (12.4) | 44/445 (9.9) | |
| Relative risk reduction, % | … | 45.2 | 6.2 | 25.5 | |
| 95% CI, % | … | 1.7 to 69.5 | −52.9 to 42.5 | −16.2 to 52.2 | |
| P value | … | .0431 | .7975 | .2048 | |
| 3. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 22/147 (15.0) | 8/162 (4.9) | 19/179 (10.6) | 27/341 (7.9) | |
| Relative risk reduction, % | … | 67.0 | 29.1 | 47.1 | |
| 95% CI, % | … | 28.2 to 84.8 | −25.9 to 60.0 | 10.2 to 68.8 | |
| 4. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in overall mFAS | ||||
| N/total n (%) | 39/367 (10.6) | 21/387 (5.4) | 41/383 (10.7) | 62/770 (8.1) | |
| Relative risk reduction, % | … | 48.9 | −0.7 | 24.2 | |
| 95% CI, % | … | 14.9 to 69.4 | −52.5 to 33.4 | −10.9 to 48.2 | |
| 5. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 43/229 (18.8) | 23/231 (10.0) | 34/236 (14.4) | 57/467 (12.2) | |
| Relative risk reduction, % | … | 47.0 | 23.3 | 35.0 | |
| 95% CI, % | … | 15.0 to 66.9 | −15.8 to 49.2 | 6.6–54.8 | |
| 6. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 31/160 (19.4) | 14/172 (8.1) | 23/188 (12.2) | 37/360 (10.3) | |
| Relative risk reduction, % | … | 58.0 | 36.9 | 47.0 | |
| 95% CI, % | … | 24.0 to 76.8 | −3.7 to 61.6 | 17.7 to 65.8 | |
| 7. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in overall mFAS | ||||
| N/total n (%) | 58/393 (14.8) | 32/406 (7.9) | 50/398 (12.6) | 82/804 (10.2) | |
| Relative risk reduction, % | … | 46.6 | 14.9 | 30.9 | |
| 95% CI, % | … | 19.6 to 64.5 | −21.0 to 40.1 | 5.4 to 49.5 | |
| Hierarchy . | Endpoint . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|---|
| Primary virologic outcomea,b | |||||
| 1. | Time-weighted average change in viral load from baseline (day 1) to day 7 in seronegative mFAS | ||||
| Patients, n | 131 | 150 | 160 | 310 | |
| LS mean change (SE), log10 copies/mL | —1.03 (0.10) | 1.28 (0.09) | 1.34 (0.09) | 1.31 (0.06) | |
| 95% CI | 1.22 to .84 | 1.46 to 1.10 | 1.51 to 1.16 | 1.43 to 1.18 | |
| Difference versus placebo at day 7, log10 copies/mL | |||||
| LS mean (SE) | … | 0.25 (0.13) | 0.31 (0.13) | 0.28 (0.12) | |
| 95% CI | … | .51 to .02 | .57 to .05 | .51 to .05 | |
| P value | … | .0663 | .0204 | .0172 | |
| Primary clinical outcomea,c,d | |||||
| 2. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 28/211 (13.3) | 16/220 (7.3) | 28/225 (12.4) | 44/445 (9.9) | |
| Relative risk reduction, % | … | 45.2 | 6.2 | 25.5 | |
| 95% CI, % | … | 1.7 to 69.5 | −52.9 to 42.5 | −16.2 to 52.2 | |
| P value | … | .0431 | .7975 | .2048 | |
| 3. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 22/147 (15.0) | 8/162 (4.9) | 19/179 (10.6) | 27/341 (7.9) | |
| Relative risk reduction, % | … | 67.0 | 29.1 | 47.1 | |
| 95% CI, % | … | 28.2 to 84.8 | −25.9 to 60.0 | 10.2 to 68.8 | |
| 4. | Proportion of patients who died or went on mechanical ventilation from day 6 to day 29 in overall mFAS | ||||
| N/total n (%) | 39/367 (10.6) | 21/387 (5.4) | 41/383 (10.7) | 62/770 (8.1) | |
| Relative risk reduction, % | … | 48.9 | −0.7 | 24.2 | |
| 95% CI, % | … | 14.9 to 69.4 | −52.5 to 33.4 | −10.9 to 48.2 | |
| 5. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in high viral load mFAS | ||||
| N/total n (%) | 43/229 (18.8) | 23/231 (10.0) | 34/236 (14.4) | 57/467 (12.2) | |
| Relative risk reduction, % | … | 47.0 | 23.3 | 35.0 | |
| 95% CI, % | … | 15.0 to 66.9 | −15.8 to 49.2 | 6.6–54.8 | |
| 6. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in seronegative mFAS | ||||
| N/total n (%) | 31/160 (19.4) | 14/172 (8.1) | 23/188 (12.2) | 37/360 (10.3) | |
| Relative risk reduction, % | … | 58.0 | 36.9 | 47.0 | |
| 95% CI, % | … | 24.0 to 76.8 | −3.7 to 61.6 | 17.7 to 65.8 | |
| 7. | Proportion of patients who died or went on mechanical ventilation from day 1 to day 29 in overall mFAS | ||||
| N/total n (%) | 58/393 (14.8) | 32/406 (7.9) | 50/398 (12.6) | 82/804 (10.2) | |
| Relative risk reduction, % | … | 46.6 | 14.9 | 30.9 | |
| 95% CI, % | … | 19.6 to 64.5 | −21.0 to 40.1 | 5.4 to 49.5 | |
Abbreviations: CAS + IMD, casirivimab and imdevimab; CI, confidence interval; IV, intravenous; LS, least-squares; mFAS, modified full analysis set.
Pooled phase 3 cohort 1 and phase 2 cohort 1A.
LS mean, 95% CI, and P value for change from baseline on log scale for each treatment group is based on the analysis of covariance model with treatment group and the type of background standard of care (antiviral therapies and non-antiviral therapies) as fixed effects, and baseline viral load and treatment baseline as covariates. Negative changes imply improvement in viral load.
The 95% CI for the relative risk and relative risk reduction (1 − relative risk) uses the Farrington-Manning method.
P value is derived from the Cochran-Mantel-Haenszel test stratified by the type of background standard of care (antiviral therapies and non-antiviral therapies). If np ≤ 5 or n (1 − p) ≤ 5 in any treatment group, P value is based on Fisher exact test.
Both doses of CAS + IMD exhibited similar viral load reductions, showing improvement over placebo starting at day 3 and reaching significance at day 7, after which viral load in the CAS + IMD groups continued to fall relative to placebo (Figure 2 and Supplementary Figure 3). The overall population least-squares mean fell below the lower limit of quantification (2.85 log10 copies/mL) 2 days earlier with CAS + IMD (day 9) versus placebo (day 11) (Supplementary Figure 3). Reductions of viral load were observed in all populations (Figure 2 and Supplementary Figure 3), with greater reductions in seronegative patients.
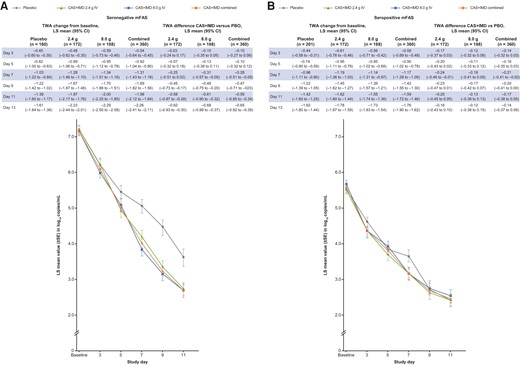
Viral load by serostatus. A, Graph shows LS mean viral load following administration of CAS + IMD (2.4 g, 8.0 g, or combined analysis of 2.4 and 8.0 g) or placebo for patients who tested negative for all SARS-CoV-2 antibodies at baseline (seronegative). B, The same but for patients who tested positive for any SARS-CoV-2 antibody at baseline (seropositive). A and B, Lower limit of quantification was 2.85 log10 copies/mL. Corresponding tables list values for time-weighted average change from baseline and difference for CAS+IMD versus placebo. Abbreviations: CAS + IMD, casirivimab and imdevimab; CI, confidence interval; IV, intravenous; mFAS, modified full analysis set; LS, least-squares; PBO, placebo; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SE, standard error; TWA, time-weighted average.
Clinical Efficacy
Death or Mechanical Ventilation
Death or mechanical ventilation was examined from days 1 to 29 and days 6 to 29, and evaluated in the seronegative, high-viral load, and overall populations using a statistical hierarchy. While the analyses presented herein examine the pooled CAS + IMD dose group and pooled cohorts for low-flow and no supplemental oxygen (Figure 3 and Figure 4), individual dose groups of 2.4 g and 8.0 g of CAS + IMD (Supplementary Figure 4) and separate cohorts by respiratory status (Supplementary Figure 5) also showed trends of benefit in seronegative patients across all clinical endpoints.
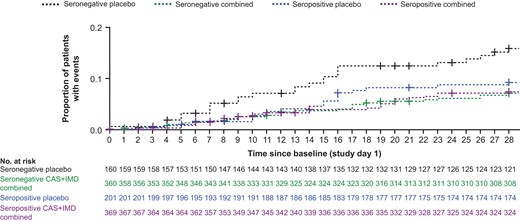
Mortality outcomes by serostatus for combined dose CAS + IMD from day 1 though day 29. The Kaplan-Meier curve shows the proportion of patients who died through study day 29, after administration of CAS + IMD (combined analysis of 2.4 g or 8.0 g) or placebo. Results were analyzed separately for patients who were seronegative or seropositive at baseline; + indicates censoring. Abbreviations: CAS + IMD, casirivimab and imdevimab.
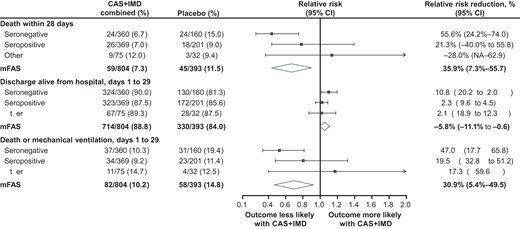
Efficacy outcomes by serostatus for combined dose CAS + IMD from day 1 though day 29. Forest plot shows relative risk and relative risk reduction with 95% CIs for CAS + IMD combined dose analysis (2.4 g and 8.0 g) versus placebo. Parameters examined included death within 28 days, discharge alive from hospital from days 1 to 29, and death or mechanical ventilation from days 1 to 29. For all populations, the mFAS comprised patients who tested positive for SARS-CoV-2 at baseline. Populations analyzed included patients who tested negative for all SARS-CoV-2 antibodies at baseline (seronegative mFAS), patients who tested positive for any SARS-CoV-2 antibody at baseline (seropositive mFAS), those with borderline, inconclusive, or missing baseline serology (other), and the overall population regardless of serostatus (overall mFAS). For the proportion of death within 28 days and the proportion of death or mechanical ventilation with 28 days, the lower bounds of the CI of the relative risk reduction were –342.0% and –241.0%, respectively (NA in the figure). Abbreviations: CAS + IMD, casirivimab and imdevimab; CI, confidence interval; mFAS, modified full analysis set; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In the statistical hierarchy (Supplementary Table 3), the first test for clinical efficacy on the endpoint of death or mechanical ventilation in the high viral load population from days 6 to 29 showed a numerically lower risk versus placebo but did not reach statistical significance (relative risk reduction [RRR], 25.5%; 95% CI, −16.2% to 52.2%; P = .2048; Table 2); accordingly, all subsequent clinical efficacy analyses are considered descriptive. The endpoint of death or mechanical ventilation in the seronegative population from days 6 to 29 showed an RRR of 47.1% (95% CI, 10.2%–68.8%; Table 2); similar trends of improvement were also observed in the overall population (RRR, 24.2%; 95% CI, −10.9% to 48.2%; Table 2).
Treatment with CAS + IMD showed a trend in reduction in the proportions of patients who died or required mechanical ventilation, with improvement from days 1 to 29 in the high viral load (RRR, 35.0%; 95% CI, 6.6%–54.8%), seronegative (RRR, 47.0%; 95% CI, 17.7%–65.8%), and overall (RRR, 30.9%; 95% CI, 5.4%–49.5%) populations (Table 2). While seronegative patients exhibited the greatest benefit from CAS + IMD treatment, no meaningful benefit or harm was observed in seropositive patients (RRR, 19.5%; 95% CI, −32.8% to 51.2%; Figure 4).
All-Cause Mortality
Treatment with CAS + IMD led to numeric improvement in all-cause mortality through day 29 in the seronegative, high-viral load, and overall populations in a pooled analysis of patients on low-flow or no supplemental oxygen receiving 2.4 g or 8.0 g CAS + IMD versus placebo. The greatest reduction in the relative risk of death occurred in seronegative patients; 24/360 (6.7%) died within 28 days in the CAS + IMD group versus 24/160 (15.0%) in the placebo group (RRR, 55.6%; 95% CI, 24.2%–74.0%; Figure 4). No harm or meaningful benefit was observed in the seropositive population (Figure 3). For the overall population, driven by the seronegative group, a numerical reduction in death was observed; 59/804 patients (7.3%) died within 28 days in the CAS + IMD combined dose group versus 45/393 patients (11.5%) in the placebo group (RRR, 35.9%; 95% CI, 7.3%–55.7%; Figure 4). The improvement with CAS + IMD persisted through study day 57 (Supplementary Figure 6).
Similar benefits with CAS + IMD treatment were also observed in hospital discharge (Figure 4 and Supplementary Table 5) and readmission (Supplementary Table 6); see Supplementary Results.
Safety
SAEs were experienced by more patients in the placebo group than the CAS + IMD group for patients on low-flow oxygen (131/469 [27.9%] placebo versus 224/941 [23.8%] CAS + IMD) and no supplemental oxygen (43/198 [21.7%] placebo versus 61/399 [15.3%] CAS + IMD; Table 3). More patients experienced treatment-emergent adverse events that resulted in death in the placebo group versus CAS + IMD for patients on low-flow oxygen (72/469 [15.4%] placebo versus 108/941 [11.5%] CAS + IMD; Supplementary Table 7) and no supplemental oxygen (15/198 [7.6%] placebo versus 15/399 [3.8%] CAS + IMD Supplementary Table 8). These events were generally considered by the sponsor as associated with COVID-19 and its complications.
| Adverse Event . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|
| Low-flow oxygena | n = 469 | n = 470 | n = 471 | n = 941 |
| Patients with any TEAEb | 132 (28.1) | 118 (25.1) | 131 (27.8) | 249 (26.5) |
| Patients with any grade 3 or 4 TEAE | 93 (19.8) | 68 (14.5) | 82 (17.4) | 150 (15.9) |
| Patients with any treatment-emergent SAE | 131 (27.9) | 106 (22.6) | 118 (25.1) | 224 (23.8) |
| Patients with any treatment-emergent AESI | 6 (1.3) | 10 (2.1) | 14 (3.0) | 24 (2.6) |
| Patients with any treatment-emergent serious AESI | 2 (0.4) | 4 (0.9) | 6 (1.3) | 10 (1.1) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 5 (1.1) | 7 (1.5) | 11 (2.3) | 18 (1.9) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.2) | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Patients with any TEAE leading to study infusion interruption | 1 (0.2) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| No supplemental oxygend | n = 198 | n = 202 | n = 197 | n = 399 |
| Patients with any TEAEb | 48 (24.2) | 31 (15.3) | 37 (18.8) | 68 (17.0) |
| Patients with any grade 3 or 4 TEAE | 31 (15.7) | 24 (11.9) | 23 (11.7) | 47 (11.8) |
| Patients with any treatment-emergent SAE | 43 (21.7) | 29 (14.4) | 32 (16.2) | 61 (15.3) |
| Patients with any treatment-emergent AESI | 2 (1.0) | 4 (2.0) | 6 (3.0) | 10 (2.5) |
| Patients with any treatment-emergent serious AESI | 1 (0.5) | 1 (0.5) | 3 (1.5) | 4 (1.0) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 1 (0.5) | 4 (2.0) | 4 (2.0) | 8 (2.0) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.5) | 0 | 2 (1.0) | 2 (0.5) |
| Patients with any TEAE leading to study infusion interruption | 0 | 0 | 2 (1.0) | 2 (0.5) |
| Adverse Event . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|
| Low-flow oxygena | n = 469 | n = 470 | n = 471 | n = 941 |
| Patients with any TEAEb | 132 (28.1) | 118 (25.1) | 131 (27.8) | 249 (26.5) |
| Patients with any grade 3 or 4 TEAE | 93 (19.8) | 68 (14.5) | 82 (17.4) | 150 (15.9) |
| Patients with any treatment-emergent SAE | 131 (27.9) | 106 (22.6) | 118 (25.1) | 224 (23.8) |
| Patients with any treatment-emergent AESI | 6 (1.3) | 10 (2.1) | 14 (3.0) | 24 (2.6) |
| Patients with any treatment-emergent serious AESI | 2 (0.4) | 4 (0.9) | 6 (1.3) | 10 (1.1) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 5 (1.1) | 7 (1.5) | 11 (2.3) | 18 (1.9) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.2) | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Patients with any TEAE leading to study infusion interruption | 1 (0.2) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| No supplemental oxygend | n = 198 | n = 202 | n = 197 | n = 399 |
| Patients with any TEAEb | 48 (24.2) | 31 (15.3) | 37 (18.8) | 68 (17.0) |
| Patients with any grade 3 or 4 TEAE | 31 (15.7) | 24 (11.9) | 23 (11.7) | 47 (11.8) |
| Patients with any treatment-emergent SAE | 43 (21.7) | 29 (14.4) | 32 (16.2) | 61 (15.3) |
| Patients with any treatment-emergent AESI | 2 (1.0) | 4 (2.0) | 6 (3.0) | 10 (2.5) |
| Patients with any treatment-emergent serious AESI | 1 (0.5) | 1 (0.5) | 3 (1.5) | 4 (1.0) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 1 (0.5) | 4 (2.0) | 4 (2.0) | 8 (2.0) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.5) | 0 | 2 (1.0) | 2 (0.5) |
| Patients with any TEAE leading to study infusion interruption | 0 | 0 | 2 (1.0) | 2 (0.5) |
Data are n(%).
Abbreviations: AESI, adverse event of special interest; CAS + IMD, casirivimab and imdevimab; IV, intravenous; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Phase 1/2/3 cohort 1.
TEAEs collected include treatment-emergent SAEs, AESIs, and grade 3/4 TEAEs, as well as ad hoc/voluntarily reported TEAEs by some sites.
Deemed treatment-related as per investigator assessment.
Phase 2 cohort 1A.
| Adverse Event . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|
| Low-flow oxygena | n = 469 | n = 470 | n = 471 | n = 941 |
| Patients with any TEAEb | 132 (28.1) | 118 (25.1) | 131 (27.8) | 249 (26.5) |
| Patients with any grade 3 or 4 TEAE | 93 (19.8) | 68 (14.5) | 82 (17.4) | 150 (15.9) |
| Patients with any treatment-emergent SAE | 131 (27.9) | 106 (22.6) | 118 (25.1) | 224 (23.8) |
| Patients with any treatment-emergent AESI | 6 (1.3) | 10 (2.1) | 14 (3.0) | 24 (2.6) |
| Patients with any treatment-emergent serious AESI | 2 (0.4) | 4 (0.9) | 6 (1.3) | 10 (1.1) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 5 (1.1) | 7 (1.5) | 11 (2.3) | 18 (1.9) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.2) | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Patients with any TEAE leading to study infusion interruption | 1 (0.2) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| No supplemental oxygend | n = 198 | n = 202 | n = 197 | n = 399 |
| Patients with any TEAEb | 48 (24.2) | 31 (15.3) | 37 (18.8) | 68 (17.0) |
| Patients with any grade 3 or 4 TEAE | 31 (15.7) | 24 (11.9) | 23 (11.7) | 47 (11.8) |
| Patients with any treatment-emergent SAE | 43 (21.7) | 29 (14.4) | 32 (16.2) | 61 (15.3) |
| Patients with any treatment-emergent AESI | 2 (1.0) | 4 (2.0) | 6 (3.0) | 10 (2.5) |
| Patients with any treatment-emergent serious AESI | 1 (0.5) | 1 (0.5) | 3 (1.5) | 4 (1.0) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 1 (0.5) | 4 (2.0) | 4 (2.0) | 8 (2.0) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.5) | 0 | 2 (1.0) | 2 (0.5) |
| Patients with any TEAE leading to study infusion interruption | 0 | 0 | 2 (1.0) | 2 (0.5) |
| Adverse Event . | Placebo . | CAS + IMD 2.4 g IV . | CAS + IMD 8.0 g IV . | CAS + IMD Combined . |
|---|---|---|---|---|
| Low-flow oxygena | n = 469 | n = 470 | n = 471 | n = 941 |
| Patients with any TEAEb | 132 (28.1) | 118 (25.1) | 131 (27.8) | 249 (26.5) |
| Patients with any grade 3 or 4 TEAE | 93 (19.8) | 68 (14.5) | 82 (17.4) | 150 (15.9) |
| Patients with any treatment-emergent SAE | 131 (27.9) | 106 (22.6) | 118 (25.1) | 224 (23.8) |
| Patients with any treatment-emergent AESI | 6 (1.3) | 10 (2.1) | 14 (3.0) | 24 (2.6) |
| Patients with any treatment-emergent serious AESI | 2 (0.4) | 4 (0.9) | 6 (1.3) | 10 (1.1) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 5 (1.1) | 7 (1.5) | 11 (2.3) | 18 (1.9) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.2) | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Patients with any TEAE leading to study infusion interruption | 1 (0.2) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| No supplemental oxygend | n = 198 | n = 202 | n = 197 | n = 399 |
| Patients with any TEAEb | 48 (24.2) | 31 (15.3) | 37 (18.8) | 68 (17.0) |
| Patients with any grade 3 or 4 TEAE | 31 (15.7) | 24 (11.9) | 23 (11.7) | 47 (11.8) |
| Patients with any treatment-emergent SAE | 43 (21.7) | 29 (14.4) | 32 (16.2) | 61 (15.3) |
| Patients with any treatment-emergent AESI | 2 (1.0) | 4 (2.0) | 6 (3.0) | 10 (2.5) |
| Patients with any treatment-emergent serious AESI | 1 (0.5) | 1 (0.5) | 3 (1.5) | 4 (1.0) |
| Patients with any treatment-emergent AESI of infusion-related reactions, grade ≥ 2, through day 4c | 1 (0.5) | 4 (2.0) | 4 (2.0) | 8 (2.0) |
| Patients with any treatment-emergent AESI of hypersensitivity reactions, grade ≥ 2, through day 29 | 1 (0.5) | 0 | 2 (1.0) | 2 (0.5) |
| Patients with any TEAE leading to study infusion interruption | 0 | 0 | 2 (1.0) | 2 (0.5) |
Data are n(%).
Abbreviations: AESI, adverse event of special interest; CAS + IMD, casirivimab and imdevimab; IV, intravenous; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Phase 1/2/3 cohort 1.
TEAEs collected include treatment-emergent SAEs, AESIs, and grade 3/4 TEAEs, as well as ad hoc/voluntarily reported TEAEs by some sites.
Deemed treatment-related as per investigator assessment.
Phase 2 cohort 1A.
Grade ≥ 2 infusion-related reactions occurred in few patients on low-flow (5/469 [1.1%] placebo versus 18/941 [1.9%] CAS + IMD) and no supplemental oxygen (1/198 [0.5%] placebo versus 8/399 [2.0%] CAS + IMD; Table 3). Grade ≥ 2 hypersensitivity reactions also occurred in few patients on low-flow (1/469 [0.2%] placebo versus 7/941 [0.7%] CAS + IMD) and no supplemental oxygen (1/198 [0.5%] placebo versus 2/399 [0.5%] CAS + IMD; Table 3). AESIs are further detailed in Supplementary Tables 9 and 10.
DISCUSSION
Hospitalized patients with COVID-19 experience high mortality rates, ranging from 10% to 30% [4, 17–19]. Until the recent results from the RECOVERY platform trial [15], it was unknown whether treatment with CAS + IMD in patients who were already hospitalized would meaningfully impact clinical outcomes. The current placebo-controlled randomized international trial (with approximately 55% of patients receiving concomitant remdesivir and 75% receiving steroids) demonstrated and extended the benefit reported in the RECOVERY trial among seronegative patients, and also documented no harm signals among seropositive patients receiving low-flow or no supplemental oxygen. When added to standard-of-care treatment, CAS + IMD may reduce all-cause mortality. While the primary clinical endpoint of death or mechanical ventilation from day 6 to 29 in the high viral load population had a strong positive trend but did not reach significance, all clinical endpoints demonstrated numeric improvements, predominantly driven by results in the seronegative population. CAS + IMD also improved the rates of hospital discharge and death or readmission to hospital at day 29, which persisted through day 57, showing possible benefit to patients as well as the overburdened health care system.
In the current variant-rich world with widespread COVID-19 vaccination, the utility of serostatus is unclear; numerous publications cite that even vaccinated patients with high antibody titers may have little to no neutralizing activity to emerging variants [20–23]. Future studies are needed to further explore the potential clinical benefit in seropositive patients and, in particular, seropositive patients whose antibodies lack neuralization potential for the circulating strain.
CAS + IMD is the first monoclonal antibody therapy, and the first SARS-CoV-2 antiviral, that significantly lowers viral load and may reduce mortality in hospitalized patients with COVID-19 [15]. Other monoclonal antibodies against the SARS-CoV-2 in hospitalized populations have failed to show such benefit [24, 25]. Very few treatments have demonstrated a mortality benefit in hospitalized COVID-19 patients, and most are designed to modulate the immune response late in the disease course after damage has occurred, rather than to clear SARS-CoV-2. The corticosteroid dexamethasone showed a 17% improvement in 28-day mortality in the RECOVERY trial, with the greatest benefit in patients receiving mechanical ventilation [26]. Baricitinib, a Janus kinase inhibitor, improved 28-day mortality by 38% in hospitalized patients [27]. Interleukin-6 inhibitors such as tocilizumab and sarilumab were recommended by the World Health Organization for use in hospitalized patients, in whom they reduced mortality by 13% [28, 29]. The Food and Drug Administration-approved medication remdesivir has shown some benefit against death and progression to ventilation in hospitalized patients with COVID-19 [30]. CAS + IMD’s mechanism of action and safety profile should allow combination approaches with any or all of these other agents.
CAS + IMD in patients on low-flow or no supplemental oxygen was well-tolerated and the safety profile was consistent with that observed previously [13, 15], showing low rates of infusion-related and hypersensitivity reactions. The placebo group experienced a greater frequency of SAEs and adverse events leading to death than the CAS + IMD group, consistent with the clinical benefit of treatment.
The absence of full representation across the spectrum of hospitalized patients on varying degrees of oxygen support is a limitation of this study. The respiratory status of the population in this article includes only those receiving low-flow or no supplemental oxygen, as the study did not enroll sufficient numbers of patients on high-intensity oxygen or mechanical ventilation prior to pausing of these cohorts early during the conduct of the study due to an imbalance in mortality observed in interim data. This imbalance was not observed in the much larger RECOVERY trial, where efficacy was seen across all hospitalized patients regardless of respiratory status [15]. The study was prematurely terminated due to slow recruitment resulting in smaller than planned sample size. As a result, key analyses pooled the 2 remaining patient cohorts (no supplemental oxygen/low-flow oxygen) as well as the 2 doses. Sensitivity analyses did not reveal major efficacy differences across the cohorts or doses. Observed variability in the magnitude of risk reductions, with greater effects for the 2.4-g dose compared to the 8.0-g dose, was likely due to small numbers within each group suggesting either dose can be utilized in hospitalized individuals requiring low-flow or no supplemental oxygen.
As an additional limitation, this study was conducted prior to widespread circulation of the Delta and Omicron variants of SARS-CoV-2. CAS + IMD, which contains 2 distinct neutralizing antibodies [5, 6], retains neutralizing potency against most viral variants of concern including Delta [31], but has been shown to have diminished neutralization activity against Omicron-lineage variants [10], which is the most prevalent lineage at the time of publication. Nonetheless, the results of this trial are informative for use of CAS + IMD against current or future circulating variants susceptible to CAS + IMD and also show promise for future SARS-CoV-2 antibodies that retain neutralizing capacity in the hospitalized population.
Taken together with reports from the RECOVERY trial, these data support CAS + IMD monoclonal antibody therapy as a well-tolerated treatment option to reduce viral load and likely reduce the risk of mortality in hospitalized patients with susceptible variants of SARS-CoV-2.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the patients who participated in this study, as well as their families; the study investigators; the members of the Independent Data Monitoring Committee; Kaitlyn Scacalossi, PhD, and Caryn Trbovic, PhD, from Regeneron Pharmaceuticals for assistance with development of the manuscript; and Prime, Knutsford, United Kingdom, for formatting and copyediting suggestions. Study sites and investigators as well as Regeneron study team members are listed in the Supplementary Material.
Regeneron study team. A. Thomas DiCioccio, Adebiyi Adepoju, Adnan Mahmood, Aisha Mortagy, Ajla Dupljak, Alison Brown, Alpana Waldron, Amanda Cook, Amra Arslanagic, Amy Froment, Andrea T. Hooper, Andrea Margiotta, Anita Islam, Anne Smith, Arvinder Dhillon, Aurora Breazna, Bari Kowal, Barry Silverstein, Bret Musser, Brian Bush, Brian Head, Bryan Zhu, Camille Debray, Careta Phillips, Carmella Simiele, Carol Lee, Carolyn Nienstedt, Caryn Trbovic, Catherine Elliott, Chad Fish, Charlie Ni, Charlotte Lyon, Christa Polidori, Christina Perry, Christine Enciso, Christopher Chamak, Christopher Powell, Cynthia Pan, Dana Wolken, Danise Subramaniam, David Liu, David M. Weinreich, David Stein, Dawlat Hassan, Daya Gulabani, Deborah Fix, Deborah Leonard, Deepshree Sarda, Denise Bonhomme, Denise Kennedy, Derrick Bramble, Devin Darcy, Dhanalakshmi Barron, Diana Hughes, Diana Rofail, Dipinder Kaur, Dominique Atmodjo Watkins, Dona Bianco, Donna Gambaccini, Eduardo Forleo Neto, Edward Jean-Baptiste, Ehsan Bukhari, Elizabeth Bucknam, Emily Nanna, Esther Huffman O’Keefe, Evelyn Gasparino, Evonne Fung, Flonza Isa, Fung-Yee To, Gary Herman, Gayatri Anand, George D. Yancopoulos, Georgia Bellingham, Giane Sumner, Grainne Moggan, Grainne Power, Gregory P. Geba, Gwyn Dixon, Haixia Zeng, Heath Gonzalez, Helen Cicirello, Helen Kang, Hibo Noor, Ian Minns, James Donohue, Jamie Rusconi, Janice Austin, Janie Parrino, Jeannie Yo, Jenna McDonnell, Jennifer D. Hamilton, Jessica Boarder, Jianguo Wei, Jing Xiao, Jingchun Yu, Jingning Mei, Joanne Malia, Joanne Tucciarone, Jodie Tyler-Gale, John D. Davis, John Rembis, John Strein, Jonathan Cohen, Jonathan Meyer, Jordan Ursino, Joseph Im, Joseph Tramaglini, Joseph Wolken, Jutta Miller, Kaitlyn Potter, Kaitlyn Scacalossi, Kamala Naidu, Kara Ford, Karen Browning, Karen Yau, Katherine Woloshin, Kelly Lewis-Amezcua, Kenneth C. Turner, Kit Chiu, Kristina McGuire, Kristy Macci, Kurt Ringleben, Kyle Foster, Lacey Douthat, Latora Knighton, Leah Lipsich,† Lillian Brener, Linda Kelly, Lindsay Darling, Lisa Boersma, Lisa Cowen, Lisa Cupelli, Lisa Hersh, Lisa Jackson, Lisa Purcell, Lisa Sherpinsky, Lori Geissler, Louise Boppert, Lyra Fiske, Mahesh Vadyala, Manika Bista, Marc Dickens, Maureen Weimer, Meagan O’Brien, Michael Batchelder, Michael Partridge, Michel Tarabocchia, Mivia Rodriguez, Moetaz Albizem, Muriel O’Byrne, Nagaratna Medapati, Ned Braunstein, Neena Sarkar, Neil Stahl, Ngan Trinh, Nicholas Moore, Nicole Deitz, Nicole Memblatt, Nirav Shah, Nitin Kumar, Nkechi Moghalu, Olga Herrera, Oluchi Adedoyin, Ori Yellin, Pamela Snodgrass, Patrick Floody, Paul D’Ambrosio, Peter Boutros, Prankur Krishnatry, Qin Li, Rafia Bhore, Rakiyya Ali, Ramya Iyer, Rinol Alaj, Rita Pedraza, Robert Hamlin, Romana Hosain,† Ruchin Gorawala, Ryan White, Ryan Yu, Rylee Fogarty, S. Balachandra Dass, Sagarika Bollini, Samit Ganguly, Sandra DeCicco, Sandra Osbild, Sara Dale, Selin Somersan-Karakaya, Sharon Henkel, Shazia Ali, Shelley Geila Shapiro, Soraya Nossoughi, Steve Chen, Steven Elkin, Steven Long, Sumathi Sivapalasingam,† Susan Irvin, Susan Wilt, Suzanne Luther, Tami Min, Tatiana Constant, Theresa Devins, Travis Bernardo, Viet Pham, Violet Vincent, Xin Chen, Yanmei Tian, Yasmin Khan, Yiping Sun, Yuhwen Soo, Yuming Zhao, Yunji Kim. †Former employee of Regeneron Pharmaceuticals, Inc.
Author contributions. Conceptualization was by S. S.-K., S. A., S. S., Y. Sun, R. B., J. Mei, E. F.-N., A. T. H., J. D. H., R. H., A. M., J. D. D., K. C. T., B. K., A. T. D., G. P. G., N. S., L. L., N. B., G. A. H., G. D. Y., and D. M. W. Data curation was performed by V. P. M., J. C. W., Y. Sun, R. B., J. Mei, L. C., A. T. H., J. D. H., C. P., V. P., Y. Z., Y. K., A. C., and Y. Soo. Formal analysis was performed by S. S.-K., S. A., S. S., Y. Sun, R. B., J. Mei, R. H., A. M., J. D. D., K. C. T., Y. Soo, A. T. D., G. P. G., L. L., N. B., G. A. H., G. D. Y., and D. M. W. Investigation was by E. M., C. P., V. P., Y. Z., E. F.-N., B. K., and G. P. G. Methodology was by Y. Sun, R. B., J. Mei, E. F.-N., A. T. H., J. D. H., and G. P. G. Project administration was by A. T. H., J. D. H., C. P., V. P., Y. Z., Y. K., and A. C. Resourcing was by S. S.-K., S. A., S. S., J. Miller, C. P., V. P., Y. Z., Y. K., and A. C. Software and visualization was by Y. Sun, R. B., and J. Mei. Validation was performed by Y. Sun, R. B., J. Mei, A. T. H., and J. D. H. Supervision was by Y. Sun, R. B., J. Mei, E. F.-N., R. H., A. M., B. K., Y. Soo, A. T. D., L. L., N. B., G. A. H., G. D. Y., and D. M. W. The original draft was written by S. S.-K., S. A., G. D. Y., and D. M. W. with review and editing by S. S.-K., E. M., V. P. M., J. C. W., S. A., S. S., Y. Sun, R. B., J. Mei, J. Miller, L. C., E. F.-N., A. T. H., J. D. H., R. H., A. M., J. D. D., K. C. T., B. K., Y. Soo, A. T. D., G. P. G., N. S., L. L., N. B., G. A. H., G. D. Y., and D. M. W.
Financial support. This work was supported by Regeneron Pharmaceuticals, Inc. Certain aspects of this project were supported by federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, and Biomedical Advanced Research and Development Authority (grant number HHSO100201700020C).
Data availability
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (eg, Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency, etc.), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Requests should be submitted to https://vivli.org/.
References
Author notes
S. S.-K. and E. M. contributed equally.
Former employee of Regeneron Pharmaceuticals, Inc.
Presented in part: 2022 American Society for Clinical Pharmacology and Therapeutics, virtual meeting, 16–18 March 2022; and 2021 IDWeek, virtual conference, 30 September 2021.
Potential conflicts of interest. S. S.-K., S. A., Y. Sun, R. B., J. Mei, J. Miller, E. F.-N., C. P., V. P., Y. Z., A. M., J. D. D., Y. K., A. C., B. K., Y. Soo, A. T. D., G. P. G., L. L., N. B., and D. M. W. are employees/stockholders of Regeneron Pharmaceuticals, Inc, and report grants from Biomedical Advanced Research and Development Authority (BARDA). E. M. reports payments to his institution received from National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases, NIH/National Institute of General Medical Sciences, SciClone Pharmaceuticals, Regeneron Pharmaceuticals, Inc, Pfizer, Chemic Labs/KODA Therapeutics, Cidara, and Leidos Biomedical Research Inc/NCI. V. P. M. and J. C. W. report grants from BARDA. S. S. is an Excision BioTherapeutics employee/stockholder and former Regeneron Pharmaceuticals, Inc, employee and current stockholder, and reports grants from BARDA. L. C. is a Regeneron Pharmaceuticals, Inc employee and reports grants from BARDA. A. T. H. is a Regeneron Pharmaceuticals, Inc employee/stockholder, a former Pfizer employee and current stockholder, has a patent pending with Regeneron Pharmaceuticals, Inc and reports grants from BARDA. J. D. H., K. C. T., and G. A. H. are employees/stockholders of Regeneron Pharmaceuticals, Inc and have a patent pending, which has been licensed and receiving royalties, with Regeneron Pharmaceuticals, Inc. R. H. is a former employee and current stockholder of Regeneron Pharmaceuticals, Inc, and reports grants from BARDA. N. S. and G. D. Y. are employees/stockholders of Regeneron Pharmaceuticals, Inc, and have issued patents (US Patent Nos. 10 787 501, 10 954 289, and 10 975 139) and pending patents, which have been licensed and receiving royalties, with Regeneron Pharmaceuticals, Inc, and reports grants from BARDA. Funding to pay the Open Access publication charges for this article was provided by Regeneron Pharmaceuticals, Inc.




